Abstract
Background
Increased D-dimer levels have been shown to correlate with adverse outcomes in various clinical conditions. However, few studies with a large sample size have been performed thus far to evaluate the prognostic value of D-dimer in patients with infective endocarditis (IE).
Methods
613 patients with IE were included in the study and categorized into two groups according to the cut-off of D-dimer determined by receiver operating characteristic (ROC) curve analysis for in-hospital death: > 3.5 mg/L (n = 89) and ≤ 3.5 mg/L (n = 524). Multivariable regression analysis was used to determine the association of D-dimer with in-hospital adverse events and six-month death.
Results
In-hospital death (22.5% vs. 7.3%), embolism (33.7% vs 18.2%), and stroke (29.2% vs 15.8%) were significantly higher in patients with D-dimer > 3.5 mg/L than in those with D-dimer ≤ 3.5 mg/L. Multivariable analysis showed that D-dimer was an independent risk factor for in-hospital adverse events (odds ratio = 1.11, 95% CI 1.03–1.19, P = 0.005). In addition, the Kaplan–Meier curve showed that the cumulative 6-month mortality was significantly higher in patients with D-dimer > 3.5 mg/L than in those with D-dimer ≤ 3.5 mg/L (log-rank test = 39.19, P < 0.0001). Multivariable Cox regression analysis showed that D-dimer remained a significant predictor for six-month death (HR 1.11, 95% CI 1.05–1.18, P < 0.001).
Conclusions
D-dimer is a reliable prognostic biomarker that independently associated with in-hospital adverse events and six-month mortality in patients with IE.
Similar content being viewed by others
Background
Despite improvements in the diagnostic approach and management strategy, infective endocarditis (IE) remains associated with high rates of in-hospital and long-term mortality, and significant complications [1,2,3,4,5,6]. Therefore, rapid identification of patients at high-risk of death could help clinical decision-making with respect to the timing of surgery and intensity of in-hospital care in order to improve prognosis.
Systemic embolization (especially stroke) is a severe complication of IE and is associated with increased morbidity and mortality [7]. Early identification of coagulation activation and thrombus formation with associated biomarkers such as D-dimer may be of prognostic value. D-dimer is a fibrin-degradation product that is released when a blood clot disintegrates, indicating thrombosis or fibrinolysis [8]. It is a valuable blood marker for the diagnosis and evaluation of a vast array of thrombosis-related clinical conditions such as venous thromboembolism, pulmonary thromboembolism, and myocardial infarction [9,10,11]. Turak et al. reported that increased D-dimer level at admission was associated with high in-hospital mortality in patients with IE [12]. However, the sample size of their study was small (n = 157), and the impact of D-dimer on long-term outcomes in patients with IE was not discussed. Therefore, in this study, we aimed to evaluate the association of D-dimer at admission with in-hospital and six-month outcomes in a relatively large series of IE patients.
Method
Study population
In this observational, 613 patients diagnosed with definite IE at Guangdong Provincial People’s Hospital between January 2009 and February 2017 were included. The diagnosis of definite IE was in accordance with the current guideline [2]. We excluded patients who were < 18 years old, lack data regarding on-admission D-dimer level, and had concomitant disseminated intravascular coagulation at admission. This study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital with a waiver of written informed consent because of the retrospective study design (No. GDREC2020098H). Oral informed consent was obtained from patients or their relatives by telephone and recorded by trained nurses during the follow-up period.
Measurement and data collection
D-dimer level was measured using quantitative latex turbidimetric test in our hospital. All subjects underwent either transthoracic (TTE) or transesophageal echocardiography (TEE) within 24 h of admission. Left ventricular ejection fraction (LVEF) was evaluated using the Simpson’s biplane method. Echocardiographic results of the type, severity of valvular involvement, perivalvular complications were documented. Estimated glomerular filtration rate (eGFR) was calculated using the four-variable Modification of Diet in Renal Disease formula for the Chinese population [13]. Surgical treatment was performed based on current guideline recommendations [2]. Patients who could not tolerate or afford the operation received conservative therapy. Patients’ clinical and demographic data including age, sex, predisposing factors, history of illness, and comorbid conditions, common laboratory results were collected from the electronic medical record by one researcher and reviewed by another researcher.
Follow-up and outcome
All patients were followed-up by telephonic interviews at 6 months. We also reviewed the outpatient and readmission medical records of these patients. The primary outcome was in-hospital adverse events, defined as the composite of in-hospital death, stroke, and embolism. The secondary outcome was 6-month death. Stroke is diagnosed based on the presence clinical symptoms and signs of neurological deficits and radiographic evidence of ischemic or hemorrhagic changes of the brain, therefore including both ischemic and hemorrhagic stroke. Embolism was defined as the combination of ischemic stroke, pulmonary embolism or lung infarction, and arterial embolism suggested by clinical or radiographic findings.
Statistical analysis
Statistical analyses were performed using SPSS software version 24.0 (SPSS Inc., Chicago, IL, USA). Normally distributed continuous data were expressed as mean ± standard deviation (SD) and compared using Student’s t-test, and non-normally distributed data were presented as median and interquartile range (IQR) and compared by the Wilcoxon rank-sum test. Categorical data were expressed as proportions and compared using the chi-squared or Fisher’s exact test. The optimal cut-off of D-dimer for predicting in-hospital death was determined by receiver operating characteristic (ROC) curve analysis. Univariable and multivariable logistic regression were used to determine risk factors for events. Variables with P value less than 0.05 in the univariable logistic regression analysis were included in the multivariable logistic regression analysis for in-hospital outcomes. The adjusted odds ratio (OR) and 95% confidence interval (CI) were calculated. Univariable analyses of 6-month mortality were performed using a log-rank test for patients categorized by D-dimer cutoff. Multivariable Cox regression analyses were also performed for six-month death. P < 0.05 was considered statistically significant for all analysis.
Results
Baseline clinical characteristics
In total, 613 patients (69.0% female; aged 44.9 ± 16.0 years) were included for analysis. Table 1 shows the baseline clinical characteristics of including patients. In-hospital death occurred in 58 (9.4%) patients. Patients who died during hospitalization tend to be older with a higher prevalence of diabetes mellitus (DM), rheumatic heart disease (RHD), previous valve replacement. More advanced stage of heart failure was observed in patients who expired. Dead patients had significantly higher level of CRP, SCr, D-dimer and the lower level of platelets count as compared with those who survived. Perivalvular complications under echocardiography were more common in the death group. Further, a higher proportion of patients who survived received surgical treatment than those who died.
D-dimer and in-hospital outcomes
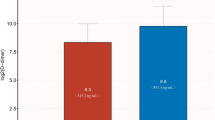
The ROC curve analysis showed that D-dimer levels > 3.5 mg/L had a sensitivity of 36.2% and specificity of 87.2% (AUC = 0.622, 95% CI = 0.543–0.702, P = 0.002) for predicting in-hospital death. Patients were categorized into two groups according to the cutoff value: 524 with D-dimer ≤ 3.5 mg/L, 89 with D-dimer > 3.5 mg/L. Primary outcome occurred in 49.4% of patients with D-dimer > 3.5 mg/L, as compared with 26.3% in those with D-dimer ≤ 3.5 mg/L. The rate of in-hospital death (22.5% vs. 7.3%, P < 0.001, Fig. 1), embolic events (33.7% vs 18.2%, P = 0.001, Fig. 1) and stroke (29.2% vs 15.8%, P = 0.002, Fig. 1) was significantly higher in patients with D-dimer levels > 3.5 mg/L than in those without elevated D-dimer levels.
Univariable logistic regression analysis indicated that D-dimer was associated with increased in-hospital adverse events (OR = 1.14, P < 0.001) (Table 2). Additional significant indicators included RHD, CHD, prosthetic valve, NYHA III-IV heart failure, serum creatinine > 2 mg/dL, anemia, positive blood culture, perivalvular complications and surgical treatment. After adjusting the confounding variables, increased D-dimer was an independent risk factor for in-hospital adverse events (adjusted OR = 1.11, 95% CI = 1.03–1.19, P = 0.005, Table 2).
D-dimer and six-month mortality
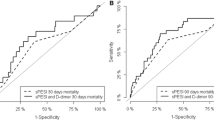
After six months, 80 patients died. Cumulative six-month mortality was significantly higher in the group with D-dimer > 3.5 mg/L than that in the other group (29.2% vs 10.3%, P < 0.001). Kaplan–Meier survival curve showed that patients with D-dimer levels > 3.5 mg/L had a lower six-month survival than those with D-dimer ≤ 3.5 mg/L (log-rank test = 30.23, P < 0.0001, Fig. 2). Multivariable Cox proportional hazard analysis showed that D-dimer remained a significant predictor for six-month death (HR 1.11, 95% CI 1.05–1.18, P < 0.001, Table 3).
Discussion
This study investigated the association of D-dimer at admission with the in-hospital adverse events and six-month mortality in a large cohort of patients with IE. Elevation of at-admission D-dimer level was associated with increased rate of in-hospital adverse events and six-month death. D-dimer was an independent predictor for both in-hospital adverse events and six-month mortality.
Early and accurate identification of patients at high risk and timely surgical intervention have been found to improve prognosis in patients with IE [14, 15]. However, the clinical history of IE is highly variable, rendering more focus on identifying predictors of adverse outcomes. Systemic embolization and in particular, CNS embolization, is one of the determinants of adverse outcome in patients with IE [2, 7, 16]. Therefore, early detection of activation of the coagulation cascade could play a pivotal role in recognizing excessive thrombus formation. Previous studies have proposed some clinical and laboratory predictors, including pro-inflammatory and hemodynamic biomarkers [17,18,19,20]. However, they are not directly related to thromboembolism. Identification of novel prognostic biomarkers on the basis of coagulation may further stratify patients with IE based on risk, providing guidance for clinical decision-making.
Elevated D-dimer level in plasma is indicative of acute thrombus formation and fibrinolysis, which is likely a valuable tool to diagnose a vast array of thrombosis-related clinical conditions [21]. Understanding the pathophysiology of IE might be helpful to completely elucidate the underlying mechanism of the prognostic role of D-dimer in IE. The characteristic endocardial lesion in IE—a vegetation—is an aggregation of platelets, fibrin, microorganisms, and inflammatory cells [2, 22]. Development of non-bacterial thrombotic endocarditis which is composed of a platelet–fibrin network is the nidus for bacterial adhesion and invasion. The bacteremia and colonization of bacteria on heart valves further promote platelet aggregation, coverage of the bacteria by a platelet–fibrin meshwork, and formation of mature vegetation [23]. This process involves recruitment of inflammatory cells, release of pro-inflammatory cytokines, and activation of the coagulation cascade. Pro-inflammatory cytokines and other mediators are capable of activating the coagulation system and down-regulating physiologic anticoagulant pathways and fibrinolysis, which reversely modulate the inflammatory process through protease-activated cell receptors and activation of platelets. Hence, down regulation of anticoagulant pathways not only promotes thrombosis but also amplifies the inflammatory process. This interplay of inflammation and coagulation, contributing significantly to the outcome, is one of the most prominent features of sepsis [24]. When the inflammation-coagulation interactions overwhelm the natural defense systems, catastrophic events such as those manifested in sepsis and IE occur. Furthermore, the continued proliferation of bacteria and deposition of platelets and fibrin result in vegetation that can embolize peripherally and cause embolic phenomena. As showed in our study, patients with elevated levels of on-admission D-dimer had a significantly higher rate of in-hospital thromboembolic events than those that did not. Embolism was the most common adverse events in our study cohort, attributing to 70% of all in-hospital events.
The prognosis of IE could be influenced by many factors. Previous studies have identified several predictors for in-hospital and long-term mortality in patients with IE, such as prosthetic heart valve, staphylococcus infection, LVEF, surgical therapy, vegetation size > 10 mm, perivalvular abscess and the presence of complications (stroke, heart failure, renal failure) [1, 5, 7, 12]. After adjusting these risk factors, D-dimer remains an independent predictor for in-hospital and 6-month all-cause mortality in our study. Besides, the presence of NYHA III-IV heart failure, negative blood culture and absence of surgical therapy were also identified as independent risk factors, which is consistent with previous studies. This finding supports the application of D-dimer in clinical practice to acquire additional prognostic information apart from traditional risk factors, especially when D-dimer > 3.5 mg/L.
Currently, many studies have been conducted to investigate the prognostic value of D-dimer in several thrombosis-related conditions. On-admission plasma D-dimer level has been reported to be a valuable marker in predicting short- and long-term outcomes in acute aortic dissection [25]. Similar results were found in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention [26]. Musicant et al. reported that baseline D-dimer level was significantly associated with the occurrence of myocardial infarction in subjects with symptomatic peripheral artery disease [27]. To date, however, only four studies have been performed to evaluate the role of D-dimer in outcome prediction in IE. In a study including 42 patients, Bakal et al. showed that plasma D-dimer levels were increased in patients with IE who suffered from clinically significant systemic embolism; D-dimer level > 425 ng/dL could predict clinical embolism with a sensitivity of 77% and specificity of 62% [28]. However, the association of D-dimer level with in-hospital mortality in patients with IE was not studied. Turak et al. included 157 patients with IE and found that on-admission D-dimer ≥ 4.2 mg/L was independently associated with IE-related in-hospital death [12]. Recently, Baris et al. enrolled 79 patients with IE, and using multiple logistic regression analysis, showed that D-dimer was a strong parameter for predicting in-hospital mortality and embolic events [29]. Nevertheless, the small sample size and lack of long-term follow-up limit the significance of their results. Our study was conducted in a relatively large sample of patients, and the long-term follow-up demonstrated that increased D-dimer level on admission was independently associated with adverse in-hospital and long-term outcome in patients with IE. Additionally, this association was still significant after adjusting for cardiac function and surgical therapy, which have been shown as strong predictors for poor outcome in the previous studies and the current guideline. Therefore, we believe our study results provide further evidence for supporting D-dimer as a reliable biomarker to predict increased risk of complications and in-hospital and long-term death in patients with IE. Patients diagnosed with IE with increased D-dimer level should be closely monitored for embolization and carefully evaluated for early surgical intervention.
Limitations
Our study has some limitations. First, it is a single center study and clinical data were retrospectively collected from electronic medical records. Our results should be interpreted with caution and require validation by prospective, multi-center studies. Second, reasons of death cannot be clearly identified by telephone follow-up. Third, Third, some peri-operative parameters that may be associated with patient outcome were not available in our study and not included into the analysis. Fourth, we only recorded on-admission D-dimer level without serial measurement. Measurement of D-dimer levels after treatment or upon discharge would be helpful to further analyze the correlation with disease progression and evaluate long-term outcome.
Conclusion
Prognostic evaluation of patients with IE is of utmost clinical interest, which often guides clinicians to develop an algorithm for risk stratification and decision-making. Our study suggested that increased on-admission D-dimer was a reliable prognostic biomarker that associated with high risk of in-hospital adverse events and six-month mortality in patients with IE.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CRP:
-
C-reactive protein
- CHD:
-
Congenital heart disease
- CI:
-
Confidence interval
- DM:
-
Diabetes mellitus
- eGFR:
-
Estimated glomerular filtration rate
- HR:
-
Hazard ratio
- IE:
-
Infective endocarditis
- LVEF:
-
Left ventricular ejection fraction
- RHD:
-
Rheumatic heart disease
- ROC:
-
Receiver operating characteristic
- SCr:
-
Serum creatinine
- TEE:
-
Transesophageal echocardiography
- TTE:
-
Transthoracic echocardiography
References
Fernández-Hidalgo N, Almirante B, Tornos P, González-Alujas MT, Planes AM, Galiñanes M, et al. Immediate and long-term outcome of left-sided infective endocarditis. A 12-year prospective study from a contemporary cohort in a referral hospital. Clin Microbiol Infect. 2012;18:E522-530.
Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European. Eur Heart J. 2015;36:3075–128.
Lecomte R, Laine JB, Issa N, Revest M, Gaborit B, Le Turnier P, et al. Long-term outcome of patients with non-operated prosthetic valve infective endocarditis: is relapse the main issue? Clin Infect Dis. 2020;22:1316–9.
Mokhles MM, Ciampichetti I, Head SJ, Takkenberg JJ, Bogers AJ. Survival of surgically treated infective endocarditis: a comparison with the general Dutch population. Ann Thorac Surg. 2011;91:1407–12.
Thuny F, Giorgi R, Habachi R, Ansaldi S, Le Dolley Y, Casalta JP, et al. Excess mortality and morbidity in patients surviving infective endocarditis. Am Heart J. 2012;164:94–101.
Vallejo Camazón N, Cediel G, Núñez Aragón R, Mateu L, Llibre C, Sopena N, et al. Short- and long-term mortality in patients with left-sided infective endocarditis not undergoing surgery despite indication. Am Heart J. 2012;164:94–101.
Thuny F, Avierinos JF, Tribouilloy C, Giorgi R, Casalta JP, Milandre L, et al. Impact of cerebrovascular complications on mortality and neurologic outcome during infective endocarditis: a prospective multicentre study. Eur Heart J. 2007;28:1155–61.
Olson JD. D-dimer: an overview of hemostasis and fibrinolysis, assays, and clinical applications. Adv Clin Chem. 2015;69:1–46.
Simes J, Robledo KP, White HD, Espinoza D, Stewart RA, Sullivan DR, et al. D-dimer predicts long-term cause-specific mortality, cardiovascular events, and cancer in patients with stable coronary heart disease. Circulation. 2018;138:712–23.
Ay C, Dunkler D, Pirker R, Thaler J, Quehenberger P, Wagner O, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012;97:1158–64.
van Es N, van der Hulle T, van Es J, den Exter PL, Douma RA, Goekoop RJ, et al. Wells rule and D-dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253–61.
Turak O, Canpolat U, Ozcan F, Yayla C, Mendi MA, Oksüz F, et al. D-dimer level predicts in-hospital mortality in patients with infective endocarditis: a prospective single-centre study. Thromb Res. 2014;134:587–92.
Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–44.
Ferrera C, Vilacosta I, Fernández C, López J, Sarriá C, Olmos C, et al. Early surgery for acute-onset infective endocarditis. Eur J Cardiothorac Surg. 2018;54:1060–6.
Anantha Narayanan M, Mahfood Haddad T, Kalil AC, Kanmanthareddy A, Suri RM, Mansour G, et al. Early versus late surgical intervention or medical management for infective endocarditis: a systematic review and meta-analysis. Heart. 2016;102:950–7.
Fabri J Jr, Issa VS, Pomerantzeff PM, Grinberg M, Barretto AC, Mansur AJ. Time-related distribution, risk factors and prognostic influence of embolism in patients with left-sided infective endocarditis. Int J Cardiol. 2006;110:334–9.
Nunes MCP, Guimarães-Júnior MH, Murta Pinto PHO, Coelho RMP, Souza Barros TL, Faleiro Maia NPA, et al. Outcomes of infective endocarditis in the current era: early predictors of a poor prognosis. Int J Infect Dis. 2018;68:102–7.
Cornelissen CG, Frechen DA, Schreiner K, Marx N, Krüger S. Inflammatory parameters and prediction of prognosis in infective endocarditis. BMC Infect Dis. 2013;13:272.
Ris T, Teixeira-Carvalho A, Coelho RMP, Brandao-de-Resende C, Gomes MS, Amaral LR, et al. Inflammatory biomarkers in infective endocarditis: machine learning to predict mortality. Clin Exp Immunol. 2019;196:374–82.
Thoker ZA, Khan KA, Rashid I. Correlation of cardiac troponin I levels with infective endocarditis & its adverse clinical outcomes. Int J Cardiol. 2016;222:661–4.
Tripodi A. D-dimer testing in laboratory practice. Clin Chem. 2011;57:1256–62.
Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387:882–93.
Holland TL, Baddour LM, Bayer AS, Hoen B, Miro JM, Fowler VG. Infective endocarditis. Nat Rev Dis Primers. 2016;2:16059.
Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res. 2016;118:1392–408.
Ohlmann P, Faure A, Morel O, Petit H, Kabbaj H, Meyer N, et al. Diagnostic and prognostic value of circulating D-dimers in patients with acute aortic dissection. Crit Care Med. 2006;34:1358–64.
Halvorsen S, Seljeflot I, Weiss T, Bøhmer E, Arnesen H. Inflammatory and thrombotic markers in patients with ST-elevation myocardial infarction treated with thrombolysis and early PCI: a NORDISTEMI substudy. Thromb Res. 2012;130:495–500.
Musicant SE, Taylor LM, Peters D, Schuff RA, Urankar R, Landry GJ, et al. Prospective evaluation of the relationship between C-reactive protein, D-dimer and progression of peripheral arterial disease. J Vasc Surg. 2006;43:772–80.
Bakal RB, Karakoyun S, Kahveci G, Ozveren O, Omaygenç O, Hatipoğlu Akpınar S, et al. Relationship between D-dimer and systemic embolism in patients with infective endocarditis. Turk Kardiyol Dern Ars. 2013;41:589–94.
Barış VÖ, Kılıçkap M, Göksülük H, Gerede Uludağ DM, Erol Ç. Dimer is a strong predictor of in-hospital mortality in patients with infective endocarditis. Anatol J Cardiol. 2019;21:124–33.
Acknowledgements
None.
Funding
This study was supported by grants from National Natural Science Foundation of China (grant no. 82002014), Natural Science Foundation of Guangdong Province (grant no. 2021A1515010107), Medical Science and Technology Research Funding of Guangdong (Grant no. A2019409), the Fundamental Research Funds for the Central Universities (Grant no. 2019MS136), and Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention (Grant no. 2017B030314041). The funders had no role in the study design, data collection and analysis, decision to publish, nor preparation of the manuscript. The work was not funded by any industry sponsors.
Author information
Authors and Affiliations
Contributions
YDQ and CJY contributed to the conception or design of the study. LYW, WXB, HJL, SZDZ, JM contributed to the acquisition, analysis, or interpretation of data. LYW, JM, WXB drafted the manuscript. YDQ and CJY critically revised the manuscript. All the authors gave final approval and agreed to be accountable for all aspects of work for ensuring integrity and accuracy. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital with a waiver of informed consent because of the retrospective study design (No. GDREC2020098H). Oral informed consents were obtained from patients or their relatives on the telephone by trained nurses during the follow-up period.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, YW., Jiang, M., Wei, Xb. et al. Prognostic value of D-dimer for adverse outcomes in patients with infective endocarditis: an observational study. BMC Cardiovasc Disord 21, 279 (2021). https://doi.org/10.1186/s12872-021-02078-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-021-02078-3