Abstract
Background
The study sought to compare Coronary Artery Disease Reporting and Data System (CAD-RADS) classification with traditional coronary artery disease (CAD) classifications and Duke Prognostic CAD Index for predicting the risk of all-cause mortality in patients with suspected CAD.
Methods
9625 consecutive suspected CAD patients were assessed by coronary CTA for CAD-RADS classification, traditional CAD classifications and Duke Prognostic CAD Index. Kaplan–Meier and multivariable Cox models were used to estimate all-cause mortality. Discriminatory ability of classifications was assessed using time dependent receiver-operating characteristic (ROC) curves and The Hosmer–Lemeshow goodness-of-fit test was employed to evaluate calibration.
Results
A total of 540 patients died from all causes with a median follow-up of 4.3 ± 2.1 years. Kaplan–Meier survival curves showed the cumulative events increased significantly associated with CAD-RADS, three traditional CAD classifications and Duke Prognostic CAD Index. In multivariate Cox regressions, the risk for the all-cause death increased from HR 0.861 (95% CI 0.420–1.764) for CAD-RADS 1 to HR 2.761 (95% CI 1.961–3.887) for CAD-RADS 4B&5, using CAD-RADS 0 as the reference group. The relative HRs for all-cause death increased proportionally with the grades of the three traditional CAD classifications and Duke Prognostic CAD Index. The area under the time dependent ROC curve for prediction of all-cause death was 0.7917, 0.7805, 0.7991for CAD-RADS in 1 year, 3 year, 5 year, respectively, which was non-inferior to the traditional CAD classifications and Duke Prognostic CAD Index.
Conclusions
The CAD-RADS classification provided important prognostic information for patients with suspected CAD with noninvasive evaluation, which was non-inferior than Duke Prognostic CAD Index and traditional stenosis-based grading schemes in prognostic value of all-cause mortality. Traditional and simplest CAD classification should be preferable, given the more number of groups and complexity of CAD-RADS and Duke prognostic index, without using more time consuming classification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Cardiovascular disease (CVD) remains the leading cause of premature mortality and rising health care costs [1, 2]. A multinational collaborative research study recently reported the prevalent case of CVD nearly doubled and the number of CVD deaths increased 6.5 million in the world from 1990 to 2019 [3]. Coronary computed tomography angiography (CTA) has been proved to be reliable for triage patients with stable or acute chest pain of ischemic origin [4]. UK National Institute for Health and Clinical Excellence (NICE) and European Society of Cardiology (ESC) recommend coronary CTA as a first-line strategy for evaluation of patients presenting with non-acute chest pain [5] or with chest pain and low to intermediate likelihood of having obstructive coronary artery disease (CAD) [6]. It seems likely that coronary CTA will play an increasingly dominant role for the evaluation of suspected CAD based on these developments.
The Coronary Artery Disease-Reporting and Data System (CAD-RADS) is a new standardized method for classification of CAD, which is then integrated into patient-specific clinical care [7]. The international CONFIRM registry recently reported that CAD-RADS was associated with 5-year outcome in 5039 patients [8]. Another recent study revealed that CAD-RADS added incremental prognostic value beyond atherosclerotic cardiovascular disease (ASCVD) risk score and coronary artery calcification scores (CACS) with a median follow-up of 2 years [9]. However, this prognostic value of CAD-RADS has been reported only in the US and other western countries with a relative short term follow-up. Whether there is incremental prognostic value of CAD-RADS beyond existing traditional stenosis classifications or Duke Prognostic CAD Index in the Chinese population with long term follow-up is unknown. Thus, the aim of this study was to evaluate the prognostic value of CAD-RADS classifications to predict the risk of all-cause mortality in patients with suspected CAD compared with traditional stenosis classifications and Duke Prognostic CAD Index.
Methods
This retrospective study was approved by the Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology. We confirmed that all methods were performed in accordance with the related guidelines and the principles of the Declaration of Helsinki. Written informed consent was waived because of its retrospective observational nature by Institutional Review Board of the Clinical Research Institute at The Central Hospital of Wuhan.
Study population
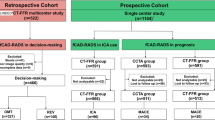
This study population consisted of 11,356 consecutive suspected CAD patients who underwent coronary CTA for evaluation from January 2012 to December 2019 (Fig. 1). Patients included in our analysis meet the following inclusion criteria: (1) adults who were 18 years old or older; (2) refer for coronary CTA using a ≥ 64-detector row scanner; (3) good quality images acquired that could be diagnosed with standardized reporting of segmental coronary stenosis [10]. The following exclusion criteria were used: (1) repeated coronary CTA examination in the database (n = 876) and patients younger than 18 years (n = 7); (2) patients had congenital heart disease (n = 44); (3) patients had known CAD (prior MI, angiographically confirmed CAD, PCI or CABG, n = 561); (4) Coronary CTA datasets were not available (n = 44) or non-diagnostic image quality (including insufficient image quality, motion of one or more vessels, bad contrast and wrong bolus timing, n = 144) and (5) loss of follow-up (n = 86). At last, a total of 9625 patients were included in the final analysis.
Coronary CTA protocol, CAD-RADS, traditional CAD classifications and Duke Prognostic CAD Index definitions
Coronary CTA was performed using one of the four CT systems: Aquilion 64, Toshiba Medical Systems, Otawara, Japan; Philips Brilliance iCT, Philips Medical Systems, Best, the Netherlands; uCT 760, United Imaging, China; SOMATOM Definition AS, Siemens Healthineers, Germany which has been reported previously [11]. Heart rate control (HR ≥ 65 beats/min) was performed with beta-blockers before the scan. Scanning parameters were as following: tube voltage 120 kV, tube current 280–300 mAs. For contrast enhancement, 60–80 mL of iopromide (370 mgI/mL, Bayer Schering Pharma, Germany) followed by 30–40 mL of pure saline with a flow rate of 4–5 mL/s. The iodine contrast agent was automatically triggered into descending aorta of 100 HU threshold units. Then the scanning was performed during an inspiratory breath hold of 8–14 s after delay of 2 s. The reconstruction images were automatic send to one of the four workstations VITREA 2 (version 6.1, Vital Images, Inc, Minnesota, America), Intelligence space portal (Version 6.0.4, Philips Medical Systems, Best, the Netherlands), UIH Advanced Workstation (uWS-CT, R004, United Imaging Healthcare, Shanghai, China), SyngoVIA (Siemens Medical Solutions, Forchheim, Gemany).
First, according to the CAD-RADS consensus document [7], the standardized CAD-RADS classification was based on the highest degree of coronary stenosis and defined as follows: CAD-RADS 0: 0% stenosis, CAD-RADS 1: 1–24% stenosis, CAD-RADS 2: 25–49% stenosis, CAD-RADS 3: 50–69% stenosis, CAD-RADS 4A: 70–99% stenosis in 1 or 2 vessels, CAD-RADS 4B: Left main > 50% stenosis or 3-vessel disease, 70–99% stenosis, CAD-RADS 5: 100% stenosis or total occlusion. In order to restrict our analysis to patients without previously known CAD or revascularization, we did not include CAD-RADS modifiers to describe patients with stents (modifier S), or grafts (modifier G) in our analysis. Second, three traditional stenosis classifications and Duke Prognostic CAD Index were described as following [8, 9, 12]: Traditional CAD Classification 1, no CAD: 0% stenosis, mild CAD: 1–49% stenosis, moderate CAD: 50–69% stenosis in any major vessels/branch, severe CAD: ≥ 50% stenosis in LM or ≥ 70% stenosis in any major vessels/branch. Traditional CAD Classification 2, normal: 0% stenosis, mildly abnormal: 1–69% stenosis in any major vessels/branch, or 1–49% stenosis in LM, moderately abnormal: ≥ 70% stenosis in any major vessels/branch, severely abnormal: ≥ 70% stenosis in 2 or more vessel, or ≥ 50% stenosis in LM, or ≥ 70% stenosis in proximal left anterior descending (pLAD). Traditional CAD Classification 3, no CAD: 0% stenosis, nonobstructive CAD: 1–49% stenosis in any major vessels/branch, 1 vessel obstructive CAD: ≥ 50% stenosis in 1 major vessels/branch, 2 vessel obstructive CAD: ≥ 50% stenosis in 2 major vessels/branch, 3 vessel/LM obstructive CAD: ≥ 50% stenosis in 3 major vessels/branch, or ≥ 50% stenosis in LM. Duke Prognostic CAD Index, Duke CAD 0: 0% stenosis in all vessels, Duke CAD 1: 1–24% stenosis, or at most 1 with 25–49% stenosis, Duke CAD 2: ≥ 2 stenosis 25–49%, Duke CAD 3: 1 vessel with 50–69% stenosis, Duke CAD 4: 2 stenosis 50–69%, or 1 vessel with ≥ 70% stenosis, Duke CAD 5: 3 stenosis 50–69%, or 2 vessels with ≥ 70% stenosis, or pLAD stenosis ≥ 70%, Duke CAD 6: 3 vessels ≥ 70% stenosis, 2 vessels with ≥ 70% stenosis with pLAD, Duke CAD 7: LM stenosis ≥ 50%.
Follow-up
The primary endpoint was all-cause mortality. Follow-up procedures were approved by our hospital’s institutional review board (the Institutional Review Board of the Clinical Research Institute at The Central Hospital of Wuhan). Death status was ascertained by querying the local Community Health Service Centers. For death outside of the city, event was determined through telephone call, or review of medical records. The deadline date of follow-up was December 31, 2020.
Statistical analysis
Continuous variables were presented as mean ± SD. Categorical variables are presented as frequencies and percentages. We used Student’s t test for continuous variables between groups. Categorical variables were compared using a chi-square test or Fisher’s exact test as appropriate. Cox proportional hazards regression models were used to calculated time to death of all cause and hazard ratios (HR) with 95% confidence intervals (95% CI). Kaplan–Meier method was used to estimate cumulative event-free survival. Time dependent Receiver-operating characteristic (ROC) curves were used to evaluate the discriminatory value of traditional CAD classifications, Duke Prognostic CAD Index and CAD-RADS grading for the outcome. The calibration of models compares the agreement between the predicted and observed event rates. The Hosmer–Lemeshow goodness-of-fit test was employed to evaluate calibration. This test divided patients into deciles according to the risk scores of models and compared the predicted versus the observed rates of mortality. A significant P value indicates a lack-of-fit and suboptimal calibration. A two-tailed P < 0.05 was considered statistically significant. All statistical analyses were performed using Stata version 16 (StataCorp LP, College Station, Texas) and R statistical package (version 3.6.3, R foundation for Statistical Computing, Vienna, Austria).
Results
Baseline clinical and coronary CTA characteristics of the study
Overall, 9625 patients were included in the final analysis. Figure 1 showed the reason for exclusion. Of the 9625 patients, the average age was 59.8 ± 10.7 years, and 44.3% (4262 of 9619) were male, the baseline clinical characteristics and coronary CTA characteristics of the study population in each group based on three traditional CAD classifications, Duke Prognostic CAD Index or CAD-RADS classification were shown in Table 1.
Estimating death from all causes
A total of 540 patients died from all causes with a median follow-up of 4.3 ± 2.1 years. Kaplan–Meier survival curves showed the cumulative events increased significantly associated with CAD-RADS, three traditional CAD classifications and Duke Prognostic CAD Index (log-rank test, all P < 0.001, Fig. 2). The univariate Cox regression analysis showed that compared to CAD-RADS 0 group, CAD-RADS 1, 2, 3, 4A, 4B, 5 were significantly associated with all-cause mortality (all P < 0.001).
In multivariate Cox regressions that were adjusted for gender and age, the risk for the all-cause death increased from HR 0.861 (95% CI 0.420–1.764) for CAD-RADS 1 to HR 2.761 (95% CI 1.961–3.887) for CAD-RADS 4B&5, using CAD-RADS 0 as the reference group. The relative HRs for all-cause death increased proportionally with the grades of the three traditional CAD classifications and Duke Prognostic CAD Index (Table 2).
Discriminatory ability of classifications
The prognostic performance of CAD-RADS compared to the three traditional CAD classification of characterizing CAD extent/severity and Duke Prognostic CAD Index were shown in Fig. 3. The area under the time dependent ROC curve for prediction of all-cause death was 0.7917, 0.7805, 0.7991 for CAD-RADS in 1 year, 3 year, 5 year, respectively, which was similar compared to the traditional CAD classification 1 (0.7928 in 1 year, 0.7819 in 3 year, 0.7987 in 5 year; all P > 0.05), traditional CAD classification 2 (0.7945 in 1 year, 0.78 in 3 year, 0.7965 in 5 year; all P > 0.05), traditional CAD classification 3 (0.7926 in 1 year, 0.7812 in 3 year, 0.7996 in 5 year; all P > 0.05) and Duke Prognostic CAD Index (0.7930 in 1 year, 0.7811 in 3 year, 0.799 in 5 year; all P > 0.05). The five Classifications showed good calibration in the overall cohort (Hosmer–Lemeshow P value were 0.732, 0.71, 0.39, 0.505 and 0.91 for traditional CAD classification 1 traditional CAD classification 2, traditional CAD classification 3, Duke Prognostic CAD Index and CAD-RADS, respectively) (Additional file 1: Figure S1).
Discussion
The present study examine a long-term prognosis associated with the novel CAD-RADS classification in the Chinese population, which were strongly predictive of all-cause death among suspected CAD patients in a real-world and were non-inferior to Duke Prognostic CAD Index and other coronary CTA prognostic classifications.
As a collaborative effort, CAD-RADS can now guide for clinical management with standardize CAD classifications after noninvasive imaging and may enhance communication between test abnormalities and optimal patient care. As a new classification system, high consistency of diagnosis is an essential prerequisite for its application. In a recent study [13], Inter-observer reproducibility of CAD-RADS categories was evaluation. The agreement among expert readers (ICC 0.925, 95% CI 0.884–0.954) and early career readers (ICC 0.904, 95% CI 0.852–0.941) were both excellent. However, the concordance for modifier “V” (high-risk plaque) is fair (kappa = 0.40), with poor-to-fair agreement for each of the high-risk plaque features. These findings were supported by another recent study [14]. The CAD-RADS category is the most important, regardless of the presence of a modifier [15]. Based on these results, the present study did not incorporated modifier (V) in the analysis.
From the large retrospective study, CAD-RADS grades were strongly associated with all-cause mortality, ranging from a 2- to fivefold increase in risk with progressively higher grades compared to a normal coronary CTA. We further found that CAD-RADS did not improve CAD risk discrimination for future events compared to the traditional CAD classifications and Duke Prognostic CAD Index. This findings was in keeping with the CONFIRM (Coronary CT Angiography Evaluation For Clinical Outcomes) registry study which has shown that increasing CAD-RADS scores were correlated with an increased risk of all-cause mortality up to a HR of 3.09 (95% CI 1.87–4.92) for patients with CAD-RADS 5 after adjusted for patient demographics and cardiac risk factors [8]. Analysis from the PROMISE (PROMISE [Prospective Multicenter Imaging Study for Evaluation of Chest Pain) study [9] showed that CAD-RADS had significantly higher discriminatory value than traditional CAD classifications (C-statistic 0.747 vs. 0.698–0.717; all P ≤ 0.001) which was different from the present study and the CONFIRM registry study findings. However, although with a randomized comparative effectiveness trial design from 193 sites, the relative smaller sample of 3840 patients were included in the analysis compared with the present study. Moreover, high-risk plaque (HRP) was added in the analysis and the agreement among expert readers for HRP (kappa = 0.56) is lower than that of ≥ 70% stenosis or left main ≥ 50% stenosis (kappa = 0.69). In addition, the definition of the endpoint was difference from the two studies that our primary outcome was all-cause mortality, yet nonfatal myocardial infarction was added in the CONFIRM registry study and myocardial infarction and hospitalization for unstable angina in the PROMISE study. The present study revealed that the patients with CAD-RADS 4B&5 classifications were at the highest risk of subsequent events. These patients had 3 vessel disease ≥ 70% or left main stem stenosis ≥ 50%. This finding was in keeping with other researches which have reported that the presence of obstructive CAD is associated with a poorer prognosis [16,17,18,19].
The Duke Prognostic CAD Index was originally derived for important prognostic aspects with a more detailed information of coronary anatomy than the traditional CAD classification of normal, one, two and three-vessel disease, and has been widely used to assess the mortality risk for treatment modalities based on the severity of coronary disease severity [20, 21]. The present study revealed similar prognostic value for predicting all-cause death among suspected CAD patients as those in the CONFIRM registry study, the PROMISE study and a multidetertor CCTA study [12]. However, compared with the Duke Prognostic CAD Index, CAD-RADS classification is more concise and thus is more conducive to enhance communication between interpreting and referring clinicians. Moreover, automated classification of CAD-RADS based on structured reporting systems may improve data quality and then establishing standard databases with education, patient care and research purposes [22,23,24].
The quantification of CAD scoring systems were initially used as a tool for both clinical practice and scientific investigation by invasive coronary angiography (ICA) [25]. Coronary CTA is a relatively new test that enables noninvasive and direct visualization of the presence and extent of coronary stenosis. Increasing amounts of CAD scoring based on coronary CTA including CACS are used in clinical and research [8, 9, 19, 26]. As a standardized reporting system of CCTA, the primary aim of CAD-RADS is to facilitate the consistent fashion among physicians, including recommendations for further investigations and management. Despite of performing similar as well as traditional CAD classifications and Duke prognostic index in the present, a limitation of CAD-RADS is that only the highest grade of stenosis is considered and more number of groups and complexity than the traditional CAD classifications and CACS. Duke prognostic index based on coronary CTA is also a valuable classification [12]. However, complex classifications also exit than traditional CAD classifications and CACS. Traditional CAD classifications are simple and widely used in clinic. Nevertheless, modifiers with nonevaluable, stent, coronary bypass graft, and high-risk vulnerable plaque features may not be included in the standardized reporting system compared with CAD-RADS.
Despite the import findings and clinical implications for CAD-RADS prognostic value in patients with suspected CAD in the present study, the study had several limitations. First, the study contains a relative larger sample size; however, this was conducted at a single center. In addition, the selection bias may be present with the retrospective nature of this study. Second, the numbers of classes are different according to the classifications that may lead inconsistent of the proportion between complex classifications and simple classifications. Larger samples and multicenter researches are needed to reduce bias. Third, as we restricted our analysis to suspected CAD patients without previously known CAD, CAD-RADS modifiers to describe patients with stents (modifier S), grafts (modifier G), or vulnerable plaque features (modifier V) were not included in the present study. Finally, the present study had limited data on coronary artery calcium (CAC) which was recently shown with high correlation with the presence of obstructive stenosis and suggested to be the main predictor of risk for death by the WDHR (Western Denmark Heart Registry) study [27].
In conclusion, the CAD-RADS classification provided important prognostic information for patients with suspected CAD with noninvasive evaluation, which was non-inferior than Duke Prognostic CAD Index and traditional stenosis-based grading schemes in prognostic value of all-cause mortality. Traditional and simplest CAD classification should be preferable, given the more number of groups and complexity of CAD-RADS and Duke prognostic index, without using more time consuming classification.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CAD-RADS:
-
Coronary artery disease reporting and data system
- CAD:
-
Coronary artery disease
- CTA:
-
Computed tomography angiography
- ROC:
-
Receiver-operating characteristic
- AUC:
-
Area under curve
- CVD:
-
Cardiovascular disease
- ASCVD:
-
Atherosclerotic cardiovascular disease
- CACS:
-
Coronary artery calcification scores
- HR:
-
Hazard ratios
- CI:
-
Confidence intervals
- PCI:
-
Percutaneous coronary intervention
- CABG:
-
Coronary artery bypass graft
- HRP:
-
High-risk plaque
- CAC:
-
Coronary artery calcium
References
Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22.
Mensah GA, Roth GA, Fuster V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J Am Coll Cardiol. 2019;74(20):2529–32.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021.
Rubinshtein R, Hamdan A. Coronary CTA-based CAD-RADS reporting system and the PROMISE to predict cardiac events. JACC Cardiovasc Imaging. 2020;13(7):1546–8.
National Institute for Health and Clinical Excellence. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin (update). CG95 London, UK: National Institute for Health and Clinical Excellence; 2016. https://www.nice.org.uk/guidance/cg95. Accessed October 2019.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–77.
Cury RC, Abbara S, Achenbach S, Agatston A, Berman DS, Budoff MJ, Dill KE, Jacobs JE, Maroules CD, Rubin GD, et al. CAD-RADS(TM) coronary artery disease—reporting and data system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr. 2016;10(4):269–81.
Xie JX, Cury RC, Leipsic J, Crim MT, Berman DS, Gransar H, Budoff MJ, Achenbach S, Hartaigh B, Callister TQ, et al. The coronary artery disease-reporting and data system (CAD-RADS): prognostic and clinical implications associated with standardized coronary computed tomography angiography reporting. JACC Cardiovasc Imaging. 2018;11(1):78–89.
Bittner DO, Mayrhofer T, Budoff M, Szilveszter B, Foldyna B, Hallett TR, Ivanov A, Janjua S, Meyersohn NM, Staziaki PV, et al. Prognostic value of coronary CTA in stable chest pain: CAD-RADS, CAC, and cardiovascular events in PROMISE. JACC Cardiovasc Imaging. 2020;13(7):1534–45.
Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, Nieman K, Pontone G, Raff GL. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8(5):342–58.
Huang Z, Xiao J, Li Z, Hu Y, Xie Y, Zhang S, Wang X. Evaluation of LVDD by CCTA with dual-source CT in type 2 diabetes mellitus patients. Curr Med Imaging. 2020;16(3):214–23.
Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, Lippolis NJ, Berman DS, Callister TQ. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50(12):1161–70.
Maroules CD, Hamilton-Craig C, Branch K, Lee J, Cury RC, Maurovich-Horvat P, Rubinshtein R, Thomas D, Williams M, Guo Y, et al. Coronary artery disease reporting and data system (CAD-RADS(TM)): inter-observer agreement for assessment categories and modifiers. J Cardiovasc Comput Tomogr. 2018;12(2):125–30.
Abdel Razek AAK, Elrakhawy MM, Yossof MM, Nageb HM. Inter-observer agreement of the Coronary Artery Disease Reporting and Data System (CAD-RADS(TM)) in patients with stable chest pain. Pol J Radiol. 2018;83:e151–9.
Canan A, Ranganath P, Goerne H, Abbara S, Landeras L, Rajiah P. CAD-RADS: pushing the limits. Radiographics. 2020;40(3):629–52.
Williams MC, Moss A, Dweck M, Hunter A, Pawade T, Adamson PD, Shah ASV, Alam S, Maroules CD, van Beek EJ, et al. Standardized reporting systems for computed tomography coronary angiography and calcium scoring: a real-world validation of CAD-RADS and CAC-DRS in patients with stable chest pain. J Cardiovasc Comput Tomogr. 2020;14(1):3–11.
Hadamitzky M, Taubert S, Deseive S, Byrne RA, Martinoff S, Schomig A, Hausleiter J. Prognostic value of coronary computed tomography angiography during 5 years of follow-up in patients with suspected coronary artery disease. Eur Heart J. 2013;34(42):3277–85.
Andreini D, Pontone G, Mushtaq S, Bartorelli AL, Bertella E, Antonioli L, Formenti A, Cortinovis S, Veglia F, Annoni A, et al. A long-term prognostic value of coronary CT angiography in suspected coronary artery disease. JACC Cardiovasc Imaging. 2012;5(7):690–701.
Petretta M, Daniele S, Acampa W, Imbriaco M, Pellegrino T, Messalli G, Xhoxhi E, Del Prete G, Nappi C, Accardo D, et al. Prognostic value of coronary artery calcium score and coronary CT angiography in patients with intermediate risk of coronary artery disease. Int J Cardiovasc Imaging. 2012;28(6):1547–56.
Califf RM, Harrell FE Jr, Lee KL, Rankin JS, Hlatky MA, Mark DB, Jones RH, Muhlbaier LH, Oldham HN Jr, Pryor DB. The evolution of medical and surgical therapy for coronary artery disease. A 15-year perspective. JAMA. 1989;261(14):2077–86.
Mark DB, Nelson CL, Califf RM, Harrell FE Jr, Lee KL, Jones RH, Fortin DF, Stack RS, Glower DD, Smith LR, et al. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation. 1994;89(5):2015–25.
Szilveszter B, Kolossvary M, Karady J, Jermendy AL, Karolyi M, Panajotu A, Bagyura Z, Vecsey-Nagy M, Cury RC, Leipsic JA, et al. Structured reporting platform improves CAD-RADS assessment. J Cardiovasc Comput Tomogr. 2017;11(6):449–54.
Faggioni L, Coppola F, Ferrari R, Neri E, Regge D. Usage of structured reporting in radiological practice: results from an Italian online survey. Eur Radiol. 2017;27(5):1934–43.
Huang Z, Xiao J, Xie Y, Hu Y, Zhang S, Li X, Wang Z, Li Z, Wang X. The correlation of deep learning-based CAD-RADS evaluated by coronary computed tomography angiography with breast arterial calcification on mammography. Sci Rep. 2020;10(1):11532.
Neeland IJ, Patel RS, Eshtehardi P, Dhawan S, McDaniel MC, Rab ST, Vaccarino V, Zafari AM, Samady H, Quyyumi AA. Coronary angiographic scoring systems: an evaluation of their equivalence and validity. Am Heart J. 2012;164(4):547-552.e541.
Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR, Huang M, Pencina M, Mark DB, Heitner JF, et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation. 2017;135(24):2320–32.
Mortensen MB, Dzaye O, Steffensen FH, Botker HE, Jensen JM, Ronnow Sand NP, Kragholm KH, Sorensen HT, Leipsic J, Maeng M, et al. Impact of plaque burden versus stenosis on ischemic events in patients with coronary atherosclerosis. J Am Coll Cardiol. 2020;76(24):2803–13.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The following authors contributed to the preparation of the manuscript as follows: ZH: Study design, manuscript editing, interpretation of data, literature search. SZ, NJ: Study design, Statistical analysis, manuscript editing, literature search. YH, XW: Study design and concept, manuscript editing, interpretation of data, literature search. JX, ZL, YY, RS, ZW, XL, YX: interpretation of data, literature search, manuscript editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The research was approved by the Institutional Review Board of the Clinical Research Institute at The Central Hospital of Wuhan (No. 2021-003, Supplementary textual file). We confirmed that all methods were performed in accordance with the related guidelines and the principles of the Declaration of Helsinki. Written informed consent was waived because of its retrospective observational nature.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Calibration plot of the models.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, Z., Zhang, S., Jin, N. et al. Prognostic value of CAD-RADS classification by coronary CTA in patients with suspected CAD. BMC Cardiovasc Disord 21, 476 (2021). https://doi.org/10.1186/s12872-021-02286-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-021-02286-x