Abstract
Background
Patients with Heart Failure (HF) show impaired functional capacities which have been related to their prognosis. Moreover, physical functional performance in functional tests has also been related to the prognosis in patients with HF. Thus, it would be useful to investigate how physical functional performance in functional tests could determine the prognosis in patients with HF, because HF is the leading cause of hospital admissions for people older than 65 years old. This systematic review and meta-analysis aims to summarise and synthesise the evidence published about the relationship between physical functional performance and prognosis in patients with HF, as well as assess the risk of bias of included studies and the level of evidence per outcome.
Methods
Major electronic databases, such as PubMed, AMED, CINAHL, EMBASE, PEDro, Web of Science, were searched from inception to March 2020 for observational longitudinal cohort studies (prospective or retrospective) examining the relationship between physical functional performance and prognosis in patients with HF.
Results
44 observational longitudinal cohort studies with a total of 22,598 patients with HF were included. 26 included studies reported a low risk of bias, and 17 included studies showed a moderate risk of bias. Patients with poor physical functional performance in the Six Minute Walking Test (6MWT), in the Short Physical Performance Battery (SPPB) and in the Gait Speed Test showed worse prognosis in terms of larger risk of hospitalisation or mortality than patients with good physical functional performance. However, there was a lack of homogeneity regarding which cut-off points should be used to stratify patients with poor physical functional performance from patients with good physical functional performance.
Conclusion
The review includes a large number of studies which show a strong relationship between physical functional performance and prognosis in patients with HF. Most of the included studies reported a low risk of bias, and GRADE criteria showed a low and a moderate level of evidence per outcome.
Similar content being viewed by others
Background
Cardiovascular diseases continue to be the leading cause of disability-adjusted life-years (DALYs) due to non-communicable diseases and the leading cause of death [1,2,3]. Within cardiovascular diseases, Heart Failure (HF) is the only cardiovascular disease which is increasing in incidence and prevalence due to the aging of the world population, because its prevalence increases with age [4,5,6,7,8]. In addition, heart failure constitutes the most important hospital diagnosis in older adults, is the leading cause of hospital admissions for people older than 65 years old and contributes to the increase of medical care costs [5,6,7,8,9].
Heart Failure is characterised by a weak myocardium with decreased cardiac output that is unable to meet the body metabolic demands [4,5,6, 8, 10,11,12]. There are several functional symptoms that appear in patients with HF, such as reduced aerobic capacity, decreased muscle strength, low weekly physical activity and exercise intolerance, which are accompanied by fatigue and dyspnea symptoms [12,13,14,15,16,17]. Furthermore, patients with HF show impaired functional capacities, experience a declined ability to carry out their activities of daily living and suffer a reduced quality of life [12, 14, 17]. It has also been reported that patients with chronic HF show a slower gait speed than healthy subjects of the same age [18]. The maximal aerobic capacity has been inversely correlated to the severity of HF and has been directly correlated to the prognosis and the life expectancy [14, 19, 20]. Similarly, the lower extremities muscle mass and muscle strength have also been related to long-term survival in patients with HF [14, 21].
Some functional tests have been used to predict prognosis in patients with HF. Thus, the 6-min walk test (6-MWT) has been proposed as a simple, inexpensive, safe and reproducible exercise test to assess functional capacity in patients with HF, which could also predict the prognosis of patients with HF based on distance walked [12, 22,23,24]. The Short Physical Performance Battery (SPPB) provides a useful and indirect measure of muscle functional capacity [12]. Moreover, the SPPB and the Timed Up and Go test (TUG) could be used to assess physical or functional frailty in patients with HF, which has been associated with an increased risk of hospitalisation and mortality in chronic heart failure [25, 26]. The utility of Gait Speed has also been shown to predict functional independence loss, cardiovascular disease, hospitalisation, and mortality in older adults [27,28,29,30,31]. The 6-MWT measures the distance which patients can walk during 6 min [32]. The test is usually conducted in a closed corridor of 30 m where two marks are placed on the ground at a distance of 30 m, and patients walk from one end to the other, during 6 min [32]. The SPPB includes 3 tests: balance (feet together, semitándem and tandem during 10 s each), gait speed (4 m) and standing up and sitting on a chair 5 times. Each test is scored from 0 (worst performance) to 4 (best performance). The total score for the whole battery that is the addition of the 3 tests and ranges from 0 to 12 [33]. In the TUG test patients are sat down in a chair, and at the order to “go”, they stand up from the chair, walk 3 m until a reach a line that is on the floor. Then, patients should turn, return to the chair walking and sit again [34].
Hence, it would be necessary to conduct a synthesis of evidence that explores the relationship between the physical functional performance in functional tests and the prognosis in patients with HF. A systematic review may permit the formation of firm conclusions through an exhaustive synthesis of data [35]. Thus, the aim of this study was to answer the following PECOS (P, participant; E, exposure; C, comparator; O, outcome; S, study design) question through a systematic review of the literature on observational longitudinal cohort studies (prospective or retrospective) (S): Do older patients with HF (P), who have poor physical functional performance in some functional tests, such as 6-MWT, SPPB, TUG or Gait Speed (E), show a worse prognosis (O) than those patients with good physical functional performance (C)?
Methods
The Systematic Review and Meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [36]. The systematic review protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO: CRD42020177427).
Data sources and search strategy
Two independent reviewers (IJF-A and AIC-V) conducted a systematic search using relevant search terms that were developed from Medical Subject Headings (MeSH) and keywords from other similar studies from inception to March, 24th 2020 using optimised search strategies in the following electronic databases: PubMed, AMED, CINAHL, EMBASE, PEDro, Web of Science (Additional file 1). A manual search of relevant eligible studies, to select any studies missed during the electronic search, was also conducted using cross-references identified in the reference lists within both original and review articles. The grey literature databases, such as New York Academy of Medicine Grey Literature Report, Open Grey and Google Scholar [37] were examined to identify any relevant unpublished data. References were exported, and duplicates were removed using the Mendeley desktop V.1.19.2 citation management software.
Eligibility criteria
The aforementioned PECOS framework was followed to determine which studies were included in the present systematic review and meta-analysis. Each study had to meet the following inclusion criteria:
-
1.
Observational longitudinal cohort studies (prospective or retrospective)(S) examining whether older patients with HF (P), who have a poor physical functional performance in some functional tests, such as 6-MWT, SPPB, TUG or Gait Speed (E), show worse prognosis, assessed as larger risk of hospitalisation or mortality, (O) than those patients with good physical functional performance (C).
-
2.
No restriction was applied on the participants’ age, ethnicity, gender, HF diagnosis or on the New York Heart Association (NYHA) scale score.
-
3.
No restriction was applied on the language.
-
4.
Studies recruiting participants from any setting (general population, primary or secondary care).
-
5.
Studies providing Odds Ratio (OR) or Hazard Ratio (HR) data.
The exclusion criteria were as follows:
-
1.
All studies that did not include an observational longitudinal cohort design (e.g cross-sectional studies, randomised controlled trials).
-
2.
Studies exploring the prognosis value of functional tests in patients with other cardiovascular diseases different from HF.
-
3.
Studies examining the relationship between physical functional performance in functional tests and other outcomes different from mortality or hospitalisation.
-
4.
Studies investigating the prognosis value of physical activity assessed as daily activity, exercise time per week or physical activity scales.
Study selection
Two independent reviewers (IJF-A and AIC-V) carried out the screening of titles and abstracts to detect potentially relevant records and also excluded those documents that were not original papers. The same reviewers conducted the screening of those articles that met all inclusion criteria. A short checklist was carried out and followed in order to select the relevant studies (Additional file 2). In case of disagreements, the articles were always included.
Data extraction
Two independent reviewers (IJF-A and AIC-V) identified the following relevant data from each study: study details (first author and year of publication), region, setting, study design, sample size, functional tests with their cut-off points and characteristics of participants (mean age, %males), HF diagnosis, follow-up, outcome and main results. When necessary, an email was sent to the original authors to try to get OR or HR data that was not included in their original articles.
Quality assessment
The same two reviewers (IJF-A and AIC-V) assessed the risk of bias of the included observational longitudinal cohort studies using the Newcastle Ottawa Scale (NOS) [38]. The NOS has been decribed as a reliable and valid tool for assessing the quality of observational longitudinal cohort studies [38, 39].
Data synthesis and analysis
To assess the overall quality and the strength of the evidence per outcome, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used [40, 41]. Two researchers (IJF-A and AIC-V) judged whether these factors were present for each outcome reported at least in two studies. Meta-analysis was conducted for each outcome reported in two or more studies, as long as studies assessed the same outcome with the same functional test and the same measurement unit, that is, HR or OR. Outcomes not included in the meta-analysis were reported using a descriptive quantitative analysis. Thus, the most relevant summary measure with the 95% Confidence Interval (95%CI) for each study was provided. The most relevant summary measure with its 95%CI was extracted of adjusted multivariate models when it was possible. In each meta-analysis it was decided to use the inverse variance as statistical method, fixed effects as analysis model and the HR or OR as effect measures. Heterogeneity was assessed using I2 statistic [42, 43]. Values of > 25% is considered as low heterogeneity, > 50% moderate heterogeneity, and > 75% high heterogeneity [42, 43]. When heterogeneity was moderate or high, random effects were used as analysis model. Moreover, when meta-analyses included patients with HF with reduced (HFrEF) and preserved (HFpEF) ejection fraction or meta-analyses revealed high heterogeneity, as long as the outcome was reported by three or more studies, sensitivity analyses were conducted including studies dealing only with patients with HFrEF because the inclusion of patients with different ejection fraction could be a source of heterogeneity or could bias the results. The mean effect sizes, 95% CI, and I2 were calculated for each outcome and used to create forest plots for visualization of each meta-analysis using the Review Manager (RevMan) version 5.3 [44].
Results
Characteristics of included studies
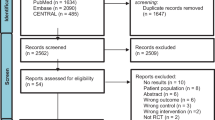
A total of 3881 citations were identified through electronic databases, with 263 additional studies identified through Grey Literature Sources and 14 studies identified through manual search. One thousand six hundred seventy-one titles and abstracts were screened and 110 original papers were assessed. The number of studies retrieved from each database and the number of studies excluded in each screening phase are shown in Fig. 1. The full reference of excluded studies in the second stage (n = 66) is reported in Additional file 3. The conflict of interest of included studies is shown in Additional file 4. Of these, 44 observational longitudinal cohort studies (prospective or retrospective) with a total of 22,598 patients with HF were included. Twenty of the included studies (45.45%) reported only patients with HFrEF. Twenty one of the included studies (47.72%) showed patients with HFrEF and HFpEF. The 6MWT was the most used test (n = 33) followed by the Gait Speed test (n = 8) and the SPPB (n = 4). The characteristics of the included observational longitudinal cohort studies are reported in Table 1.
Flow-Diagram. PRISMA 2009. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:https://doi.org/10.1371/journal.pmed1000097. For more information, visit www.prisma-statement.org
Meta-analyses
The outcomes assessed by each study, as well as the main results, the risk of bias summary and the GRADE summary are shown in Table 2. Forest plots and effect sizes of each meta-analysis can also be seen in Additional file 5.
Patients with HFrEF, HFpEF and acute HF who showed a poor physical functional performance in the 6MWT reported a larger risk of All-Cause of Mortality [HR = 2.29 95%CI (1.86–2.82), p < 0.001] than those patients who showed a good physical functional performance (Fig. 2a). Moreover, patients with HFrEF who decreased the meters (m) they walked in the 6MWT during follow-up showed larger risk of All-Cause of Mortality [HR = 1.22 95%CI (1.10–1.36), p < 0.001], although there was no lower risk of All-Cause of Mortality between patients with HFrEF, patients with HFpEF and patients with acute HF who increased the meters they walked in the 6MWT during follow-up (Additional file 5). Patients with HFrEF and HFpEF who showed a poor physical functional performance in the 6MWT also reported a larger risk of HF Mortality [HR = 2.39 95%CI (2.21–2.59), p < 0.001] than those patients who showed a good physical functional performance (Fig. 2b). Patients with HFrEF who showed a poor physical functional performance in the 6MWT also reported a larger risk of the combined endpoint of Hospitalisation and Mortality for any cause [HR = 1.80 95%CI (1.45–2.23), p < 0.001] or [OR = 2.07 95%CI (1.41–3.02), p < 0.001] than those patients who showed a good physical functional performance (Fig. 2c and Fig. 2d, respectively). Furthermore, patients with HFrEF, HFpEF and acute HF who showed a poor physical functional performance in the 6MWT reported a larger risk of HF Hospitalisation [HR = 1.68 95%CI (1.20–2.33), p = 0.002] than those patients who showed a good physical functional performance (Additional file 5). On the other hand, patients with HFrEF, HFpEF and acute HF who showed a slower gait speed reported a larger risk of All-Cause of Mortality [HR = 1.49 95%CI (1.24–1.79), p < 0.001] than those patients who showed a faster gait speed (Fig. 3), above all, when gait speed was slower than 0.65 m/s [HR = 1.59 95%CI (1.10–2.30), p = 0.01] (Additional file 5). Moreover, patients with HFrEF, HFpEF and acute HF who increased their gait speed during follow-up showed a lower risk of All-Cause of Mortality [HR = 0.85 95%CI (0.81–0.91) (Additional file 5). Patients with HFrEF and HFpEF who showed a slower gait speed (< 0.80 m/s) also reported a larger risk of All-Cause of Hospitalisation [HR = 1.32 95%CI (1.10–1.57), p = 0.002] than patients with a faster gait speed (> 0.80 m/s) (Additional file 5).
Forest Plots ilustrating the risk of All-Cause Mortality (a), the risk of HF Mortality (b) and the risk of the combined endpoint of Hospitalisation and Mortality for any cause (c and d) in the 6MWT. Patients with Poor Physical Functional Performance Versus Patients with Good Physical Functional Performance
Sensitivity analyses
The risk of All-Cause of Mortality in the 6MWT was larger when only patients with HFrEF and poor physical functional performance were assessed [HR = 2.46 95%CI (1.94–3.12), p < 0.001] (Additional file 6). However, the risk of HF Mortality [HR = 2.39 95%CI (2.21–2.58), p < 0.001] as well as the risk of All-Cause of Mortality in the 6MWT per increased units did not change when only patients with HFrEF were assessed (Additional file 6).
Descriptive quantitative analysis
Physical functional performance and mortality
A score between 1 and 4 points on the SPPB was associated with a larger risk of All-Cause of Mortality (HR = 4.78 95%CI [1.63–14.02, p < 0.05]) in patients with HFrEF and HFpEF [80], while a score below 7 points on the SPPB was not associated with a larger risk of All-Cause of Mortality in patients with acute HF [78].
Physical functional performance and the combined endpoint of hospitalisation and mortality
A score below 7 points on the SPPB was associated with a larger risk of the combined endpoint of hospitalisation and mortality for any cause (OR = 3.6 95%CI [1.0–12.9, p < 0.05]) in patients with acute HF [78]. However, per each 1-unit improved in SPPB the risk of the combined endpoint of hospitalisation and mortality for any cause could be reduced OR = 0.81 95%CI [0.69–0.94, p < 0.05] in patients with HFpEF [79]. Patients with HFrEF and HFpEF with a gait speed slower than 0.8 m/s also showed a larger risk of the combined endpoint of hospitalisation and mortality for any cause (HR = 1.31 95%CI [1.08–1.58, p < 0.05]) [84].
Physical functional performance and hospitalisation
Patients with HFrEF with poor physical performance in the 6MWT showed a larger risk of All-Cause of Hospitalisation [OR = 14.02 95%CI (4.90–40.14), p = 0.001] [48] as patients with HFrEF and HFpEF [HR = 1.41 95%CI (1.01–1.99), p < 0.05] [51]. A score below 7 points on the SPPB was also associated with a larger risk of HF Hospitalisation (OR = 6.7 95%CI [1.5–30.4, p < 0.05]) in patients with acute HF [78].
Risk of Bias assessment
The risk of bias of included observational longitudinal cohort studies is shown in Table 3. In summary, 26 studies (59.10%) reported a low risk of bias, and 17 studies (38,63%) showed a moderate risk of bias. Selection bias (97,72%) were usual across the included studies. Using GRADE criteria, observational longitudinal cohort studies reported a low evidence in most of the prognostic outcomes. However, HF mortality and all-cause mortality showed a moderate evidence in the 6-MWT (Table 4).
Discussion
Main findings and comparison with other studies
The current systematic review and meta-analysis showed that patients with HFrEF and HFpEF who reported a poor physical functional performance in 6-MWT have an increased risk of all-cause of mortality and an increased risk of HF mortality. There was consistency in the risk of all-cause of mortality and HF mortality between the studies included in each meta-analysis (Fig. 2a and Fig. 2b) and the GRADE criteria also reported a moderate level of evidence per otucome. Although patients with HFrEF who decreased the meters they walked in the 6MWT during follow-up showed an increased risk of all-cause of mortality, there was no decreased risk of all-cause of mortality between patients with HFrEF and HFpEF who increased the meters they walked in the 6MWT during follow-up [52, 53, 59, 60, 65, 67, 68, 70, 73, 75, 77]. Maybe this is beacuse the most of included studies in the meta-analysis reported a decreased risk of mortality for every 1 m increased [53, 65, 67, 68, 70, 73] or every 10 m [52, 60, 77] increased, while a systematic review determined that 45 m is the clinically meaningful change in the 6MWT [89]. Patients with HF who showed a poor physical functional performance in the 6MWT also reported an increased risk of the combined endpoint of hospitalisation and mortality for any cause (Fig. 2c and Fig. 2d), an increased risk of HF hospitalisation (Additional file 5) and an increased risk of all-cause of hospitalisation [48, 51]. However, the level of evidence of those outcomes was low according to the GRADE criteria. Moreover, there was a lack of homogeneity regarding which cut-off point should be used to stratify patients with HF based on their physical functional performance in the 6MWT. A distance traveled < 300 m was the most used distance to define patients with poor physical performance in the 6MWT in this study [47, 49, 55, 56, 58, 59, 61, 62, 64, 69, 74], while a previous review reported that a distance traveled ≤350 m in 6-MWT could be the most indicative distance of poor physical functional performance and worse prognosis in patients with HF [24].
A score between 1 and 4 points on the SPPB was associated with an increased risk of all-cause of mortality in this systematic review [80]. However, in the current study a score below 7 points on the SPPB seems to be the most indicative of a worse prognosis in patients with HF since it was associated with a larger risk of the combined endpoint of hospitalisation and mortality for any cause and a larger risk of HF hospitalisation [78]. GRADE criteria showed a very low level of evidence per outcome in each outcome examined by the SPPB. Moreover, meta-analysis on physical functional performance on the SPPB and prognosis in patients with HF could not be performed. As the present review, a score below 7 points on the SPPB was also associated with large risk of all-cause mortality in older adults [90]. However, other studies reported a large risk of mortality or hospitalisation in older adults who showed a score below 5 points [80, 91,92,93].
Patients who showed a slower gait speed also reported an increased risk of all-cause of mortality (Fig. 3), above all, when gait speed was slower than 0.65 m/s (Additional file 5). Moreover, patients with HF who showed a slower gait speed also reported an increased risk of all-cause of hospitalisation (Additional file 5) and an increased risk of the combined endpoint of hospitalisation and mortality for any cause [84], specially when gait speed was slower than 0.80 m/s [83, 84, 86]. GRADE criteria reported a low level of evidence per outcome in each prognostic outcome in Gait Speed Test. Other studies have shown the relationship between gait speed and survival, death and hospitalisation due to HF [27, 94]. In fact, Dodson et al. [95] revealed that patients who showed a gait speed slower than 0.8 m/s were more likely to experience one-year mortality or hospitalisation than patients with gait speed faster than 0.8 m/s. Alfredsson et al. [96] also reported that patients with a gait speed slower than 0.8 m/s after a transcatheter aortic valve replacement, had 35% higher 30-day mortality than patients with faster gait speed. Chainani et al. [97] reported that gait speed and handgrip strength are associated with increased risk of cardiovascular mortality.
A meta-analysis published by Yamamoto et al. [98] reported that 6MWT were significantly associated with mortality and cardiovascular disease. Frailty has also been associated with larger risk of mortality and hospitalisation in patients with chronic HF [25, 26, 30, 31, 99]. Bagnall et al. [100] revealed that frailty patients had a risk of mortality 2- to 4-fold compared with non-frail patients after acardiac surgery or transcatheter aortic valve implantation. Gait speed is a marker of frailty, although frailty could be also assessed by the 6MWT, the SPPB or the TUG [25, 26, 30, 31, 99]. In this way, the use of functional tests seem to be useful to stratify patients with HF based on their physical functional performance and to determine their prognosis.
To our knowledge, our review is the first systematic review reporting the level of evidence per each prognostic outcome using GRADE criteria. Other reviews showed the prognostic role of the 6MWT test or the impact of the physical performance on prognosis in patients with HF, but not reported the risk of bias of included studies or the level of evidence per outcome according to GRADE criteria [22, 23, 98, 101,102,103].
Implications for clinical practice
The current findings may be useful to promote functional assessments that allow stratify patients with HF according to their functional impairment. Furthermore, accurate prognostic stratification could be essential for optimizing clinical management and treatment decision making, with the aim of maintaining functionality, improving quality of life and reducing the number of hospitalisations, as well as increasing the life expectancy of patients with HF.
Adjusted medical-pharmacological treatment, in addition to improve symptoms, could prevent further cardiovascular accidents and prolong the life expectancy of patients with HF [13]. Moreover, adjusted exercise programs could reduce mortality, may improve functional capacity and quality of life, and may reduce hospitalisations [5, 8]. It has also been shown that patients with more physical activity performed weekly reported a lower risk of mortality [104,105,106]. Functional tests such as 6MWT, Gait Speed or SPPB may provide incremental prognostic value and could help to individualize the exercise prescription [107].
Future research
Future research should aim to determine the optimal cut-off points for prognostic prediction and to determine the utility of functional assessments in the management and treatment of patients with HF. The following recommendations should guide future research: 1) use the same cut off point in functional tests; 2) include a large sample size with patients with HF who show different characteristics.
Strengths and limitations of the study
The strengths of this systematic review and meta-analysis included the use of a pre-specified protocol registered on PROSPERO, the PRISMA checklist, the NOS to determine the risk of bias of each study, the GRADE criteria to assess the overall quality and the strength of the evidence per outcome, a robust search strategy complemented by a manual search, so that all studies that met the eligibility criteria could have been identified. Thus, our systematic review included 44 studies, while a previous similar review carried out by Yamamoto et al. [98] included only 22 studies.
However, there are several limitations that should be mentioned. First, the lack of uniformity among included studies, which included different cut-off points in functional tests, should be taken into account when interpreting the results. Finally, most of prognositc outcomes showed a low level of evidence per outcome according to GRADE criteria.
Conclusion
Patients with HF who report a poor physical functional performance in the 6MWT, in the SPPB or in the Gait Speed Test, show worse prognosis than patients who report a good physical functional performance in terms of an increased risk of hospitalisation or an increased risk of mortality. However, there is a lack of homogeneity regarding which cut-off point should be used to stratify patients with HF based on their physical functional performance in the different functional tests and GRADE criteria show a low level of evidence per outcome in most of examined prognostic outcome variables.
Availability of data and materials
Not Applicable.
Abbreviations
- HF:
-
Heart failure
- 6MWT:
-
Six minute walking test
- SPPB:
-
Short physical performance battery
- DALYs:
-
Disability-adjusted life-years
- TUG:
-
Timed up and go test
- PECOS:
-
Participant, exposure, comparator, outcome, study design
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses statement
- PROSPERO:
-
International prospective register of systematic reviews
- NYHA:
-
New York heart association
- OR:
-
Odds ratio
- HR:
-
Hazard ratio
- NOS:
-
The Newcastle Ottawa scale
- GRADE:
-
Grading of recommendations assessment, development and evaluation
- HFrEF:
-
Heart failure with reduced ejection fraction
- HFpEF:
-
Heart failure with preserved ejection fraction
- CI:
-
Confidence interval
- m:
-
Meters
References
Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abdulkader RS, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1260–344.
Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, Aboyans V, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1151–210.
Wang H, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970-2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1084–150.
Bui A, Horwich T, Fonarow G. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(3):30–41.
Rogers C, Bush N. Heart failure: pathophysiology, diagnosis, medical treatment guidelines, and nursing management. Nurs Clin North Am. 2015;50(4): 787–799. DOI: http://dx.doi.org/https://doi.org/10.1016/j.cnur.2015.07.012.
Tendera M. Epidemiology, treatment, and guidelines for the treatment of heart failure in Europe. Eur Heart J. 2005;7(1):5–9.
Smith AC. Effect of telemonitoring on re-admission in patients with congestive heart failure. Medsurg Nurs. 2013;22(1):39–44.
Yancy C, Jessup M, Bozkurt B, et al. ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;62(16):147.
Wheeler EC, Plowfield L. Clinical education initiative in the community: caring for patients with congestive heart failure. Nurs Educ Perspect. 2004;25(1):16–21.
Fletcher L, Thomas D. Heart failure: understanding the pathophysiology and management. J Am Acad Nurse Pract. 2001;13(6):249–57.
Siracuse J, Chaikof E. The pathogenesis of diabetic atherosclerosis. Diab Peripher Vasc Dis Diagn Manage. 2012;158(5):13–26.
Kaminsky LA, Tuttle MS. Functional assessment of heart failure patients. Heart Fail Clin. 2015;11(1):29–36. doi: http://dx.doi.org/https://doi.org/10.1016/j.hfc.2014.08.002.
Ponikowski P, Voors AA, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task forcé for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200.
Gutekunst DJ. Isokinetic torque timing parameters and ceramides as markers of muscle dysfunction in systolic heart failure. J Card Fail. 2016;22(5): 356–357. doi: http://dx.doi.org/https://doi.org/10.1016/j.cardfail.2016.03.018.
Watson RD, Gibbs CR, Lip GY. ABC of heart failure. Clinical features and complications. BMJ. 2000;320(7229):236–9.
Barker J, Byrne KS, Doherty A, Foster C, Rahimi K, Ramakrishnan R, et al. Physical activity of UK adults with chronic disease: cross-sectional analysis of accelerometer-measured physical activity in 96 706 UK biobank participants. Int J Epidemiol. 2019;48(4):1167–74.
Kinugawa S, Takada S, Matsushima S, Okita K, Tsutsui H. Skeletal muscle abnormalities in heart failure. Int Heart J. 2015;56(5):475–84.
Bona RL, Bonezi A, da Silva PF, Biancardi CM, de Souza Castro FA, Clausel NO. Effect of walking speed in heart failure patients and heart transplant patients. Clin Biomech. 2017;42: 85–91. doi: http://dx.doi.org/https://doi.org/10.1016/j.clinbiomech.2017.01.008.
Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds L, Wilson J. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83(3):778–86.
Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801.
Hülsmann M, Quittan M, Berger R, Crevenna R, Springer C, Nuhr M, et al. Muscle strength as a predictor of long-term survival in severe congestive heart failure. Eur J Heart Fail. 2004;6(1):101–7.
Rostagno C, Gensini GF. Six minute walk test: a simple and useful test to evaluate functional capacity in patients with heart failure. Intern Emerg Med. 2008;3(3):205–12.
Du H, Wonggom P, Tongpeth J, Clark RA. Six-minute walk test for assessing physical functional capacity in chronic heart failure. Curr Heart Fail Rep. 2017;14(3):158–66.
Rasekaba T, Lee AL, Naughton MT, Williams TJ, Holland AE. The six-minute walk test: A useful metric for the cardiopulmonary patient. Intern Med J. 2009;39:495–501.
Díez-Villanueva P, Arizá-Solé A, Vidán MT, Bonanad C, Formiga F, Sanchis J, et al. Recomendaciones de la Sección de Cardiología Geriátrica de la Sociedad Española de Cardiología Para la valoración de la fragilidad en el anciano con cardiopatía. Rev Esp Cardiol. 2019;72(1):63–71.
Yang X, Lupón J, Vidán MT, Ferguson C, Gastelurrutia P, Newton PJ, et al. Impact of frailty on mortality and hospitalisation in chronic heart failure: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7(23):e008251.
Studenski S, Perera S, Kushang P, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–8.
Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an international academy on nutrition and aging (IANA) task force. J Nutr Health Aging. 2009;13:881–9.
Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–62.
Singh M, Stewart R, White H. Importance of frailty in patients with cardiovascular disease. Eur Heart J. 2014;35:1726–31.
Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–66.
Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL. Pugsley S0, Taylor DW, et al. the 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919–23.
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):85–94.
Podsiadlo D, Richardson S. The timed “up and go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8.
Chan ME, Arvey RD. Meta-analysis and the development of knowledge. Perspect Psychol Sci. 2012;7:79–92. https://doi.org/10.1177/1745691611429355.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Haddaway NR, Collins AM, Coughlin D, et al. The role of google scholar in evidence reviews and its applicability to grey literature searching. PLoS One. 2015;10:e0138237–17. https://doi.org/10.1371/journal.pone.0138237.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. 2008 [Accessed September 24, 2019]. Available from URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Zeng X, Zhang Y, Kwong JSW, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2–10. https://doi.org/10.1111/jebm.12141.
Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. https://doi.org/10.1136/bmj.328.7454.1490.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE working group: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. https://doi.org/10.1136/bmj.39489.470347.AD.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Brenyo A, Goldenberg I, Moss AJ, Rao M, McNitt S, Huang DT, et al. Baseline functional capacity and the benefit of cardiac resynchronization therapy in patients with mildly symptomatic heart failure enrolled in MADIT-CRT. Hear Rhythm 2012;9(9):1454–1459. Available from: http://dx.doi.org/https://doi.org/10.1016/j.hrthm.2012.04.018.
Ferreira JP, Metra M, Anker SD, Dickstein K, Lang CC, Ng L, et al. Clinical correlates and outcome associated with changes in 6-minute walking distance in patients with heart failure: findings from the BIOSTAT-CHF study. Eur J Heart Fail. 2019;21(2):218–26.
Wegrzynowska-Teodorczyk K, Rudzinska E, Lazorczyk M, Nowakowska K, Banasiak W, Ponikowski P, et al. Distance covered during a six-minute walk test predicts long-term cardiovascular mortality and hospitalisation rates in men with systolic heart failure: an observational study. J Physiother. 2013;59(3):177–87.
Bittner V, Weiner DH, Yusuf S, Rogers WJ, Mcintyre KM, Bangdiwala SI, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. JAMA. 1993;270(14):1702–7.
Arslan S, Erol MK, Gundogdu F, Sevimli S, Aksakal E, Senocak H, et al. Prognostic value of 6-minute walk test in stable outpatients with heart failure. Tex Heart Inst J. 2007;34(2):166–9.
Lee R, Chan YH, Wong J, Lau D, Ng K. The 6-minute walk test predicts clinical outcome in Asian patients with chronic congestive heart failure on contemporary medical therapy: a study of the multiracial population in Singapore. Int J Cardiol. 2007 Jul 10;119(2):168–75.
Curtis JP, Rathore SS, Wang Y, Krumholz HM. The association of 6-minute walk performance and outcomes in stable outpatients with heart failure. J Card Fail. 2004;10(1):9–14.
Ingle L, Cleland JG, Clark AL. The long-term prognostic significance of 6-minute walk test distance in patients with chronic heart failure. Biomed Res Int. 2014;2014:505969.
Alahdab MT, Mansour IN, Napan S, Stamos TD. Six minute walk test predicts long-term all-cause mortality and heart failure Rehospitalisation in African-American patients hospitalized with acute decompensated heart failure. J Card Fail 2009;15(2): 130–135. Available from: http://dx.doi.org/https://doi.org/10.1016/j.cardfail.2008.10.006.
Mangla A, Kane J, Beaty E, Richardson D, Powell LH, Calvin JE. Comparison of predictors of heart failure-related hospitalisation or death in patients with versus without preserved left ventricular ejection fraction. Am J Cardiol. 2013;112(12):1907–12.
Hasin T, Topilsky Y, Kremers WK, Boilson BA, Schirger JA, Edwards BS, et al. Usefulness of the six-minute walk test after continuous axial flow left ventricular device implantation to predict survival. Am J Cardiol 2012;110(9): 1322–1328. Available from: http://dx.doi.org/https://doi.org/10.1016/j.amjcard.2012.06.036.
Passantino A, Lagioia R, Mastropasqua F, Scrutinio D. Short-term change in distance walked in 6 min is an Indicator of outcome in patients with chronic heart failure in clinical practice. J Am Coll Cardiol. 2006;48(1):99–105.
Howie-Esquivel J, Dracup K. Does oxygen saturation or distance walked predict rehospitalisation in heart failure? J Cardiovasc Nurs. 2008 Jul;23(4):349–56.
Zotter-Tufaro C, Mascherbauer J, Duca F, Koell B, Aschauer S, Kammerlander AA, et al. Prognostic significance and Determinantsof the 6-min walk test inPatients WithHeart failure and preserved EjectionFraction. JACC Hear Fail. 2015;3(6):459–66.
Boxer R, Kleppinger A, Ahmad A, Annis K, Hager D, Kenny A. The 6-minute walk is associated with frailty and predicts mortality in older adults with heart failure. Congest Hear Fail. 2010;16(5):208–13.
Ingle L, Cleland JG, Clark AL. The relation between repeated 6-minute walk test performance and outcome in patients with chronic heart failure. Ann Phys Rehabil Med. 2014;57(4):244–53.
Guazzi M, Dickstein K, Vicenzi M, Arena R. Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: a comparative analysis on clinical and prognostic insights. Circ Hear Fail. 2009;2(6):549–55.
McCabe N, Butler J, Dunbar SB, Higgins M, Reilly C. Six-minute walk distance predicts 30-day readmission after acute heart failure hospitalisation. Heart Lung. 2017;46(4):287–92.
Vegh EM, Kandala J, Orencole M, Upadhyay GA, Sharma A, Miller A, et al. Device-measured physical activity versus six-minute walk test as a predictor of reverse remodeling and outcome after cardiac resynchronization therapy for heart failure. Am J Cardiol. 2014;113(9):1523–8.
Roul G, Germain P, Bareiss P. Does the 6-minute walk test predict the prognosis in patients with NYHA class II or III chronic heart failure? Am Heart J. 1998;136(3):449–57.
Frankenstein L, Remppis A, Graham J, Schellberg D, Sigg C, Nelles M, et al. Gender and age related predictive value of walk test in heart failure: Do anthropometrics matter in clinical practice? Int J Cardiol. 2008;127(3):331–6.
Mene-Afejuku TO, Balogun MO, Akintomide AO, Adebayo RA. Prognostic indices among hypertensive heart failure patients in Nigeria: the roles of 24-hour holter electrocardiography and 6-minute walk test. Vasc Health Risk Manag. 2017;13:71–9.
Ingle L, Rigby AS, Carroll S, Butterly R, King RF, Cooke CB, et al. Prognostic value of the 6 min walk test and self-perceived symptom severity in older patients with chronic heart failure. Eur Heart J. 2007;28:560–8.
Rostagno C, Olivo G, Comeglio M, Boddi V, Banchelli M, Galanti G, et al. Prognostic value of 6-minute walk corridor test in patients with mild to moderate heart failure: comparison with other methods of functional evaluation. Eur J Heart Fail. 2003;5(3):247–52.
Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110(2):325–32.
Frankenstein L, Zugck C, Nelles M, Schellberg D, Katus H, Remppis A. Sex-specific predictive power of 6-minute walk test in chronic heart failure is not enhanced using percent achieved of published reference equations. J Hear Lung Transplant. 2008;27(4):427–34.
Rubim VSM, Neto CD, Martins-Romeo JL, Montera MW. Valor prognóstico do teste de caminhada de seis minutos na insuficiência cardíaca. Arq Bras Cardiol. 2006;86(2):120–5.
Kanagala P, Arnold JR, Cheng ASH, Singh A, Khan JN, Gulsin GS, et al. Left atrial ejection fraction and outcomes in heart failure with preserved ejection fraction. Int J Cardiovasc Imaging. 2020 Jan 1;36(1):101–10.
Zugck C, Krüger C, Kell R, Körber S, Schellberg D, Kübler W, et al. Risk stratification in middle-aged patients with congestive heart failure: prospective comparison of the heart failure survival score (HFSS) and a simplified two-variable model. Eur J Heart Fail. 2001;3(5):577–85.
Cahalin LP, Arena R, Labate V, Bandera F, Lavie CJ, Guazzi M. Heart rate recovery after the 6 min walk test rather than distance ambulated is a powerful prognostic Indicator in heart failure with reduced and preserved ejection fraction: a comparison with cardiopulmonary exercise testing. Eur J Heart Fail. 2013;15:519–27.
Reibis RK, Treszl A, Wegscheider K, Ehrlich B, Dissmann R, Völler H. Exercise capacity is the most powerful predictor of 2-year mortality in patients with left ventricular systolic dysfunction. Herz. 2010 Mar;35(2):104–10.
Castel MA, Méndez F, Tamborero D, Mont L, Magnani S, Tolosana JM, et al. Six-minute walking test predicts long-term cardiac death in patients who received cardiac resynchronization therapy. Europace. 2009;11:338–42.
Kamiya K, Hamazaki N, Matsue Y, Mezzani A, Corrà U, Matsuzawa R, et al. Gait speed has comparable prognostic capability to six-minute walk distance in older patients with cardiovascular disease. Eur J Prev Cardiol. 2018;25(2):212–9.
García GL, Sánchez SM, García-Briñón MÁ, Fernández-Alonso C, González del Castillo J, Martín-Sánchez FJ. El efecto de la fragilidad física en el pronóstico a largo plazo en los pacientes mayores con insuficiencia cardiaca aguda dados de Alta desde un servicio de urgencias. Emergencias. 2019;31(6):413–6.
Hornsby WE, Sareini M, Golbus JR, Willer J, Mcnamara JL, Konerman MC, et al. Lower extremity function is independently associated with hospitalisation burden in heart failure with preserved ejection fraction. J Card Fail. 2019;25(1):2–9.
Chiarantini D, Volpato S, Sioulis F, Bartalucci F, Del Bianco L, Mangani I, et al. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Card Fail. 2010;16(5):390–5.
Zaharias E, Cataldo J, Mackin L, Howie-Esquivel J. Simple measures of function and symptoms in hospitalized heart failure patients predict short-term cardiac event-free survival. Nurs Res Pract. 2014;815984..
Lo AX, Donnelly JP, McGwin G, Bittner V, Ahmed A, Brown CJ. Impact of gait speed and instrumental activities of daily living on all-cause mortality in adults ≥65 years with heart failure. Am J Cardiol. 2015;115(6):797–801.
Pulignano G, Del Sindaco D, Di Lenarda A, Alunni G, Senni M, Tarantini L, et al. Incremental value of gait speed in predicting prognosis of older adults with heart failure: insights from the IMAGE-HF study. JACC Hear Fail. 2016;4(4):289–98.
Chaudhry SI, McAvay G, Chen S, Whitson H, Newman AB, Krumholz HM, et al. Risk factors for hospital admission among older persons with newly diagnosed heart failure: findings from the cardiovascular health study. J Am Coll Cardiol. 2013;61(6):635–42.
Tanaka S, Kamiya K, Hamazaki N, Matsuzawa R, Nozaki K, Maekawa E, et al. Incremental value of objective frailty assessment to predict mortality in elderly patients hospitalized for heart failure. J Card Fail. 2018;24(11):723–32.
Tanaka S, Kamiya K, Hamazaki N, Matsuzawa R, Nozaki K, Nakamura T, et al. Short-term change in gait speed and clinical outcomes in older patients with acute heart failure. Circ J. 2019;83(9):1860–7.
Rodríguez-Pascual C, Paredes-Galán E, Ferrero-Martínez AI, González-Guerrero JL, Hornillos-Calvo M, Menendez-Colino R, et al. The frailty syndrome is associated with adverse health outcomes in very old patients with stable heart failure: a prospective study in six Spanish hospitals. Int J Cardiol. 2017;236:296–303.
Vidán MT, Blaya-Novakova V, Sánchez E, Ortiz J, Serra-Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail. 2016;18(7):869–75.
Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG. Clinically meaningful change estimates for the six-minute walk test and daily activity in individuals with chronic heart failure. Cardiopulm Phys Ther J. 2013;24(3):21–9.
Fox KR, Ku P-W, Hillsdon M, Davis MG, Simmonds BAJ, Thompson JL, et al. Objectively assessed physical activity and lower limb function and prospective associations with mortality and newly diagnosed disease in UK older adults: an OPAL four-year follow-up study. Age Ageing. 2015;44(2):261–8.
Corsonello A, Lattanzio F, Pedone C, Garasto S, Laino I, Bustacchini S, et al. Prognostic significance of the short physical performance battery in older patients discharged from acute care hospitals. Rejuvenation Res. 2012;15(1):41–8.
Legrand D, Vaes B, Matheï C, Adriaensen W, Van Pottelbergh G, Degryse J-M. Muscle strength and physical performance as predictors of mortality, hospitalisation, and disability in the oldest old. J Am Geriatr Soc. 2014;62(6):1030–8.
Volpato S, Cavalieri M, Sioulis F, Guerra G, Maraldi C, Zuliani G, et al. Predictive value of the short physical performance battery following hospitalisation in older patients. J Gerontol A Biol Sci Med Sci. 2011;66(1):89–96.
Reeves GR, Forman DE. Gait speed: stepping towards improved assessment of heart failure patients. JACC Hear Fail. 2016;4(4): 299–300. doi: http://dx.doi.org/https://doi.org/10.1016/j.jchf.2016.02.002.
Dodson JA, Arnold SV, Gosch KL, Gill TM, Spertus J, Krumholz HM, et al. Slow gait speed and risk of mortality or hospital readmission following myocardial infarction in the TRIUMPH registry. J Am Geriatr Soc. 2016;64(3):596–601.
Alfredsson J, Stebbins A, Brennan JM, Matsouaka R, Afilalo J, Peterson ED, et al. Gait speed predicts 30-day mortality after Transcatheter aortic valve replacement: results from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation. 2016;133(14):1351–9.
Chainani V, Shaharyar S, Dave K, Choksi V, Ravindranathan S, Hanno R, et al. Objective measures of the frailty syndrome (hand grip strength and gait speed) and cardiovascular mortality: a systematic review. Int J Cardiol. 2016;215:487–93.
Yamamoto S, Yamaga T, Nishie K, Sakai Y, Ishida T, Oka K, et al. Impact of physical performance on prognosis among patients with heart failure: systematic review and meta-analysis. J Cardiol. 2020;76(2):139–46.
Boxer RS, Wang Z, Walsh SJ, Hager D, Kenny AM. The utility of the 6-minute walk test as a measure of frailty in older adults with heart failure. Am J Geriatr Cardiol. 2008;17(1):7–12.
Bagnall NM, Faiz O, Darzi A, Athanasiou T. What is the utility of preoperative frailty assessment for risk stratification in cardiac surgery? Interact Cardiovasc Thorac Surg. 2013;17(2):398–402.
Giannitsi S, Bougiakli M, Bechlioulis A, Kotsia A, Michalis LK, Naka KK. 6-minute walking test: a useful tool in the management of heart failure patients. Ther Adv Cardiovasc Dis. 2019;13:1–10.
Zielinska D, Bellwon J, Rynkiewicz A, Elkady MA. Prognostic value of the six-minute walk test in heart failure patients undergoing cardiac surgery: a literature review. Rehabil Res Pract. 2013;2013:965494.
Pollentier B, Irons SL, Benedetto CM, Dibenedetto A-M, Loton D, Seyler RD, et al. Examination of the six minute walk test to determine functional capacity in people with chronic heart failure: a systematic review. Cardiopulm Phys Ther J. 2010;21(1):13–21.
Jeong SW, Kim SH, Kang SH, Kim HJ, Yoon CH, Youn TJ, et al. Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur Heart J. 2019;40(43):3547–55.
Kraus WE, Powell KE, Haskell WL, Janz KF, Campbell WW, Jakicic JM, et al. Physical activity, all-cause and cardiovascular mortality, and cardiovascular disease. Med Sci Sports Exerc. 2019;51(6):1270–81.
Blond K, Brinkløv CF, Ried-Larsen M, Crippa A, Grøntved A. Association of high amounts of physical activity with mortality risk: a systematic review and meta-analysis. Br J Sports Med. 2019:1–8.
Afilalo J. Evaluating and treating frailty in cardiac rehabilitation. Clin Geriatr Med. 2019;35(4):445–57.
Acknowledgments
Not Applicable.
Funding
Brendon Stubbs is supported by a Clinical Lectureship (ICA-CL-2017-03-001) jointly funded by Health Education England (HEE) and the National Institute for Health Research (NIHR). Brendon Stubbs is part funded by the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust. Brendon Stubbs is also supported by the Maudsley Charity, King’s College London and the NIHR South London Collaboration for Leadership in Applied Health Research and Care (CLAHRC) funding. This paper presents independent research. The views expressed in this publication are those of the authors and not necessarily those of the acknowledged institutions. María Rosa Bernal-López was supported by “Miguel Servet Type I” program (CP15/00028) from the ISCIII-Madrid (Spain), cofinanced by the Fondo Europeo de Desarrollo Regional-FEDER.
Author information
Authors and Affiliations
Contributions
IJF-A and AC-V contributed to the conception of this study. IJF-A and AC-V were involved in the selection and analysis of the included studies. IJF-A, AC-V, B-S, LMP-B, MRB-L and RG-H were involved in the writing and in the review of the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fuentes-Abolafio, I.J., Stubbs, B., Pérez-Belmonte, L.M. et al. Physical functional performance and prognosis in patients with heart failure: a systematic review and meta-analysis. BMC Cardiovasc Disord 20, 512 (2020). https://doi.org/10.1186/s12872-020-01725-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-020-01725-5