Abstract
Background
Comparative studies regarding the long-term clinical outcomes of statin intensity between acute myocardial infarction (AMI) patients with prediabetes and those with type 2 diabetes mellitus (T2DM), after successful implantation of newer-generation drug-eluting stents (DES) with statin treatment, are limited. We compared the 2-year clinical outcomes between these patients.
Methods
A total of 11,612 AMI patients were classified as statin users (n = 9893) and non-users (n = 1719). Thereafter, statin users were further divided into high-intensity (n = 2984) or low-moderate-intensity statin (n = 6909) treatment groups. Those in these two groups were further classified into patients with normoglycemia, prediabetes, and T2DM. The major outcomes were the occurrence of major adverse cardiac events (MACE), defined as all-cause death, recurrent myocardial infarction (Re-MI), or any repeat coronary revascularization.
Results
After adjusting for both high-intensity and low-moderate-intensity statin users, the cumulative incidences of MACE (p = 0.737, p = 0.062, respectively), all-cause death, Re-MI, and any repeat revascularization were similar between the prediabetes and T2DM groups. In the total study population, both high-intensity and low-moderate-intensity statin treatments showed comparable results. However, in the patients who enrolled after October 2012, the cumulative incidences of MACE (aHR 1.533; 95% CI 1.144–2.053; p = 0.004) and any repeat revascularization (aHR, 1.587; 95% CI 1.026–2.456; p = 0.038) were significantly lower in high-intensity statin users than in low-moderate intensity statin users. The beneficial effects of high-intensity compared to low-moderate-intensity statin therapy were more apparent in the normoglycemia group than hyperglycemia group, as it reduced the cumulative incidences of MACE (aHR 1.903; 95% CI 1.203–3.010; p = 0.006) and any repeat revascularization (aHR 3.248; 95% CI 1.539–6.854; p = 0.002).
Conclusions
In this retrospective registry study, prediabetes and T2DM groups showed comparable clinical outcomes, after administering both high-intensity and low-moderate-intensity statin treatments. However, these results are likely to be clearly proved by further studies, especially in patients with AMI who are being treated in contemporary practice.
Trial registration
Retrospectively registered.
Similar content being viewed by others
Background
Previous studies showed that high-intensity statin treatment effectively reduced major adverse cardiac events (MACE), cardiac death (CD), recurrent myocardial infarction (Re-MI), and revascularization, in patients with stable angina or acute coronary syndrome [1,2,3,4]. Moreover, current guidelines recommend that high-intensity statin treatment should be initiated or continued in all patients with acute MI (AMI), as a class I recommendation [5,6,7,8]. However, in many previous studies, the patients were not confined to AMI [2,3,4], and they received first-generation drug-eluting stents (DES) [2, 9]. Moreover, in actual practice, moderate-dose statin treatment is more commonly administered due to lower bodyweights in Asian population [10]. Prediabetes is not an uncommon population to interventional cardiologists [11]. Recent studies reported that those with prediabetes had worse outcomes compared to normoglycemia and comparable to those with diabetes mellitus (DM) [12, 13]. To reflect contemporary practice in Asian patients and to clarify the different effects of statin-intensity between prediabetes and type 2 DM (T2DM), in patients with AMI, we investigated a two-year clinical outcome in these two groups, especially in Korean AMI patients who underwent successful percutaneous coronary intervention (PCI) using newer-generation DES.
Methods
Study design and population
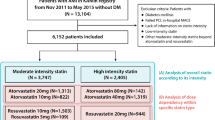
From the Korea AMI Registry (KAMIR) [14], a total of 23,391 AMI patients aged ≥ 30 years at diabetes onset, who underwent successful DES implantation from November 2005 to June 2015, were evaluated. KAMIR [14] is a prospective, observational, and on-line registry with a multicenter cohort study in South Korea, established in November 2005. Details of the registry can be found at the KAMIR website (http://www.kamir.or.kr). In this study, we tried to confine T2DM patients to diabetes cases. Therefore, we defined T2DM based on a previous study [15] which also included patients from the KAMIR. In our study, patients with incomplete laboratory results such as unidentified results of blood hemoglobin (Hb) A1c and blood glucose (n = 8432, 36.1%), patients lost to follow-up (n = 1069, 4.6%), patients treated with first-generation DES (n = 1928, 8.2%), and patients treated with uncertain doses of statins (n = 350, 1.5%) were excluded. Thus, 11,612 AMI patients who received newer-generation DES were included. The types of newer-generation DES used are listed in Table 1. The patients were classified as statin users (n = 9893, 85.2%) and statin non-users (n = 1719, 14.8%). Thereafter, statin users were further divided into high-intensity (n = 2984, 30.2%) and low-moderate-intensity statin users (n = 6909, 69.8%). Finally, those in these two groups (A and B, respectively) were further classified as patients with normoglycemia (group A1 [n = 806, 27.0%] and B1 [n = 1815, 26.3%]), prediabetes (group A2 [n = 935, 31.3%] and B2 [n = 2145, 31.0%]), and T2DM (group A3 [n = 1243, 41.7%] and B3 [n = 2949, 42.7%]) (Fig. 1, Table 1, and Additional file 1: 1). Additionally, over time, patients enrolled later may have benefited from innovative therapies that may have impacted prognosis. To assess how much the results are influenced by this point, we stratified patients into two groups before and after October 2012 according to the enrolled date of individual patient (Additional file 1: 2, 3, 4, and 5). Because a European Society of Cardiology guideline for management of AMI [16] was published in October 2012, and many treatment strategies could be changed according to the newly published guidelines, October 2012 became the cutoff point for our classification. The study protocol was approved by the Institutional Review Board of each participating center, and it was conducted in compliance with the ethical standards of the Declaration of Helsinki 1975. Informed consent was obtained from all patients prior to their inclusion in the study, and we followed up all enrolled patients through face-to-face interviews, phone calls, and chart reviews. All 11,612 patients completed a 2-year clinical follow-up, and all clinical events were evaluated by an independent event adjudication committee. The processes of event adjudication have been described previously by the KAMIR investigators [14].
Percutaneous coronary intervention (PCI) procedure and medical treatment
Diagnostic coronary angiography and PCI were performed using standard techniques [17]. All patients received loading doses of aspirin (200–300 mg) and other antiplatelet agents such as clopidogrel (300–600 mg), ticagrelor (180 mg), or prasugrel (60 mg), before PCI was performed. It was recommended that the duration of dual antiplatelet therapy (DAPT; a combination of aspirin 100 mg/day with clopidogrel 75 mg/day, ticagrelor 90 mg twice daily, or prasugrel 5–10 mg/day) should be for at least 1 year after the index PCI. Based on previous reports [18, 19], triple antiplatelet therapy (TAPT; cilostazol [100 mg twice daily] combined with DAPT) was determined by the individual operator’s discretion. In this study, the patients who received atorvastatin, rosuvastatin, simvastatin, pitavastatin, pravastatin, and fluvastatin were included (Table 1), and the kind and dose of statins to be used was left at the physicians’ discretion.
Study definitions and clinical outcomes
In this study, as mentioned [10], because moderate-dose statin treatment is more commonly administered due to lower bodyweights in Asian population, atorvastatin (≥ 40 mg), rosuvastatin (≥ 20 mg), simvastatin (≥ 40 mg), pitavastatin (≥ 4 mg), and pravastatin (≥ 40 mg) were considered as high-intensity statins, while others were considered as low-moderate-intensity statins [20], compared with current guideline [21]. Glycemic status was determined by the clinical practice recommendations of the American Diabetes Association [22]. T2DM was defined as either known T2DM for which patients received medical treatment (insulin or antidiabetics) or newly diagnosed T2DM defined as an HbA1c level ≥ 6.5%, a fasting plasma glucose (FPG) level ≥ 126 mg/dL (7 mmol/L), and/or random plasma glucose (RPG) level ≥ 200 mg/dL (11.1 mmol/L), during the index hospitalization or according to their medical history. Prediabetes was defined as an HbA1c level of 5.7%–6.4% and an FPG of 100–125 mg/dL (5.6–6.9 mmol/L). Moreover, in the case of discrepancies between HbA1c and FPG or RPG levels, we made HbA1c level a priority [12]. AMI was defined according to the current guidelines [5,6,7,8]. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) study equation [23]. The major outcome was the occurrence of MACE defined as all-cause death, Re-MI, or any repeat coronary revascularization. All-cause death was classified as CD or non-CD. Any repeat revascularization comprised of target lesion revascularization (TLR), target vessel revascularization (TVR), and non-TVR. The definitions of Re-MI, TLR, TVR, and non-TVR have been published previously [24].
Statistical analysis
The normality test was conducted using the Kolmogorov–Smirnov test. Categorical data were reported as numbers and percentages, and they were compared using the chi-square or Fisher’s exact test, as appropriate. For continuous variables, differences among the three groups are evaluated using an analysis of variance or the Jonckheere-Terpstra test, while a post-hoc analysis was performed using the Hochberg test or Dunnett T3 test. The data were expressed as mean ± standard deviation. To determine meaningful variables, all variables with p < 0.001 were included in the univariate analysis (Additional file 1: 6). After univariate analysis, variables with p < 0.001 and known conventional risk factors of poor outcomes in the AMI population were considered potential confounding factors, and were entered into the multivariate analysis [25]. Various clinical outcomes were estimated using the Kaplan–Meier method, and intergroup differences were compared using the log-rank test. For all analyses, a two-sided p value < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS software version 20 (IBM, Armonk, NY, USA).
Results
Baseline characteristics
Table 1, Additional file 1: 1, 7, 8, and 9 show the baseline characteristics of the study population. Both in high-intensity (group A) and in low-moderate-intensity (group B) statin users, the number of men, single-vessel disease, and the prescription rates of ticagrelor and angiotensin-converting enzyme inhibitors (ACEIs) were the highest in normoglycemia groups (group A1 and B1). The number of current smokers and peak creatine kinase-MB level and the levels of total and low-density lipoprotein (LDL) cholesterols were the highest in prediabetes groups (group A2 and B2). The mean age and the number of patents with hypertension, dyslipidemia, previous history of PCI and cerebrovascular accidents; levels of N-terminal pro-brain natriuretic peptide, serum creatinine, and triglyceride; the prescription rates of cilostazole and calcium channel blockers; the number of cases with right coronary artery (RCA) as infarc-related artery (IRA) and treated vessel, multivessel disease, and deployed stents, were the highest in T2DM group (group A3 and B3).
Clinical outcomes
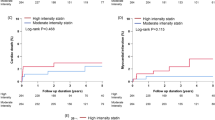
In both high-intensity and low-moderate intensity statin users, the comparisons of clinical outcomes among the three glycemic groups during the 2-year follow-up period are presented in Tables 2, 3, and Fig. 2. In high-intensity statin users, the cumulative incidences of MACE (adjusted hazard ratio [aHR]: 2.187; 95% confidence interval [CI]: 1.341–3.569; p = 0.002) and any repeat revascularization (aHR 3.009; 95% CI 1.342–6.745; p = 0.006) were higher in group A2 (prediabetes) than in group A1 (normoglycemia). Similarly, the cumulative incidences of MACE (aHR 2.368; 95% CI 1.480–3.788; p = 0.001) and any repeat revascularization (aHR 3.619; 95% CI 1.659–7.898; p = 0.001) were significantly higher in group A3 (T2DM) than in group A1. However, the cumulative incidences of MACE, all-cause death, CD, Re-MI, and any repeat revascularization were similar between groups A2 and A3 (Table 2). In low-moderate-intensity statin users, the cumulative incidences of MACE, all-cause death, CD, Re-MI, and any repeat revascularization were not significantly different between groups B1 (normoglycemia) and B2 (prediabetes) as well as between groups B2 and B3 (T2DM). However, the cumulative incidences of MACE (aHR 1.285; 95% CI 1.014–1.629; p = 0.038) and all-cause death (aHR 1.784; 95% CI 1.156–2.751; p = 0.009) were significantly higher in group B3 than in group B1. In normoglycemia groups (Table 3), the cumulative incidences of MACE (aHR 1.903; 95% CI 1.203–3.010; p = 0.006) and any repeat revascularization (aHR 3.248; 95% CI 1.539–6.854; p = 0.002) were significantly lower in high-intensity than in low-moderate-intensity statin users. However, both in the prediabetes (groups A2 and B2) and T2DM (group A3 and B3) groups, the cumulative incidences of MACE, all-cause death, CD, Re-MI, and any repeat revascularization were similar between high-intensity (group A2 vs. B2) and low-moderate-intensity statin users (group A3 vs. B3). Furthermore, in the total study population, there were no significant differences in major clinical outcomes between high-intensity (A1 + A2 + A3) and low-moderate-intensity stain users (B1 + B2 + B3). In high-intensity statin users, in both before and after October 2012 groups (Additional file 1: 2 and 3), the cumulative incidences of MACE (aHR 2.635; p = 0.003 and aHR 1.845; p = 0.048, respectively) and any repeat revascularization (aHR 4.162; p = 0.002 and aHR 2.845; p = 0.044, respectively) were higher in group A2 than in group A1. The cumulative incidences of MACE (aHR 2.896; p = 0.002 and aHR 2.146; p = 0.033, respectively) and any repeat revascularization (aHR 4.666; p = 0.001and aHR 3.241; p = 0.040, respectively) were significantly higher in group A3 than in group A1. In low-moderate-intensity statin users, in before October 2012 group (Additional file 1: 2), the cumulative incidence of all-cause death was significantly higher in group B3 than in group B1 (aHR 1.621; 95% CI 1.102–2.614; p = 0.044). In after October 2012 group (Additional file 1: 3), the cumulative incidences of MACE (aHR 1.429; 95% CI 1.001–1.998; p = 0.043), all-cause death (aHR 2.940; 95% CI 1.388–6.225; p = 0.005), CD (aHR 3.319; 95% CI 1.235–8.919; p = 0.017) were significantly higher in group B3 than in group B1. Moreover, the cumulative incidence of CD (aHR 2.757; 95% CI 1.038–7.327; p = 0.042) was significantly higher in group B3 than in group B2. In before October 2012 group (Additional file 1: 4), in normoglycemia groups, the cumulative incidences of any repeat revascularization (aHR 3.025; 95% CI 1.045–8.760; p = 0.041) was significantly lower in high-intensity than in low-moderate-intensity statin users In after October 2012 group (Additional file 1: 5), in both normoglycemia groups and total study population, the cumulative incidences of MACE (aHR 2.002; p = 0.042 and aHR 1.533; p = 0.004, respectively) and any repeat revascularization (aHR 3.308; p = 0.028 and aHR 1.587; p = 0.038, respectively) were significantly lower in high-intensity than in low-moderate-intensity statin users. In the comparison of major clinical outcomes between statin users and non-users (Additional file 1: 10), statin non-users showed higher cumulative incidences of MACE, all-cause death, and CD in all three glycemic statuses. Additionally, in the T2DM group, the cumulative incidence of any repeat revascularization (aHR 1.637; 95% CI 1.171–32.290; p = 0.004) was significantly higher in statin non-users than in statin users. Moreover, in the total study population, the cumulative incidences of MACE, all-cause death, CD, and any repeat revascularization were significantly higher in statin non-users than in statin users.
Kaplan–Meier analyses for the MACE (a), all-cause death (b), cardiac death (c), Re-MI (d), any repeat revascularization (e) in statin users. MACE: major adverse cardiac events; Re-MI: recurrent myocardial infarction, high: high-intensity statin; low-moderate: low-moderate-intensity statin; NG: normoglycemia; preDM: prediabetes; T2DM: type 2 diabetes mellitus
Independent predictors for MACE in high-intensity statin users and in low-moderate-intensity statin users at 2 years are listed in Additional file 1: 11 and 12. In both high-intensity and low-moderate-intensity statin users, decreased left ventricular ejection fraction (LVEF) (< 40%), cardiogenic shock, and decreased eGFR (< 60 mL/min/1.73m2) were found to be significantly common independent predictors for MACE.
Discussion
The main findings were as follows: (1) the cumulative incidences of MACE, all-cause death, CD, Re-MI, and any repeat revascularization were similar between the prediabetes and T2DM groups in both high-intensity and low-moderate-intensity statin users; (2) in high-intensity statin users, the cumulative incidences of MACE and any repeat revascularization in both prediabetes and T2DM group were higher than those in the normoglycemia group; (3) in low-moderate-intensity statin users, the cumulative incidences of MACE and all-cause death were significantly higher in T2DM than in normoglycemia group; (4) in both patients who enrolled after October 2012 and normoglycemia group, high-intensity statin treatment was more effective in reducing MACE and any revascularization than low-moderate-intensity statin treatment; (5) in the total population, statin users showed significantly lower incidences of MACE, all-cause death, CD, and any repeat revascularization than non-users did; (6) in both high-intensity and low-moderate-intensity statin users, decreased LVEF, cardiogenic shock, and decreased eGFR were common independent predictors of MACE.
According to current guidelines [8, 21], regardless of glycemic status, early and intensive statin treatment is recommended; more intensive statin treatment greatly reduced the risks of CD, non-fatal MI, and coronary revascularization [4]. Consistent with these previous reports [4, 8, 21], our study showed that the cumulative incidences of MACE, all-cause death, CD, and any repeat revascularization were significantly lower in statin users than in statin non-users in the total study population (Additional file 1: 10). However, with respect to statin intensity, in the total statin users groups, high-intensity and low-moderate-intensity statin users showed comparable clinical outcomes. Our results were similar to those of a previous report, which also included patients from the KAMIR [20]. In that study [20], the risk of MACE was similar between high-intensity and low-moderate-intensity statin users (HR: 0.917; 95% CI 0.760–1.107; p = 0.368). A possible explanation for this similarity may be related with different definitions, which confined high-intensity statin treatment to Asian patients, compared with the current guideline [21]. However, in the patients who enrolled after October 2012, the cumulative incidences of MACE (aHR 1.533; 95% CI 1.144–2.053; p = 0.004) and any repeat revascularization (aHR, 1.587; 95% CI 1.026–2.456; p = 0.038) were significantly lower in high-intensity statin users than in low-moderate intensity statin users. This finding also could reflect the possibility that innovative therapies may have impacted prognosis. Despite this limitation, in the normoglycemia group, the cumulative incidences of MACE (aHR 1.903; 95% CI 1.203–3.010; p = 0.006) and any repeat revascularization rate (aHR 3.248; 95% CI 1.539–6.854; p = 0.002) were significantly lower in high-intensity users than in low-moderate-intensity users (Table 3). As mentioned, in this study, we compared major clinical outcomes between the before and after October 2012 groups according to the enrolled date of individual patient (Additional file 1: 2, 3, 4 and 5). The trend of change in the major clinical outcomes shown in Additional file 1: 2, 3, 4 and 5 were of similar those shown in Tables 2 and 3. In high-intensity statin users, the values of aHR for MACE and any repeat revascularization in after October 2012 group were low than those in before October 2012 group (e.g. 2.635 vs. 1.845 or 4.162 vs. 2.845, Additional file 1: 2 and 3). Hence, we can assume patients enrolled later may have benefited from innovative therapies that may have impacted prognosis. Although there are some debates [26, 27], lipophilic statins (atorvastatin, simvastatin, pitavastatin, and fluvastatin), especially at high intensity, may lead to unfavorable metabolic effects, including reduction of insulin secretion and exacerbation of insulin resistance [28, 29] and hydrophilic statins (rosuvastatin and pravastatin) could reduce the risk of cardiovascular disease compared with lipophilic statins [30, 31]. In our study, the number of patients who received atorvastatin was higher in normoglycemia group than in the prediabetes and T2DM groups (54.6% vs. 44.7% vs. 50.4%, p < 0.001, Table 1). Additionally, atorvastatin (aHR 1.578; 95% CI 1.108–2.392; p = 0.021) was independent predictors of MACE (Additional file 1: 11) in high-intensity statin users. However, although the number of patients who received rosuvastatin was lower in normoglycemia group than in the prediabetes and T2DM (38.7% vs. 47.1% vs. 40.3%, p < 0.001, Table 1), rosuvastatin was not independent predictor of MACE in this high-intensity statin users (aHR 1.301; 95% CI 1.100–1.775; p = 0.101, Additional file 1: 11). Despite the fact that statin treatments can improve endothelial function, increase the bioavailability of nitric oxide, and produce antioxidant and anti-inflammatory effects [32], hyperglycemia accelerates the formation of advanced glycation end products (AGEs) by nonenzymatic glycation reactions [33]. Therefore, hyperglycemia and increased AGE formation lead to tissue damage and cardiovascular complications [34]. Hence, our results suggest that hyperglycemic status may be more related to poor clinical outcomes than with normoglycemia, even after higher-intensity statin treatment. However, this hypothesis is likely to be proved by further studies.
Patients with DM are at intermediate or high risk of atherosclerotic cardiovascular disease [35, 36]. In contrast, in the era of newer-generation DES, the clinical significance of prediabetes in patients with AMI is not well understood. Huang et al. [37] reported that prediabetes defined by HbA1c was associated with an increased risk of composite cardiovascular events (relative risk: 1.21, 95% CI 1.01–1.44), and the health risk increased in patients with an FPG concentration as low as 5.6 mmol/L (100 mg/dL) in their meta-analysis study. Chronically elevated glucose leads to pan-vascular damage, which is present in the prediabetic state, and its severity is determined by the time of hyperglycemia onset [38, 39]. The period between waiting for hyperglycemia to reach the currently accepted cutoff levels for the diagnosis of T2DM and to intervene, may allow vascular damage to advance and become irreversible [40]. Therefore, patients with prediabetes could show worse outcomes compared with those with normoglycemia. Hence, our results showing comparable clinical outcomes between the prediabetes and T2DM groups in both high-intensity and low-moderate-intensity statin users are consistent with recent reports [12, 13]. According to a recent published report [41] after statin treatment, the cumulative incidences of MACE (p = 0.314), all-cause death (p = 0.530), cardiac death (p = 0.873), Re-MI (p = 0.170), and any repeat revascularization (p = 0.548) were similar between the prediabetes and T2DM groups regardless of statin intensity.
Radial access has proved to be beneficial in reducing the incidence of hemorrhagic events, mortality and acute kidney injury compared to femoral access [42]. In our study, the number of cases with transradial or transfemoral approaches was not significantly different between high-intensity and low-moderate-intensity statin treatment or between statin users and nonusers.
Finally, Gragnano et al. [43] suggested that proprotein convertase subtilisin/kexin 9 inhibitors (PCSK 9i) may represent an attractive strategy to overcome nonadherence barriers in selected high-risk patients. In the recent report [44], PCSK9i improved the quality of life and global health status of patients at high or very high cardiovascular risk, beyond their LDL-cholesterol lowering and positive prognostic impact. Therefore, the use of non-statin drugs may help in increasing adherence to statins.
In this retrospective registry study, more than 50 high-volume university or community hospitals of South Korea were included [14]. As mentioned, in many previous studies, the patients were not confined to AMI [2,3,4], and they received first-generation DES [2, 9]. Therefore, their results might not reflect contemporary practice using second-generation DES. However, our study population was strictly confined to patients who received newer-generation DES. Moreover, studies concerning the long-term effects of statin therapy in patients with AMI and prediabetes are very limited. Hence, we believe that our study can provide useful information to interventional cardiologists performing PCI with new-generation DES in AMI patients, regarding the importance of hyperglycemia (especially prediabetes) and the relationship with worse cardiovascular outcomes after both high-intensity and low-moderate-intensity statin treatment.
This study has several limitations. First, because of the lack of information in the KAMIR data, we could not present the cumulative events of statin-related new-onset DM during the follow-up period. This is a major weakness of this study. Second, Gragnano et al. [45] mentioned that insufficient LDL-C reduction and high residual risk in a significant proportion of statin-treated patients signify that additional therapies are required to deliver more effective coronary care. Therefore, LDL-cholesterol levels are important during the follow-up period. However, we could not provide these values due to the limitation of this registry data. Third, we did not perform oral glucose tolerance to define prediabetes, which is an important bias. Fourth, there may have been some under-reporting and/or missing data due to the registry nature of this study. Fifth, treatment adherence remains essential in the management of patients with AMI undergoing PCI [45, 46]. Especially, DAPT is recommended for at least 12 months in patients after an acute coronary syndrome (ACS). Underuse or premature discontinuations of DAPT are common in clinical practice. Currently, Crisci et al. [46] are investigating the impact of a dedicated follow-up strategy with clinical visits and counseling on adherence levels to ticagrelor in patients with ACS through a PROGRESS (PROmotinG dual antiplatelet therapy adheREnce in the setting of acute coronary Syndromes) prospective randomized trial. However, because this study was based on discharge medications, we could not precisely determine the adherence or non-adherence of the enrolled patients to their prescribed discharge medications during the follow-up period; this might constitute an additional bias. Moreover, recent antidiabetic medications have been shown to improve cardiovascular outcomes [47,48,49]. Especially, sodium-glucose co-transporter 2 (SGLT-2) inhibitors, initially introduced for the treatment of DM, demonstrates cardiovascular and renal benefit in patients with heart failure (HF) [47]. Lu et al. [48] demonstrated that beneficial effects SGLT-2 inhibitors were robust in HF patients regardless of T2DM status, and a strong trend to be effective in HF with preserved EF. Recent review [49] also introduced that the hypotheses on SGLT-2 inhibitors mechanisms of action have changed: from simple glycosuric drugs, with consequent glucose lowering, erythropoiesis enhancing and ketogenesis stimulating, to intracellular sodium-lowering molecules. However, unfortunately, this registry data did not include information concerning SGLT-2 inhibitors. Hence, we could not provide comparative cardiovascular effects of SGLT-2 inhibitors between high-intensity and low-moderate-intensity statin treatment or between statin users and nonusers in our study. In addition, diabetic patients may benefit from long-term antiplatelet therapy, and diabetes is a key criterion for choosing continuation of DAPT in life [50]. Cesaro et al. [50] showed that in a real-world study, including patients with previous MI, low-dose ticagrelor for prolonged dual antiplatelet therapy showed to be effective and safe, with no major bleeding occurring at follow-up. Therefore, the duration and kinds of DAPT in patients with AMI is very important. However, because of limitation on registry data, the information requested was not available. This might constitute an important shortcoming of this study. Sixth, although multivariate analysis was performed to strengthen our results, variables not included in the KAMIR may cause a bias. Finally, this study encompasses a very broad time frame (2005 to 2015). Although we stratified patients into two groups before and after October 2012 according to the enrolled date of individual patient, this factor may lead to a bias.
Conclusions
In this retrospective registry study, prediabetes and T2DM groups showed comparable clinical outcomes, after both high-intensity and low-moderate-intensity statin treatments. Moreover, the beneficial effects of high-intensity compared to low-moderate-intensity statin therapy were more apparent in the normoglycemia group than in the prediabetes and T2DM groups. However, these results are likely to be clearly proved by further studies, especially in patients with AMI who are being treated in contemporary practice.
Availability of data and materials
All data generated or analysed during this study are included in this published article. And any additional data/files may be obtained from the corresponding author on reasonable request.
Abbreviations
- AMI:
-
Acute myocardial infarction
- KAMIR:
-
Korea AMI registry
- PCI:
-
Percutaneous coronary intervention
- T2DM:
-
Type 2 diabetes mellitus
- MACE:
-
Major adverse cardiac events
- DES:
-
Drug-eluting stent
- Re-MI:
-
Recurrent myocardial infarction
- ACEIs:
-
Angiotensin-converting enzyme inhibitors
- LVEF:
-
Left ventricular ejection fraction
- eGFR:
-
Estimated glomerular filtration rate
- HbA1c:
-
Hemoglobin A1c
References
Armitage J, Bowman L, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Peto R, Collins R. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010;376:1658–69.
Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–45.
Taguchi I, Iimuro S, Iwata H, Takashima H, Abe M, Amiya E, Ogawa T, Ozaki Y, Sakuma I, Nakagawa Y, et al. High-dose versus low-dose pitavastatin in japanese patients with stable coronary artery disease (REAL-CAD): a randomized superiority trial. Circulation. 2018;137:1997–2009.
Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81.
Tong ST, Sabo RT, Hochheimer CJ, Brooks EM, Jiang V, Huffstetler AN, Lail Kashiri P, Krist AH. Uptake of statin guidelines to prevent and treat cardiovascular disease. J Am Board Fam Med. 2021;34:113–22.
Goldberg RB, Stone NJ, Grundy SM. The 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guidelines on the management of blood cholesterol in diabetes. Diabetes Care. 2020;43:1673–8.
Yao X, Shah ND, Gersh BJ, Lopez-Jimenez F, Noseworthy PA. Assessment of trends in statin therapy for secondary prevention of atherosclerotic cardiovascular disease in US adults from 2007 to 2016. JAMA Netw Open. 2020;3:e2025505.
Harris DE, Lacey A, Akbari A, Torabi F, Smith D, Jenkins G, Obaid D, Chase A, Gravenor M, Halcox J. Achievement of European guideline-recommended lipid levels post-percutaneous coronary intervention: a population-level observational cohort study. Eur J Prev Cardiol. 2020;31:2047487320914115. https://doi.org/10.1177/2047487320914115.
de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD, Rouleau JL, Pedersen TR, Gardner LH, Mukherjee R, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–16.
Choi JY, Choi CU, Hwang SY, Choi BG, Jang WY, Kim DY, Kim W, Park EJ, Lee S, Na JO, et al. Effect of pitavastatin compared with atorvastatin androsuvastatin on new-onset diabetes mellitus in patientswith acute myocardial infarction. Am J Cardiol. 2018;122:922–8.
Carris NW, Magness RR, Labovitz AJ. Prevention of diabetes mellitus in patients with prediabetes. Am J Cardiol. 2019;2019(123):507–12.
Kok MM, von Birgelen C, Sattar N, Zocca P, Löwik MM, Danse PW, Schotborgh CE, Scholte M, Hartmann M, Kant GD, et al. Prediabetes and its impact on clinical outcome after coronary intervention in a broad patient population. EuroIntervention. 2018;14:e1049–56.
Kim YH, Her AY, Jeong MH, Kim BK, Hong SJ, Kim S, Ahn CM, Kim JS, Ko YG, Choi D, et al. Two-year clinical outcomes between prediabetic and diabetic patients with STEMI and multivessel disease who underwent successful PCI using drug-eluting stents. Angiology. 2021;72:50–61.
Kim JH, Chae SC, Oh DJ, Kim HS, Kim YJ, Ahn Y, Cho MC, Kim CJ, Yoon JH, Park HY, et al. Multicenter cohort study of acute myocardial infarction in Korea-Interim Analysis of the Korea Acute Myocardial Infarction Registry-National Institutes of Health Registry. Circ J. 2016;80:1427–36.
Lee SA, Cho SJ, Jeong MH, Kim YJ, Kim CJ, Cho MC, Kim HS, Ahn Y, Koh G, Lee JM, et al. Hypoglycemia at admission in patients with acute myocardial infarction predicts a higher 30-day mortality in patients with poorly controlled type 2 diabetes than in well-controlled patients. Diabetes Care. 2014;37:2366–73.
Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, et al. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–619.
Grech ED. ABC of interventional cardiology: percutaneous coronary intervention. II: the procedure. BMJ. 2003;326:1137–40.
Chen KY, Rha SW, Li YJ, Poddar KL, Jin Z, Minami Y, Wang L, Kim EJ, Park CG, Seo HS, et al. Korea acute myocardial infarction registry investigators. Triple versus dual antiplatelet therapy in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation. 2009;119:3207–14.
Lee SW, Park SW, Hong MK, Kim YH, Lee BK, Song JM, Han KH, Lee CW, Kang DH, Song JK, et al. Triple versus dual antiplatelet therapy after coronary stenting: impact on stent thrombosis. J Am Coll Cardiol. 2005;46:1833–7.
Hwang D, Kim HK, Lee JM, Choi KH, Kim J, Rhee TM, Park J, Park TK, Yang JH, Song YB, et al. Effects of statin intensity on clinical outcome in acute myocardial infarction patients. Circ J. 2018;82:1112–20.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-350.
American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33:S11-61.
Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghorne KR, Shankar A, Smith DH, Tonelli M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–51.
Kim YH, Her AY, Jeong MH, Kim BK, Shin DH, Kim JS, Ko YG, Choi D, Hong MK, Jang Y. Two-year outcomes of statin therapy in patients with acute myocardial infarction with or without dyslipidemia after percutaneous coronary intervention in the era of new-generation drug-eluting stents within Korean population: Data from the Korea Acute Myocardial Infarction Registry. Catheter Cardiovasc Interv. 2019;93:1264–75.
Kim YH, Her AY, Jeong MH, Kim BK, Hong SJ, Kim S, Ahn CM, Kim JS, Ko YG, Choi D, et al. Beta-Blocker and renin-angiotensin system inhibitor combination therapy in patients with acute myocardial infarction and prediabetes or diabetes who underwent successful implantation of newer-generation drug-eluting stents: a retrospective observational registry study. J Clin Med. 2020;11:3447.
Bytyçi I, Bajraktari G, Bhatt DL, Morgan CJ, Ahmed A, Aronow WS, Banach M. Hydrophilic vs lipophilic statins in coronary artery disease: a meta-analysis of randomized controlled trials. J Clin Lipidol. 2017;11:624–37.
Izawa A, Kashima Y, Miura T, Ebisawa S, Kitabayashi H, Yamamoto H, Sakurai S, Kagoshima M, Tomita T, Miyashita Y, et al. Assessment of lipophilic vs. hydrophilic statin therapy in acute myocardial infarction—ALPS-AMI study. Circ J. 2015;79:161–8.
Kanda M, Satoh K, Ichihara K. Effects of atorvastatin and pravastatin on glucose tolerance in diabetic rats mildly induced by streptozotocin. Biol Pharm Bull. 2003;26:1681–4.
Yada T, Nakata M, Shiraishi T, Kakei M. Inhibition by simvastatin, but not pravastatin, of glucose-induced cytosolic Ca2+ signalling and insulin secretion due to blockade of L-type Ca2+ channels in rat islet beta-cells. Br J Pharmacol. 1999;126:1205–13.
Li SY, Chen HH, Lin CL, Yeh SY, Kao CH. The different cardiovascular outcomes between long-term efficacy of hydrophilic and lipophilic statin therapy in both asian diabetic sexes. Dose Response. 2019;17:1559325819876766.
Sakamoto T, Kojima S, Ogawa H, Shimomura H, Kimura K, Ogata Y, Sakaino N, Kitagawa A. Usefulness of hydrophilic vs lipophilic statins after acute myocardial infarction: subanalysis of MUSASHI-AMI. Circ J. 2007;71:1348–53.
Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:III39-43.
Beisswenger PJ. Glycation and biomarkers of vascular complications of diabetes. Amino Acids. 2012;42:1171–83.
Piarulli F, Sartore G, Lapolla A. Glyco-oxidation and cardiovascular complications in type 2 diabetes: a clinical update. Acta Diabetol. 2013;50:101–10.
Wong ND, Glovaci D, Wong K, Malik S, Franklin SS, Wygant G, Iloeje U. Global cardiovascular disease risk assessment in United States adults with diabetes. Diab Vasc Dis Res. 2012;9:146–52.
Rana JS, Liu JY, Moffet HH, Jaffe M, Karter AJ. Diabetes and prior coronary heart disease are not necessarily risk equivalent for future coronary heart disease events. J Gen Intern Med. 2016;31:387–93.
Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. 2016;355:i5953.
Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab. 2009;94:3171–82.
Semenkovich CF. We know more than we can tell about diabetes and vascular disease: the 2016 edwin bierman award lecture. Diabetes. 2017;66:1735–41.
Dluhy RG, McMahon GT. Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med. 2008;358:2630–3.
Kim YH, Her AY, Jeong MH, Kim BK, Hong SJ, Kim S, Ahn CM, Kim JS, Ko YG, Choi D, et al. Effect of statin treatment in patients with acute myocardial infarction with prediabetes and type 2 diabetes mellitus: a retrospective observational registry study. Medicine (Baltimore). 2021;100:e24733.
Cesaro A, Moscarella E, Gragnano F, Perrotta R, Diana V, Pariggiano I, Concilio C, Alfieri A, Cesaro F, Mercone G. Transradial access versus transfemoral access: a comparison of outcomes and efficacy in reducing hemorrhagic events. Expert Rev Cardiovasc Ther. 2019;17:435–47.
Gragnano F, Natale F, Concilio C, Fimiani F, Cesaro A, Sperlongano S, Crisci M, Limongelli G, Calabrò R, Russo M, et al. Adherence to proprotein convertase subtilisin/kexin 9 inhibitors in high cardiovascular risk patients: an Italian single-center experience. J Cardiovasc Med (Hagerstown). 2018;19:75–7.
Cesaro A, Gragnano F, Fimiani F, Moscarella E, Diana V, Pariggiano I, Concilio C, Natale F, Limongelli G, Bossone E, et al. Impact of PCSK9 inhibitors on the quality of life of patients at high cardiovascular risk. Eur J Prev Cardiol. 2020;27:556–8.
Gragnano F, Calabrò P. Role of dual lipid-lowering therapy in coronary atherosclerosis regression: evidence from recent studies. Atherosclerosis. 2018;269:219–28.
Crisci M, Gragnano F, Di Maio M, Diana V, Moscarella E, Pariggiano I, Di Maio D, Concilio C, Taglialatela V, Fimiani F, et al. Improving adherence to ticagrelor in patients after acute coronary syndrome: results from the PROGRESS trial. Curr Vasc Pharmacol. 2020;18:294–301.
Li X, Zhang Q, Zhu L, Wang G, Ge P, Hu A, Sun X. Effects of SGLT2 inhibitors on cardiovascular, renal, and major safety outcomes in heart failure: A meta-analysis of randomized controlled trials. Int J Cardiol. 2021;332:119–26.
Lu Y, Li F, Fan Y, Yang Y, Chen M, Xi J. Effect of SGLT-2 inhibitors on cardiovascular outcomes in heart failure patients: a meta-analysis of randomized controlled trials. Eur J Intern Med. 2021;87:20–8.
Palmiero G, Cesaro A, Vetrano E, Pafundi PC, Galiero R, Caturano A, Moscarella E, Gragnano F, Salvatore T, Rinaldi L, et al. Impact of SGLT2 inhibitors on heart failure: from pathophysiology to clinical effects. Int J Mol Sci. 2021;22:5863.
Cesaro A, Taglialatela V, Gragnano F, Moscarella E, Fimiani F, Conte M, Barletta V, Monda E, Limongelli G, Severino S, et al. Low-dose ticagrelor in patients with high ischemic risk and previous myocardial infarction: a multicenter prospective real-world observational study. J Cardiovasc Pharmacol. 2020;76:173–80.
Acknowledgements
Korea Acute Myocardial infarction Registry (KAMIR) investigators. Myung Ho Jeong, MD, Youngkeun Ahn, MD, Sung Chul Chae, MD, Jong Hyun Kim, MD, Seung-Ho Hur, MD, Young Jo Kim, MD, In Whan Seong, MD, Donghoon Choi, MD, Jei Keon Chae, MD, Taek Jong Hong, MD, Jae Young Rhew, MD, Doo-Il Kim, MD, In-Ho Chae, MD, Junghan Yoon, MD, Bon-Kwon Koo, MD, Byung-Ok Kim, MD, Myoung Yong Lee, MD, Kee-Sik Kim, MD, Jin-Yong Hwang, MD, Myeong Chan Cho, MD, Seok Kyu Oh, MD, Nae-Hee Lee, MD, Kyoung Tae Jeong, MD, Seung-Jea Tahk, MD, Jang-Ho Bae, MD, Seung-Woon Rha, MD, Keum-Soo Park, MD, Chong Jin Kim, MD, Kyoo-Rok Han, MD, Tae Hoon Ahn, MD, Moo-Hyun Kim, MD, Ki Bae Seung, MD, Wook Sung Chung, MD, Ju-Young Yang, MD, Chong Yun Rhim, MD, Hyeon-Cheol Gwon, MD, Seong-Wook Park, MD, Young-Youp Koh, MD, Seung Jae Joo, MD, Soo-Joong Kim, MD, Dong Kyu Jin, MD, Jin Man Cho, MD, Sang-Wook Kim, MD, Jeong Kyung Kim, MD, Tae Ik Kim, MD, Deug Young Nah, MD, Si Hoon Park, MD, Sang Hyun Lee, MD, Seung Uk Lee, MD, Hang-Jae Chung, MD, Jang-Hyun Cho, MD, Seung Won Jin, MD, Myeong-Ki Hong, MD, Yangsoo Jang, MD, Jeong Gwan Cho, MD, Hyo-Soo Kim, MD and Seung-Jung Park, MD.
Funding
This research was supported by a fund (2016-ER6304-02) by Research of Korea Centers for Disease Control and Prevention.
Author information
Authors and Affiliations
Contributions
Y.H.K. and A.-Y.H. researched data and wrote the manuscript. Y.H.K., A.-Y.H., B.-K.K., J.-S.K., M.-K.H., and Y.J. contributed to study design. M.H.J., B.-K.K., S.-J.H., S.K., C.-M.A., J.-S.K., Y.-G.K., D.C., M.-K.H., and Y.J. contributed to the collection research data. Y.H.K. and A.-Y.H., M.H.J., B.-.KK., J.-S.K., Y.-G.K., D.C., M.-K.H., and Y.J. contributed to provide intellectual inputs for the discussion. Y.H.K., A.-Y.H., S.-J.H., contributed to data analysis and edited the manuscript. Y.H.K., M.H.J., D.C., M.-K.H., and Y.J. contributed to provide supervisor role during the full processes of manuscript submitting and editing. All authors have read and approved the manuscript, and all authors take full responsibility for this work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of each participating center and the Chonnam National University Hospital Institutional Review Board ethics committee (CNUH-2011-172) according to the ethical guideline of the Declaration of Helsinki. The written informed consent was obtained from all patients prior to their inclusion in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Supplementary Appendix.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, Y.H., Her, AY., Jeong, M.H. et al. Comparative effect of statin intensity between prediabetes and type 2 diabetes mellitus after implanting newer-generation drug-eluting stents in Korean acute myocardial infarction patients: a retrospective observational study. BMC Cardiovasc Disord 21, 386 (2021). https://doi.org/10.1186/s12872-021-02198-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-021-02198-w