Abstract
Background
Patients with acute myocardial infarction (AMI) are usually treated with angiotensin-converting enzyme inhibitors (ACEIs), or angiotensin receptor blockers (ARBs) if ACEIs are not tolerated. However, there is no data regarding the impact of switching from ACEIs to ARBs on long-term clinical outcomes in AMI patients with preserved left ventricular (LV) systolic function especially beyond 1 year. To investigate the effectiveness of treatment with ACEIs or ARBs on clinical outcomes over 3 years in AMI patients with preserved LV systolic function following percutaneous coronary intervention.
Method
It is a prospective cohort study using data from a nationwide large scale registry with 53 hospitals involved in treatment of acute myocardial infarction (AMI) in Korea. Between March 2011 and September 2015, we enrolled 6236 patients with AMI who underwent primary percutaneous coronary intervention and had a left ventricular ejection fraction ≥ 50%. Main outcome measures composite of total death or recurrent AMI over 3 years after AMI. Patients were divided into an ACEI group (n = 2945), ARB group (n = 2197), or no renin-angiotensin system inhibitor (RASI) treatment (n = 1094). We analyzed patients who changed treatment. Inverse probability of treatment weighting (IPTW) analysis was also performed.
Results
After the adjustment with inverse probability weighting, the primary endpoints at 1 year, AMI patients receiving ACEIs showed overall better outcomes than ARBs [ARBs hazard ratio (HR) compared with ACEIs 1.384, 95% confidence interval (CI) 1.15–1.71; P = 0.003]. However, 33% of patients receiving ACEIs switched to ARBs during the first year, while only about 1.5% switched from ARBs to ACEIs. When landmark analysis was performed from 1 year to the end of the study, RASI group showed a 31% adjusted reduction in primary endpoint compared to patients with no RASI group (HR, 0.74; 95% CI 0.56–0.97; P = 0.012).
Conclusions
This result suggests that certain patients got benefit from treatment with ACEIs in the first year if tolerated, but switching to ARBs beyond the first year produced similar outcomes. RASI beyond the first year reduced death or recurrent AMI in AMI patients with preserved LV systolic function.
CRIS Registration number: KCT0004990.
Similar content being viewed by others
Key points
-
Question: Do renin angiotensin system inhibitors have long term clinical beneficial effect in acute myocardial infarction (AMI) patients with preserved left ventricular systolic function?
-
Findings: In this prospective cohort study including 6236 AMI patients who underwent percutaneous coronary intervention, 33% of patients receiving angiotensin converting enzyme inhibitors switched to angiotensin receptor blockers during the first year. Renin angiotensin system inhibitors (RASI) beyond the first year reduced death or recurrent AMI in AMI patients with preserved left ventricular systolic function.
-
Meaning: Switching the medication of RASI does not impact on long term major clinical outcomes in AMI patients with preserved left ventricular systolic function. Continuation of treatment is necessary.
Introduction
Angiotensin-converting enzyme inhibitors (ACEIs) are considered as primary drugs for the secondary prevention of myocardial infarction (MI), and angiotensin receptor blockers (ARBs) are used when ACEIs are not tolerated [1, 2]. Both these types of drugs are recognized as renin-angiotensin system inhibitors (RASI), but differences in their modes of action have been reported to be associated with clinically different outcomes.
The usefulness of ACEIs for secondary prevention of cardiovascular events in patients with acute myocardial infarction (AMI) and heart failure with reduced ejection fraction (HFrEF) has been established by many randomized controlled trials (RCTs) [3,4,5]. However, the effectiveness of ACEIs and ARBs as first-line drugs in patients with AMI with preserved left ventricular (LV) function has not been established. While both classes are equally effective in patients who have vascular disease or high-risk diabetes but do not have heart failure, they may be differentially beneficial in MI patients with preserved LV systolic function [6]. Additionally, while many physicians use ACEIs as a first-line therapy, up to 20% of patients develop adverse reactions such as a cough and/or angio-edema, and change to ARBs [7], but no data is available indicating whether changing from ACEIs to ARBs in MI patients with preserved LV systolic function can affect long-term clinical outcomes including mortality.
We sought to investigate the effectiveness of treatment with ACEIs or ARBs on clinical outcomes over 3 years in AMI patients with preserved LV systolic function following percutaneous coronary intervention (PCI) from a nationwide large-scale registry of AMI patients in South Korea. We also investigated how many patients changed medication after 1 year and the impact of changing medication on long-term outcomes.
Methods
Study population
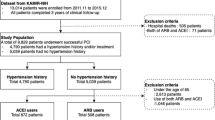
The study population was enrolled from the Korea AMI registry (KAMIR)-National Institutes of Health (NIH) registry. The design of KAMIR study has been described in our previous studies [8, 9] and the details of the registry can be found at the KAMIR website (http://www.kamir.or.kr). Briefly, it is a prospective, multicenter online registry designed to reflect the “real world” practice in a series of Korean AMI patients since November 2005 to investigate the current clinical outcomes. Participating centers have a high volume of patients and have facilities for primary PCI and onsite cardiac surgery. From November 2011 to December 2015, a total of 13,104 patients with AMI have been enrolled in a nationwide KAMIR-NIH registry. A trained study coordinator, independent research personnel collected clinical, laboratory, and clinical outcome data using a standardized case report form and protocol. Each patient was followed up during an outpatient clinic visit or contacted by telephone conversation. Inclusion criteria for the present analysis were consecutive patients aged ≥ 18; ST segment elevation or non-ST segment elevation myocardial infarction; and patients undergoing PCI. The investigators defined AMI as the criteria for the universal definition of myocardial infarction [10]. We excluded patients who died in hospital; lacked documentation of drugs prescribed at discharge and 1-year after discharge; concomitantly used an ACEI and ARB; or had a LV ejection fraction (EF) < 50% or lacked information on LVEF. From registered patients, we ultimately included 6236 in this analysis. Patients were divided into the ACEIs group and the ARBs group or the no RASI group according to the use of ACEI or ARB at discharge.
Percutaneous coronary intervention procedure and medical treatment
Physicians performed coronary angiography and stent implantation according to standard techniques and the type of the stent chosen was at the operator's discretion [11]. All patients received a 300 mg loading doses of aspirin and a 300–600 mg loading of clopidogrel or 60 mg of prasugrel or 90 mg of ticagrelor before the procedure, unless they had previously received these antiplatelet drugs. Periprocedural anticoagulation was administered according to standard regimens. Most of the patients maintained dual antiplatelet therapy at the time of PCI or discharge, and the duration of dual antiplatelet therapy was determined by the physicians. The decision to use glycoprotein IIb/IIIa inhibitors and to perform thrombus aspiration or intravascular ultrasonography was at the operator’s discretion. Drug-eluting stents (DES) were deployed after prior balloon angioplasty. During the in-hospital period, the patients received cardiovascular beneficial medications including beta-blockers, ACEI, ARB, calcium channel blockers, and statins. After discharge, the patients were encouraged to maintain the same medication they received during the hospitalization but some patients switched ACEI to ARB or ARB to ACEI and unexpectedly, some patients did not use RASI after 1 year.
Study definition and endpoint
A successful PCI was defined as normal antegrade coronary blood flow is achieved in > 95% of patients without major adverse cardiac events (MACE) in the presence of thrombolysis in myocardial infarction (TIMI) flow grade 3 [12, 13]. The key combined primary endpoint was as defined as the composite of all-cause death or recurrent AMI. The key combined secondary endpoint was MACE, as defined as the composite of all-cause death, recurrent AMI, and any repeat revascularization. Also, the secondary endpoints were the occurrence of any clinical events such as all-cause death, recurrent AMI, any repeat revascularization including surgical coronary artery bypass graft (CABG), or repeat PCI, stroke, and re-hospitalization due to heart failure (HF). All deaths are considered to be cardiac in origin unless a non-cardiac origin was definitely documented. Recurrent MI is defined as recurrent symptoms with new ST-segment elevation or re-elevation of cardiac markers to at least twice the upper limit of normal [13]. Target lesion revascularization (TLR) is defined as repeat PCI within the index procedure stent or 5 mm edge. Target vessel revascularization (TVR) is defined as any repeat PCI or surgical CABG of any segment in the target vessel. Any repeat revascularization is defined as any repeat PCI or CABG of the target vessel or non-target vessel. Target lesion failure is defined as the composite of clinically driven TLR, recurrent AMI, or cardiac death related to the target vessel [14]. All participants are required to visit the outpatient department of cardiology at the end of the first month and then every three to six months after the PCI procedure, as well as whenever angina-like symptoms occurred [15].
Ethics statement
This study was conducted in compliance with the Declaration of Helsinki regarding investigations in humans, and the study protocol was reviewed and approved by the each Institutional Review Board (IRB) of participating hospitals (Chonnan National Hospital, IRB 2011-E63002-00, 2012-E63003-00, 2013-E63005-00, 2014-E63005-01, 2013-E63005-02). Written informed consent was obtained from each patient when they were enrolled. This registry has not been registered on ClinicalTrials.gov due to observational study.
Statistical analysis
For continuous variables, differences among the three groups are evaluated using the one-way ANOVA or Kruskal–Wallis test. Data are expressed as mean ± standard deviations. For discrete variables, differences are expressed as counts and percentages and analyzed with the χ2 or Fisher’s exact test among the three groups. To adjust for any potential confounders, an inverse probability of treatment weighting (IPTW) analysis is performed [16]. We utilized generalized boosted models to estimate the propensity score weight of each treatment using methods developed for comparison of multiple treatments.
The average treatment effect on the population weights was estimated using the multinomial propensity scores (MNPS) function in the Twang package (http://www.rand.org/statistics/twang/downloads.html/mnps_turorial_code.r.) in R Statistical Software (R Foundation for Statistical Computing, Vienna, Austria). We tested all available variables that could be of potential relevance: age, sex (male), LVEF, cardiovascular risk factors (e.g., hypertension, diabetes, dyslipidemia, stroke), co-medication treatment (e.g., aspirin, other anti-platelets, calcium channel blockers, beta-blockers, and statins), angiographic and procedural characteristics (e.g., target vessel, number of diseased vessels, and DES type). Various clinical outcomes for up to 3 years are estimated by Cox-proportional hazards models analysis. Binary logistic regression analysis is used to assess the hazard ratio (HR) of the ACEIs group compared to the ARBs group and the non-RASI user group in the IPTW population. For all analyses, a two-sided P < 0.05 was considered statistically significant. All data are processed with SPSS (version 24.0, SPSS-PC, Inc. Chicago, Illinois) and R statistical software.
Results
Baseline and procedural characteristics
Overall population
The baseline, laboratory, angiographic, and procedural characteristics of the study population are summarized in Table 1. Among the 6236 eligible patients, at discharge ACEIs were prescribed to 2945 patients (47%) and ARBs to 2197 patients (35%). Renin angiotensin system blockers were not prescribed to 1094 patients (18%). Compared with patients receiving ACEIs, those in the group receiving ARBs were on average older, had a higher rate of diabetes mellitus (most of which was well controlled), had a lower rate of right coronary artery as the infarct-related artery, and had a lower rate of multi-vessel disease. Overall, patients who did not receive RASI, apart from having a slightly higher rate of low blood pressure, were on average not higher-risk patients when compared to patients in the group which received RASI. In addition, patients who did not receive RASI were less likely to have received β blockers and statins, but more likely to have received Ca-channel blockers.
After adjustment using inverse probability of treatment weighting (IPTW), there were no statistically significant differences in baseline clinical, angiographic, and procedural characteristics among the different groups (ACEIs, ARBs, and no RASI treatment), with the exception of blood pressure (Table 1). However, the observed difference in systolic blood pressure among the groups was small, and only varied by 10-15 mmHg. Most of patients had blood pressure above 100 mmHg, which is above key shock thresholds for concern.
Major clinical outcomes
Overall population at 1 year and 3 years after AMI
Table 2 shows the cumulative incidences of the major clinical outcomes during the 3-year follow-up period of the study groups. Total death or recurrent AMI occurred in 175 patients (5.9%) in the ACEI group, 164 patients (7.4%) in the ARB group (P = 0.03 for comparison with ACEIs) and 93patients (8.5%) in the no RASI group (P = 0.004 for ACEIs). Although the primary composite endpoints of ARB group seemed to be numerically lower than that of no RASI group, long-term outcomes were not statistically different between crude population (P = 0.297). When IPTW was performed at the primary endpoint (total death or recurrent AMI) after 3 years of AMI, patients receiving ACEIs had overall better outcomes than patients receiving ARBs (ACEi 6.0% vs. ARBs 7.0%, P = 0.033). Between ARBs and no RASI group, the primary endpoint were not significantly different (ARB 7.0% vs no ACEI/ARB group 7.3%, P = 0.553). However, the incidence of total death was significantly lower in the ARBs group (ARB 4.4% vs. no RASI 5.2%, P = 0.048).
When Cox regression analysis with IPTW was performed at the primary end point (total death or recurrent AMI) after 1 year of AMI, patients receiving ACEIs showed overall better outcomes than patients receiving ARBs (ARBs compared with ACEIs, hazard ratio [HR] 1.384, 95% confidence interval [CI] 1.15–1.717, P = 0.003, Table 3). There was no difference in the incidence of total death between patients in the two groups (ARBs compared with ACEIs, HR1.308, 95% CI 0.999–1.714, P = 0.051). However, when solely examining, patients receiving ACEIs were less likely to experience recurrent AMI compared to patients receiving ARBs (ARBs compared with ACEIs, HR 1.577, 95% CI 1.127–2.205, P = 0.008). We also observed that patients receiving ARBs were twice more likely to experience a stroke compared to the group receiving ACEIs (ARBs compared with ACEIs, HR 2.015, 95% CI 1.391–2.917, P < 0.001) at 1 year after AMI. Both ARB group and no RASI group showed similar risk of cardiac death or myocardial infarction (no RASI group compared with ARBs, HR 1.182, 95% CI 0.974 to 1.435; P = 0.0.089) after adjustment with IPTW at 1 year after AMI. However, treatment with ARBs was associated with a significant lower incidence of cardiac death compared with no RASI (no RASI group compared with ARBs, HR 1.182, 95% CI 0.974 to 1.435; P = 0.002). These results were similar in the 3-year follow up after AMI. Receiving ACEIs had overall better outcomes than patients receiving ARBs or no RASI. Receiving ARBs have numerically lower incidence of primary endpoint compared with no RASI group but statistically significantly lower incidence of total death at 3 years after AMI.
Subgroup analysis
We calculated the adjusted HR for total death or MI in various complex subgroups at 3 years and 1 year after AMI (Fig. 1). Non- ST elevation MI (NSTEMI) patients who were treated with ACEIs during the 3 years after MI tended to have better clinical outcomes than NSTEMI patients treated with ARBs (ARBs compared with ACEIs, HR 1.530, 95% CI 1.148–2.039, P = 0.004, Fig. 1a). Additionally, for NSTEMI patients, ACEIs were more effective than ARBs when used during the first year of treatment, and equivalent thereafter (ARBs compared with ACEIs, HR 1.824, 95% CI 1.200–2.771, P = 0.005, Fig. 1a). There were no significant difference between the use of ARB at discharge and cardiac death or MI in other subgroups (STEMI, hypertension and diabetes mellitus, normal renal function) at 1 year (Fig. 1b–d, f). However, in the group of hypertension and diabetes mellitus, the clinical outcomes of the ARB group was tend to be worse than those of the ACEI group after adjustment at 3 years. In patients with kidney disease, there were no significant differences among the three groups with regard to cardiac death or MI at both 1 and 3 years (Fig. 1e).
Inverse probability of treatment weighting score adjusted survival curves from Cox-proportional hazards models for death or myocardial infarction (MI) according to discharge treatment in overall and various subgroups. a Non-STEMI population, b STEMI population, c hypertension history population, d diabetes mellitus population, e kidney disease population, f non-kidney disease population. ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; STEMI, ST segment elevation myocardial infarction; RASI, renin angiotensin system inhibitor; eGFR-MDRD, estimates glomerular filtration rate-the original modification of diet in renal disease
Switching medication
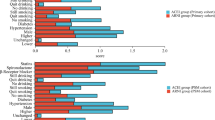
All patients were required to remain on treatment, but 19% of patients receiving treatment with RASI stopped medication in the first year (Fig. 2a). Interestingly, 18.3% of patients receiving ACEIs stopped treatment, and 14.3% of patients receiving ARBs stopped treatment in this real-world registry (Fig. 2b, c). Thirty three percent of patients receiving ACEIs switched to ARBs during the first year, while only about 1.5% switched from ARBs to ACEIs. In patients not receiving RASI treatment, 24.5% began receiving ARBs and 8% began receiving ACEIs (Fig. 2d). In total, 1,628 patients among 6536 patients either stopped treatment or were not receiving treatment after 1 year. From 1 year after AMI, 1151 patients received ACEIs for the duration of the study, 1,110 patients received ACEIs during the first year but switched to ARBs after, and 1,687 patients used ARBs for the duration of the study. Three hundred seventeen patients began not receiving any treatment but initiated treatment with ARBs after 1 year. Less than 2% of patients started, stopped, or changed treatment after the first year. Additional analysis for the primary outcomes was performed according to the timing of switch from each medications.
Changes in the use of renin-angiotensin system inhibitors after myocardial infarction in the Korean real-world. a All patients were required to remain on treatment, but 19% of patients receiving treatment with RASI stopped medication in the first year, b eighteen percent of patients receiving ACEIs stopped treatment in the first year and 33.4% of patients receiving ACEIs changed medication to ARBs, c fourteen percent of patients receiving ARBs stopped treatment in this real-world registry, while only about 1.5% switched from ARBs to ACEIs, d in patients not receiving RASI treatment, 24.5% began receiving ARBs and 8% began receiving ACEIs. RASI, renin-angiotensin system inhibitors; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; STEMI, ST segment elevation myocardial infarction
When Landmark analysis was performed from 1 year to the end of the study period (Fig. 3), groups receiving RASI showed a 31% adjusted reduction in risk of death or recurrence of MI compared to patients not receiving RASI after adjustment with IPTW (HR, 0.741; 95% CI, 0.563–0.975; P = 0.012). While all groups who received RASI showed a statistically significant reduction in death or recurrence of AMI, patients who received ACEIs in the first year but switched to ARBs after 1 year were associated with statistically significant reduction in the incidence of death or recurrent AMI, while the other treatment groups did not differ from each other significantly (Fig. 4).
Survival curves from Cox-proportional hazards models for death or myocardial infarction (MI) according to renin-angiotensin system inhibitors treatment between 1-year to 3-year after an acute myocardial infarction. RASI, renin-angiotensin system inhibitors; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker
Survival curves from Cox-proportional hazards models for death or myocardial infarction (MI) according to switching renin-angiotensin system inhibitors treatment between 1-year to 3-year after an acute myocardial infarction. RASI, renin-angiotensin system inhibitors; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker
Discussion
The main findings of our study are as follows. First, in AMI patients with preserved LV systolic function who underwent PCI, the risk of total death or recurrent AMI was lower in patients treated with ACEIs in the first year compared to patients treated with ARBs. Both groups showed lower risk compared to patients who did not receive any RASI. Second, for NSTEMI patients, treatment with ACEIs in the first year was associated with a greater reduction in risk compared to ARBs, but both were generally equivalent in risk reduction in patients with STEMI, hypertension, and diabetes mellitus. This suggests that NSTEMI patients should be treated with ACEIs in the first year. Third, AMI patients with preserved LV systolic function had a poorer prognosis if RASI were stopped after 1 year, suggesting that treatment should be continued beyond 1 year after AMI. Fourth, after 1 year, about a third of patients on ACEIs switched to ARBs, likely due to side effects, but this change in treatment did not alter their prognosis or risk of total death or recurrent AMI at least up to 3 years. This suggests that after the first year, switching the medication does not impact on long term major clinical outcomes. However, continuation of treatment is necessary.
Most randomized trials have demonstrated that ACEI therapy with captopril, enalapril, ramipril, trandolapril, or zofenopril started within 24 h to 16 days following an AMI improves the LV ejection fraction at one month to 1 year [3, 5, 17,18,19]. In these studies, the great majority of patients had an STEMI; data are limited in patients with a NSTEMI [10]. In addition, most patients were treated with either fibrinolytic therapy or no reperfusion; data in patients who underwent PCI, particularly treated with DESs for AMI are limited. ACEIs have also been reported to be beneficial in low risk patients after AMI [20], although the role of routine long term treatment in low risk patients who have undergone revascularization after STEMI is less certain. Therefore, the guidelines recommend that ACE inhibitors should be considered in all patients after STEMI in the absence of contraindications. [21]
Yang et al. showed that ARB had beneficial effects in terms of cardiac death or MI, which were comparable with those achieved with ACEIs in patients with STEMI with preserved LV systolic function [22]. They studied 6698 patients with STEMI who underwent PCI and had a LV ejection fraction ≥ 40% among the same Korean registry used in this study, but with data from a different time period (Between November 2005 and September 2010). Yang’s study differs from our study in that they enrolled only STEMI patients with EF greater than 40%, and they used a different time period and different duration of progress observation (Yang followed patients for 1 year, whereas we followed patients for 3 years). Yang’s study examined patients from a 5-year period (2005–2010) with EF greater than 40%, whereas our study examined patients from a different 5-year period (2011–2016) with EF greater than 50%, which reflect on the clinical outcomes of current contemporary PCI with DESs. In other words, our study examined a different set of lower-risk patients and examined longer-term outcomes by following them for 3 years instead of 1 year. It is notable that despite of these differences, both studies found that STEMI patients have no difference in the incidence of total death or recurrent AMI in groups treated with either ACEI or ARB at their 1-year progress observations. However, in the NSTEMI group (which was not analyzed by Yang et al.), our study found that NSTEMI patients who were treated with ACEIs during the first year after MI was associated with better clinical outcomes than NSTEMI patients treated with ARBs, suggesting the possibility that ACEIs may be more effective in the NSTEMI group in the first year than ARBs, although this requires further research. There are few studies examining RASI in NSTEMI patients. Kim et al. demonstrated that ACEIs showed more prominent ability to decrease the occurrences of MACEs, any repeat revascularization, and TVR compared to the ARB group in NSTEMI patients during a 2-year follow-up period. Additionally Byun et al. found that compared to ARB, ACEIs did not impact on causes of death, cardiac death, or recurrence of MI, but ACEIs did reduce the incidence of revascularization and MACE in diabetic NSTEMI patients [1, 23]. However, their study’s population included all NSTEMI patients, including patients with reduced EF. Our study only looked at AMI patients with EF greater than 50%, and suggests that the ACEI group had better outcomes than the ARB group in the first year. However, in our study, 33% of patients switched from ACEIs to ARBs during the first year, and patients may have changed drugs as early as the three to six-month points if they experienced side effects of ACEI, so it is not possible to conclude with certainty that one is more effective than the other.
Although the participants in our study had AMI with preserved LV systolic function, they also had a risk of LV remodeling post MI. LV remodeling is one of the major determinants of survival after recovery from MI, and the mechanism of LV remodeling within a few weeks after myocardial infarct reperfusion is well known[24]. The three main biomechanisms that contribute to the increase in the LV chamber volume post MI are (1) expansion of the infarct in the subacute phase, (2) subsequent non-ischemic infarct extension into the adjacent non-infarcted region, and (3) hypertrophy and dilatation of the non-infarcted myocardium in the chronic phase. Previous studies have led to a general consensus that treatment with ACEIs and ARBs has an anti-fibrotic effect on collagen formation in infarcted and remote non-infarcted regions [25]. This is the rationale for implementing drug therapy as soon as possible after MI, and the current ACC/AHA guidelines recommend ACE inhibitors as Class I drugs for use within the first 24 h after MI. When considering the obvious clinical benefits of ACEIs and ARBs, this suggests that there may be excessive of fibrosis of the infarct in untreated patients, when a smaller, more compact scar is preferred. Although our study cannot determine the superiority of either ACEIs or ARBs, the clinical results of ACEIs tend to be superior to those of ARBs. A plausible hypothesis for this is that these two drugs (ACEIs and ARBs), which are in the same system in terms of RAS inhibition, have different mechanisms of action[26]. ACEIs inhibit the conversion of angiotensin I to angiotensin II, which reduces the level of serum angiotensin II and activates the bradykinin system. In contrast, ARBs may cause prolonged elevation of angiotensin II levels and the upregulation of angiotensin I. It is well known that elevated serum levels of angiotensin II play an important role in the pathogenesis of coronary artery disease [27]. In addition, elevated angiotensin II levels may direct an increase in serum cholesterol levels via the interaction with macrophage AT1 receptors, stimulating 3-hydroxy-3-methylglutaryl coenzyme A reductase gene expression, ultimately leading to cholesterol accumulation in macrophages and foam cell formation [28].
Some data showed that treatment with ACEIs or ARBs after AMI was associated with improved long-term survival compared to a group not receiving treatment, regardless of underlying renal function, and was accompanied by low rates of adverse renal events [29]. The findings of our study support earlier studies that showed patients with kidney disease receiving RASI treatment after MI have better outcomes than patients not receiving RASI treatment. Although, the statistical significance was not confirmed, but risk of death or MI were better in both the ACEI and ARB groups than in the No RASI group. In all cases, our results suggest that RASI are effective and should be used in patients following AMI with preserved LV systolic function.
There is much controversy over the MI paradox when using ARB. Angiotensin II receptor stimulation may be less beneficial than previously proposed and may even be harmful under certain circumstances through mediation of growth promotion, fibrosis, and hypertrophy, as well as proatherogenic and proinflammatory effects. However, in a total of 19 of 24 studies of data on AMI, representing a total of 31 569 patients, use of ARBs was not associated with an increased risk of MI compared with placebo (odds ratio [OR] 0.94, 95% CI 0.75 to 1.16) nor when compared with ACE inhibitors (OR 1.01, 95% CI 0.87 to 1.16). Our data showed that 33% of patients using ACEIs changed to ARBs at the end of a year, and these patients showed an equal or improved prognosis compared to those who remained on ACEIs. This suggests that ARBs are safe and effective in a population with AMI and preserved LV function, particularly AMI patients undergoing PCI with DESs.
Limitations
First, our study did not have data on LV function at follow-up, meaning that our study cannot account for whether patient’s outcomes were impacted by changes in LV function or if they regained or lost LV function during the study period. Because it is possible that RASI have different effectiveness in populations with greater or reduced EF, it will be necessary to examine this more in the future to confirm these findings. Second, because the registry data is non-randomized, it is possible there was selection bias. Although we performed an IPWT matched analysis to account for these potential confounding factors, it was not possible to correct for unmeasured variables. For example, our database does not include information on why physicians switched patients from ACEIs to ARBs. In the future, it will be necessary to conduct randomized trials to confirm and expand the findings of this study. Third, adverse clinical events were not centrally adjudicated in our registry, and were instead identified by the patient’s physician and confirmed by the principal investigator of each hospital. As a result, it is possible that some adverse events were not captured in the database.
Availability of data and materials
All data can be checked by sending an email to Correspondence.
Abbreviations
- AMI:
-
Acute myocardial infarction
- ACEI:
-
Angiotensin-converting enzyme inhibitor
- ARB:
-
Angiotensin receptor blocker
- LV:
-
Left ventricular
- RASI:
-
Renin-angiotensin system inhibitor
- PCI:
-
Percutaneous coronary intervention
- TLR:
-
Target lesion revascularization
- TVR:
-
Target vessel revascularization
- IPTW:
-
Inverse probability of treatment weighting
References
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio AL, Crea F, Goudevenos JA, Halvorsen S. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77.
Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–228.
Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival and Ventricular Enlargement Trial. N Engl J Med. 1992;327(10):669–77.
Sharpe N, Smith H, Murphy J, Greaves S, Hart H, Gamble G. Early prevention of left ventricular dysfunction after myocardial infarction with angiotensin-converting-enzyme inhibition. The Lancet. 1991;337(8746):872–6.
Køber L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, Videbæk J, Cole DS, Auclert L, Pauly NC. A clinical trial of the angiotensin-converting–enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 1995;333(25):1670–6.
Clase CM, Gao P, Tobe SW, McQueen MJ, Grosshennig A, Teo KK, Yusuf S, Mann JF. Estimated glomerular filtration rate and albuminuria as predictors of outcomes in patients with high cardiovascular risk: a cohort study. Ann Intern Med. 2011;154(5):310–8.
Investigators TRASiAiswcD. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. The Lancet. 2008;372(9644):1174–83.
Choi SY, Choi BG, Rha S-W, Byun JK, SukShim M, Li H, Mashaly A, Choi CU, Park CG, Seo HS. Angiotensin-converting enzyme inhibitors versus angiotensin II receptor blockers in acute ST-segment elevation myocardial infarction patients with diabetes mellitus undergoing percutaneous coronary intervention. Int J Cardiol. 2017;249:48–54.
Hwang D, Lee JM, Kim HK, Choi KH, Rhee T-M, Park J, Park TK, Yang JH, Song YB, Choi J-H. Prognostic impact of β-blocker dose after acute myocardial infarction. Circ J. 2019;83(2):410–7.
Bax JJ, Baumgartner H, Ceconi C, Dean V, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti JJJotACoC. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60(16):1581–98.
Grech ED. ABC of interventional cardiology: percutaneous coronary intervention. II: the procedure. BMJ. 2003;326(7399):1137–40.
Carrick D, Haig C, Rauhalammi S, Ahmed N, Mordi I, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S. Prognostic significance of infarct core pathology revealed by quantitative non-contrast in comparison with contrast cardiac magnetic resonance imaging in reperfused ST-elevation myocardial infarction survivors. Eur Heart J. 2016;37(13):1044–59.
McCartney PJ, Berry C. Redefining successful primary PCI. Oxford University Press; 2019.
Stolker JM, Cohen DJ, Kennedy KF, Pencina MJ, Lindsey JB, Mauri L, Cutlip DE, Kleiman NS. Repeat revascularization after contemporary percutaneous coronary intervention: an evaluation of staged, target lesion, and other unplanned revascularization procedures during the first year. Circ Cardiovasc Interv. 2012;5(6):772–82.
Kim YH, Her A-Y, Rha S-W, Choi BG, Shim M, Choi SY, Byun JK, Li H, Kim W, Kang JH. Routine angiographic follow-up versus clinical follow-up after percutaneous coronary intervention in acute myocardial infarction. Yonsei Med J. 2017;58(4):720.
Sim DS, Jeong MH, Kim HS, Gwon HC, Seung KB, Rha SW, Chae SC, Kim CJ, Cha KS, Park JS. Dual antiplatelet therapy beyond 12 months versus for 12 months after drug-eluting stents for acute myocardial infarction. J Cardiol. 2020;75(1):66–73.
Pfeffer MA, Lamas GA, Vaughan DE, Parisi AF, Braunwald E. Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction. N Engl J Med. 1988;319(2):80–6.
St John Sutton M, Pfeffer MA, Plappert T, Rouleau J-L, Moyé LA, Dagenais GR, Lamas GA, Klein M, Sussex B, Goldman S. Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation. 1994;89(1):68–75.
Schulman SP, Weiss JL, Becker LC, Guerci AD, Shapiro EP, Chandra NC, Siu C, Flaherty JT, Coombs V, Taube JC. Effect of early enalapril therapy on left ventricular function and structure in acute myocardial infarction. Am J Cardiol. 1995;76(11):764–70.
Miocardico GIplSdSnI. GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Lancet. 1994;343(8906):1115–22.
O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, De Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(4):485–510.
Yang JH, Hahn J-Y, Song YB, Choi S-H, Choi J-H, Lee SH, Jeong M-H, Choi D-J, Park JS, Park HS. Angiotensin receptor blocker in patients with ST segment elevation myocardial infarction with preserved left ventricular systolic function: prospective cohort study. BMJ. 2014;349:g6650.
Byun JK, Choi BG, Rha S-W, Choi SY, Jeong MH. Investigators KAMIR: Comparison of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in patients with diabetes mellitus and non-ST-segment elevation myocardial infarction who underwent successful percutaneous coronary intervention. Atherosclerosis. 2018;277:130–5.
French BA, Kramer CM. Mechanisms of Post-Infarct Left Ventricular Remodeling. Drug Discov Today Dis Mech. 2007;4(3):185–96.
Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108(11):1395–403.
Kim YH, Her AY, Jeong MH, Kim BK, Hong SJ, Kim S, Ahn CM, Kim JS, Ko YG, Choi D, et al. Comparison of clinical outcomes between angiotensin-converting-enzyme inhibitors and ARBs in patients with acute myocardial infarction with dyslipidemia after a successful stent implantation. Anatol J Cardiol. 2020;23(2):86–98.
Ramchand J, Patel SK, Srivastava PM, Farouque O, Burrell LM. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS ONE. 2018;13(6):e0198144.
Keidar S, Attias J, Heinrich R, Coleman R, Aviram M. Angiotensin II atherogenicity in apolipoprotein E deficient mice is associated with increased cellular cholesterol biosynthesis. Atherosclerosis. 1999;146(2):249–57.
Evans M, Carrero J-J, Szummer K, Åkerblom A, Edfors R, Spaak J, Jacobson SH, Andell P, Lindhagen L, Jernberg T. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in myocardial infarction patients with renal dysfunction. J Am Coll Cardiol. 2016;67(14):1687–97.
Acknowledgements
This study was done with the support of Korean Circulation Society (KCS) to commemorate the 50th Anniversary of KCS. The KAMIR study group of the KSC was as follows: Korea University Guro Hospital, Seoul, South Korea (Seung-Woon Rha), Gachon University Gil Medical Center, Incheon, South Korea (Tae Hoon Ahn), Wonju Severance Christian Hospital, Wonju, South Korea (Junghan Yoon), Seoul National University Hospital, Seoul, South Korea (Hyo-Soo Kim), Seoul St. Mary's Hospital, Seoul, South Korea (Ki-Bae Seung), Samsung Medical Center, Seoul, South Korea (Hyeon-Cheol Gwon), Kyungpook National University Hospital, Daegu, South Korea (Shung Chull Chae), Kyunghee University Hospital At Gangdong, Seoul, South Korea (Chong-Jin Kim), Pusan National University Hospital, Busan, South Korea (Kwang Soo Cha), Yeungnam University Medical Center, Daegu, South Korea (Jung-Hee Lee), Chonbuk National University Hospital, Jeongju, South Korea (Jei Keon Chae), Jeju National University Hospital, Jeju, South Korea (Seung-Jae Joo), Seoul National University Bundang Hospital, Bundang, South Korea (Chang-Hwan Yoon), Keimyung University Dongsan Medical Center, Daegu, South Korea (Seung-Ho Hur), Chungnam National University Hospital, Daejeon, South Korea (In-Whan Seong), Chungbuk National University Hospital, Cheongju, South Korea (Kyung-Kuk Hwang), Inje University Haeundae Paik Hospital, Busan, South Korea (Doo-Il Kim), Wonkwang University Hospital, Iksan, South Korea (Seok Kyu Oh), Gyeongsang National University Hospital, Jinju, South Korea (Jin-Yong Hwang), Chonnam National University Hospital, Gwangju, South Korea (Myung Ho Jeong).
Funding
None.
Author information
Authors and Affiliations
Consortia
Contributions
KHK, BGC and SWR contributed to the idea and design of this study, prepared and verified the clinical coding, analyzed the data, wrote the first draft, and contributed to the subsequent drafts. BGC, MHJ and CUC prepared and verified the clinical coding and analyzed the data. BGC, KHK, and CUC contributed to the data collection and revised the manuscript. KHK and SWR are the principal investigators and contributed to the idea and design of this study, interpreted and analyzed the data, and contributed to the subsequent drafts. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
It is approved by the hospital’s IRB. Informed consent to participate in the study were obtained from participants.
Consent for publication
Not applicable.
Competing interests
The authors have no financial conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, KH., Choi, B.G., Rha, SW. et al. Impact of renin angiotensin system inhibitor on 3-year clinical outcomes in acute myocardial infarction patients with preserved left ventricular systolic function: a prospective cohort study from Korea Acute Myocardial Infarction Registry (KAMIR). BMC Cardiovasc Disord 21, 251 (2021). https://doi.org/10.1186/s12872-021-02070-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-021-02070-x