Abstract
Background
Myocardial flow reserve (MFR, stress/rest myocardial blood flow) is a strong marker of myocardial vasomotor function. MFR is a predictor of adverse cardiac events in patients with non-ischemic systolic heart failure and previous studies using different methods have found association between myocardial blood flow and left ventricular dilatation. The aim of this study was to investigate whether there is an association between increasing end-systolic- and end-diastolic volumes (ESV and EDV) and MFR in these patients measured with Rubidium-82 positron emission tomography computed tomography (82Rb-PET/CT) as a quantitative myocardial perfusion gold-standard.
Methods
We scanned 151 patients with non-ischemic heart failure with initial left ventricular ejection fraction ≤35% with 82Rb-PET/CT at rest and adenosine-induced stress to obtain MFR and volumes. To account for differences in body surface area (BSA), we used indexed ESV (ESVI): ESV/BSA (ml/m2) and EDV (EDVI). We identified factors associated with MFR using multiple regression analyses.
Results
Median age was 62 years (55–69 years) and 31% were women. Mean MFR was 2.38 (2.24–2.52). MFR decreased significantly with both increasing ESVI (estimate − 3.7%/10 ml/m2; 95% confidence interval [CI] -5.6 to − 1.8; P < 0.001) and increasing EDVI (estimate − 3.5%/10 ml/m2; 95% CI -5.3 to − 1.6; P < 0.001). Results remained significant after multivariable adjustment. Additionally, coronary vascular resistance during stress increased significantly with increasing ESVI (estimate: 3.1 mmHg/(ml/g/min) per (10 ml/m2); 95% CI 2.0 to 4.3; r = 0.41; P < 0.0001) and increasing EDVI (estimate: 2.7 mmHg/(ml/g/min) per (10 ml/m2); 95% CI 1.6 to 3.8; r = 0.37; P < 0.0001).
Conclusions
Impaired MFR assessed by 82Rb-PET/CT was significantly associated with linear increases in ESVI and EDVI in patients with non-ischemic systolic heart failure. Our findings support that impaired microvascular function may play a role in heart failure development. Clinical trials investigating MFR with regard to treatment responses may elucidate the clinical use of MFR in patients with non-ischemic systolic heart failure.
Trial registration
Sub study of the randomized clinical trial: A DANish randomized, controlled, multicenter study to assess the efficacy of Implantable cardioverter defibrillator in patients with non-ischemic Systolic Heart failure on mortality (DANISH), ClinicalTrials.gov Identifier: NCT00541268.
Similar content being viewed by others
Background
Myocardial flow reserve (MFR, stress/rest myocardial blood flow) is a strong marker of myocardial vasomotor function and quantification of MFR may identify microvascular dysfunction [1,2,3]. MFR is defined as the maximal myocardial blood flow (MBF) during pharmacologic stress divided by resting MBF. In patients with non-ischemic systolic heart failure, MFR is often impaired despite the absence of coronary artery disease [4,5,6]. Different explanations have been suggested, such as endothelial dysfunction and impaired angiogenesis, which may lead to small vessel disease with impaired perfusion and myocardial ischemia [7]. However, the mechanism behind the impaired perfusion remains unclear.
A detailed evaluation of the underlying pathophysiology of heart failure is important for development and application of new therapies [8]. For detection of microvascular disease without structural epicardial coronary artery disease, 82Rb-PET/CT with adenosine stress is a robust and validated method [9, 10]. Outcome studies have shown that MFR is a predictor of adverse cardiac events in patients with non-ischemic systolic heart failure [4, 5]. Moreover, it is well known that severity in left ventricular dysfunction with increased end-systolic volume (ESV) and end-diastolic volume (EDV) at the time of referral is a prognostic indicator of mortality [11].
Previous studies using echocardiography with coronary Doppler catheter or single photon emission computed tomography (SPECT) in patients with non-ischemic cardiomyopathy have shown that myocardial perfusion is negatively correlated with ESV and EDV [12,13,14]. PET is considered the gold standard of myocardial perfusion measurement. To our knowledge the association between myocardial perfusion, measured quantitatively as MFR, and ESV and EDV using 82Rb-PET/CT has not previously been investigated in patients with non-ischemic systolic heart failure of varying etiology. Therefore, the aim of our study was to investigate whether MFR was associated with increasing ESV and EDV in patients with non-ischemic systolic heart failure assessed with 82Rb-PET/CT.
Methods
Study population
In this sub study to DANISH (A DANish randomized, controlled, multicenter study to assess the efficacy of Implantable cardioverter defibrillator in patients with non-ischemic Systolic Heart failure on mortality) [15], patients were included late in the follow up period of the main trial. Inclusion criteria for DANISH were documented non-ischemic systolic heart failure (LV ejection fraction ≤35%), and increased levels (> 200 pg/mL) of N-terminal pro-brain natriuretic peptide (NT-proBNP) regardless of optimal medical treatment. Coronary artery catheterization was performed in 97.4% of patients in this sub study to exclude ischemia as the cause of heart failure and CT angiograms was performed in the remaining patients. If an extent of coronary artery disease was not considered to be sufficient to account for the reduced left ventricular systolic function, patients with one or two coronary arteries with stenoses could be included [15]. We excluded patients with severe chronic obstructive pulmonary disease (COPD)/asthma, blood pressure > 200/110 mmHg or systolic blood pressure < 90 mmHg, allergy or intolerance to theophylline or adenosine, pregnancy and inability to adhere to the protocol. Between May 2015 and September 2016, we scanned 151 patients with non-ischemic systolic heart failure with 82Rb-PET/CT. Figure 1 shows the inclusion flowchart. All patients had given informed oral and written consents, and the Scientific Ethics Committee of the Capital Region of Denmark and the Danish Data Protection Agency approved the protocol. (protocol number H-15000346). Our study was performed in accordance with the principles of the Declaration of Helsinki.
Inclusion flow chart. DANISH [15] Centre 1 and 2: Rigshospitalet and Gentofte Hospital, Copenhagen, Denmark. COPD chronic obstructive pulmonary disease
PET imaging
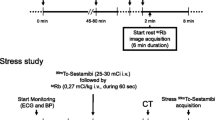
All patients underwent rest and adenosine stress PET myocardial perfusion imaging (MPI) during one scan session. Patients were instructed to abstain from caffeine and theophylline for 12 h before acquisition of the scan. Medications prescribed for heart failure were continued by all patients. Patients were scanned with a Siemens Biograph mCT/PET 128-slice scanner (Siemens Healthcare, Knoxville, TN, USA), and rest and stress images were obtained ECG-gated in list mode for 7 min from the start point of 82Rb infusion. For rest and stress imaging, patients received 1062 MBq (IQR: 1018-1273) and 1058 MBq (IQR: 1014-1269) 82Rb, supplied from a CardioGen-82Sr/82Rb generator manufactured for Bracco Diagnostics Inc., Princeton, NJ. For the stress scan we used adenosine infusion (0.14 mg/kg/min) as stressor for 6 min and we initiated the stress 82Rb infusion 2.5 min after starting the adenosine administration. Also, we performed an attenuation correcting low-dose CT before the rest study, and if necessary repeated it after the stress study. As per clinical routine, coronary artery calcium score (CACS) images from a non-contrast breath-hold CT was acquired in all patients. We calculated the CACS using the Agatston score system [16].
Quantitative and semi quantitative analyses
For quantification of MBF of dynamic rest and stress images, we used the method previous described in details by Armstrong et al. [17] Based on a single-compartment model for 82Rb tracer kinetics suggested by Lortie et al. [10], we conducted the MBF quantification with QGS/QPS v. 2015.5 (Cedars-Sinai Cardiac Suite, Los Angeles, USA). MFR was defined as MBF during adenosine induced maximal hyperemia divided by resting MBF. In order to correct the resting MBF for baseline work, we calculated the rate pressure product (RPP), defined as the systolic blood pressure times the heart rate, and divided MBF with RPP and multiplied by 10,000 [18]. Moreover, we divided MFR into normal (> 2.5), borderline (> 2.0–2.5), and low (≤2.0) [3]. Definitions of resting and stress MBF normal values were 0.82 ml/g/min ±30% and 3.3 ml/g/min ±31%, respectively [19]. Additionally, the coronary vascular resistance (CVR) was calculated as mean arterial pressure (MAP) divided by MBF at rest and during stress. Segmental perfusion scores based on a 17-segment, multi-point scale with corresponding summed scores were automatically calculated as summed rest score (SRS), summed stress score (SSS) and summed difference score (SDS=SSS-SRS) with the QPET software from Cedars-Sinai, using a 17 myocardial segment model [20]. Definition of SSS was: 0–3: normal (< 5% myocardium with perfusion abnormalities), 4–7: mildly abnormal (5–10% myocardium with perfusion abnormalities), > 8 moderately or severely abnormal (> 10% myocardium with perfusion abnormalities) [21]. Left ventricular ejection fraction (LVEF), systolic and diastolic volume measures were also computed by the software and manual corrections were made if the automatic data processing algorithm was unable to generate an accurate LV contour. Normal values for ESV and EDV at rest has been defined in previous studies by Bravo et al. (mean rest ESV ± SD: 42 ± 17 ml and mean rest EDV ± SD: 82 ± 26 ml (15% men, mean age ± SD 49 ± 9 years)) [22] and Chander et al. (mean rest ESV ± SD: 47 ± 31 ml and mean rest EDV ± SD: 81 ± 34 ml (54% men, mean age ± SD 54 ± 12 years)) [23]. To account for differences in body surface area (BSA), we used indexed ESV (ESVI): ESV/BSA (ml/m2) and EDV (EDVI): EDV/BSA (ml/m2).
Statistical analyses
Continuous variables were expressed as medians and interquartile ranges. For analyses of differences between groups we used chi-square test for categorical variables. For continuous variables, we used unpaired t-test or Wilcoxon two-sample test. When appropriate, to approximate a normal distribution, variables were log-transformed. To be able to compare our results with previously described normal values, we also calculated the mean ESV and EDV in addition to median values even though not perfectly normal distributed. Linear relations were tested for more complex models of fit using the Akaike information criterion. Multiple regression analysis of explanatory variables (sex, age, hypertension, diabetes, NT-pro-BNP, LV bundle branch block, LV ejection fraction, atrial fibrillation during scan, increases in heart rate from rest to stress and coronary calcium score) was performed with the general linear model (GLM) procedure. P-values < 0.05 were considered statistically significant. Statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA). GraphPad Prism 7.02® (GraphPad Software Inc., USA) was used for graphic presentation of results.
Results
Comparing the 151 patients in our study with the study population of the main study without COPD, we only found few differences (Table 1). Patients in our sub study had lower NT-proBNP (851 vs. 1220 pg/ml; P < 0.01) and slightly higher estimated glomerular filtration rate (78 vs. 73 ml/min/1.73m2, P = 0.03). Further, fewer of our patients had diabetes mellitus type 1 or 2 (13% vs. 20%; P = 0.04) and fewer received cardiac resynchronization therapy (50 vs. 61%; P = 0.01). Except for these variables, patients in the sub study and the remaining patients in the main study were similar. No significant difference was found between etiology in the two study populations, where the majority of patients had idiopathic cardiomyopathy; 78% in our sub study and 75% in the main study (Table 1). Measures of median ESV and EDV are shown in Table 2. Mean values for ESV and EDV were: ESV mean ± SD: 90 ± 65 and EDV mean ± SD: 148 ± 66. Median ESVI at rest was 35 ml/m2 (IQR 24 to 57) and median EDVI was 65 ml/m2 (IQR 52 to 86).
Global myocardial blood flow and myocardial flow reserve (MFR)
Perfusion results are found in Table 3. There was a significant linear decrease in MFR both with increasing ESVI (estimate − 3.7% /10 ml/m2; 95% confidence interval [CI] -5.6 to − 1.8; r = 0.30; P < 0.001), (Fig. 2a) and with increasing EDVI (estimate − 3.5% /10 ml/m2; 95% CI -5.3 to − 1.6; r = 0.29; P < 0.001), (Fig. 2b).
More complex models of relation did not improve the fit. In multivariable analyses, this relationship remained significant for both variables; ESVI: estimate − 4.5% /10 ml/m2; 95% CI -7.9 to − 0.9; P = 0.02 and EDVI: estimate − 3.8% /10 ml/m2; 95% CI -6.8 to − 0.7; P = 0.02. Table 4 and Additional file 1: Table S1 show the complete results of the multivariable analyses for ESVI and EDVI respectively. In the univariable analyses, we also found a significant association between MFR and LVEF: estimate 6.1%/10%; 95% CI 1.9 to 10.5%/10%; P < 0.01. However, this association became non-significant in multivariable analyses both when including ESVI or EDVI and when not including volumes in the statistical model (All> 0.10 for the association between MFR and LVEF).
Median global rest MBF was 0.94 ml/g/min (interquartile range [IQR] 0.76 to 1.09) and median global stress MBF was 2.18 ml/g/min (IQR 1.69–2.76). Table 5 shows associations between rest and stress MBF and ESVI and EDVI respectively. Global stress MBF decreased significantly with increasing ESVI in both the univariable (estimate − 0.10 (ml/g/min) per (10 ml/m2); P < 0.0001) and the multivariable (estimate − 0.07 (ml/g/min) per (10 ml/m2); P = 0.048) analyses. Results at rest showed significant decrease in global rest MBF with increasing ESVI in the univariable analyses both uncorrected (estimate − 0.02 (ml/g/min) per (10 ml/m2); P = 0.01) and corrected for cardiac work (RPP) (estimate − 0.02 (ml/g/min) per (10 ml/m2); P = 0.03). No significant associations were found in the corresponding multivariable adjustment analyses. In a univariable analysis, global stress MBF also decreased with increasing EDVI (estimate − 0.10 (ml/g/min) per (10 ml/m2); P < 0.0001), but with multivariable adjustment the decrease was only borderline significant (estimate − 0.06 (ml/g/min) per (10 ml/m2); P = 0.052). We also found a significant association between global rest MBF and EDVI in the univariable analysis (estimate − 0.02 (ml/g/min) per (10 ml/m2); P = 0.03), which was not significant following multivariable adjustment (estimate 0.00 (ml/g/min) per (10 ml/m2); P = 0.99) Correcting for RPP at rest, the association between global rest MBF and EDVI were non-significant in both the univariable and multivariable analyses.
Coronary vascular resistance (CVR)
Coronary vascular resistance values at rest and during stress are also shown in Table 3. In univariable analyses, we found a significant correlation between CVR at rest and ESVI (estimate: 1.6 mmHg/(ml/g/min) per (10 ml/m2); 95% CI 0.3 to 2.9; r = 0.21; P = 0.02), (Fig. 3a) and between CVR at rest and EDVI (estimate: 1.3 mmHg/(ml/g/min) per (10 ml/m2); 95% CI 0.2 to 2.6; r = 0.17; P = 0.047), (Fig. 3c). In multivariable analyses between resting CVR and ESVI and between CVR and EDVI respectively these associations were no longer significant (P = 0.92 and P = 0.88). The statistical correlations between CVR during stress and ESVI and EDVI, respectively were strong in univariable analyses: ESVI estimate: 3.1 mmHg/(ml/g/min) per (10 ml/m2); 95% CI 2.0 to 4.3; r = 0.41; P < 0.0001 (Fig. 3b) and EDVI estimate: 2.7 mmHg/(ml/g/min) per (10 ml/m2); 95% CI 1.6 to 3.8; r = 0.37; P < 0.0001), (Fig. 3d). The associations remained significant for both variables in the multivariable analyses: ESVI estimate: 3.1 mmHg/(ml/g/min) per (10 ml/m2); 95% CI 1.3 to 5.0; P < 0.001 and EDVI estimate: 2.2 mmHg/(ml/g/min) per (10 ml/m2); 95% CI 0.6 to 3.8; P < 0.01).
Sub analyses in patients with idiopathic cardiomyopathy
When investigating only the 118 patients with idiopathic cardiomyopathy, we found similar results for both MFR and CVR. MFR decreased with increasing ESVI (estimate − 3.5% /10 ml/m2; 95% CI -6.0 to − 1.1; r = 0.29; P < 0.01) and with increasing EDVI (estimate − 3.3% /10 ml/m2; 95% CI -5.7 to − 1.0; r = 0.28; P < 0.01). At stress, CVR was significantly associated with both ESVI (estimate: 3.2 mmHg/(ml/g/min) per (10 ml/m2); 95% CI 1.7 to 4.6; r = 0.42; P < 0.0001) and EDVI (estimate: 2.8 mmHg/(ml/g/min) per (10 ml/m2); 95% CI 1.3 to 4.2; r = 0.38; P < 0.001). The associations remained significant after multivariable adjustment. At rest, no significant associations were found between CVR and ESV and EDV, respectively.
Myocardial perfusion defects
Summed rest-, stress- and difference scores (SRS, SSS and SDS) were assessed in all patients (Table 3). We found that the median SSS was in the category of mildly abnormal (4.0; IQR 2.0 to 7.0) corresponding to 5–10% of the myocardium with perfusion abnormalities. In both univariable and multivariable analyses, SSS and SRS were significantly higher with increasing ESVI (SSS: estimate 0.9 per (10 ml/m2), 95% CI 0.7 to 1.3, P < 0.0001 and SRS: estimate 0.9 per (10 ml/m2), 95% CI 0.7 to 1.1, P < 0.001) and multivariable (SSS: estimate 0.9 per (10 ml/m2), 95% CI 0.3 to 1.4, P < 0.01 and SRS: estimate 0.8 per (10 ml/m2), 95% CI 0.3 to 1.2, P < 0.001). No significant association was found between ESVI and SDS either in a univariable or a multivariable analysis. Results were similar for EDVI. Association with SSS and SRS were significant univariable (SSS: estimate 0.9 per (10 ml/m2), 95% CI 0.6 to 1.2, P < 0.0001 and SRS: estimate 0.8 per (10 ml/m2), 95% CI 0.6 to 1.0, P < 0.001), whereas there were no significant relation between SDS and EDVI (SDS: estimate 0.1 per (10 ml/m2), 95% CI -0.04 to 0.3, P = 0.14). In multivariable analyses the association between EDVI and SSS as well as SRS remained significant (SSS: estimate 0.6 per (10 ml/m2), 95% CI 0.1 to 1.1, P = 0.02 and SRS: estimate 0.6 per (10 ml/m2), 95% CI 0.2 to 1.0, P < 0.01). Results for SSS and SRS remained significant associated with both ESVI and EDVI in the univariable analyses, when only including the 88 patients with more than 5% abnormal perfusion (SSS ≥4).
Discussion
This study of MFR assessed by 82Rb-PET/CT in patients with non-ischemic systolic heart failure showed that MFR decreased linearly with increases in ESVI and EDVI. These findings remained significant when adjusting for relevant covariates. Further, we found a positive association between stress CVR and ESVI as well as stress CVR and EDVI. Of our patients 64% or 46% had resting ESV higher than 59 ml or 78 ml and 72% or 66% had resting EDV higher than 108 ml or 115 ml. These values corresponded to one standard deviation above the mean normal values in the background literature.
Our results concur with previous findings of decreased MFR in patients with non-ischemic systolic heart failure [4,5,6, 24], and with findings of correlation between impaired myocardial perfusion and increases in ESV and EDV in patients with dilated cardiomyopathy [12,13,14]. The current study adds knowledge about the associations between MFR from 82Rb-PET/CT, as gold standard quantitative measurement of myocardial perfusion, and ESVI and EDVI in patients with varying types of non-ischemic systolic heart failure. In these patients, we found a linear association between increasing ESVI and EDVI and decreasing MFR. These findings have, to our knowledge, not previously been explored in patients with non-ischemic systolic heart failure using 82Rb-PET/CT. Previous studies have investigated patients with dilated cardiomyopathy using either echocardiography with coronary Doppler catheter to measure coronary flow reserve [12, 13] or SPECT with technetium-99 m methoxyisobutylisonitrile (99mTc-MIBI) washout rate as a surrogate measure [14] which are both inferior to PET in measuring myocardial perfusion.
The linear relationship between increasing ESVI and EDVI and the decreasing MFR may suggest an association between degree of dilatation and microvascular disease progression. Studies on dilated cardiomyopathy have not found any correlation between myocardial perfusion abnormalities and extent of myocardial fibrosis [25, 26] indicating that more complex mechanisms determine the myocardial perfusion.
Several experimental observations about myocardial perfusion have previously been made. Tsagalou et al. found that in patients with dilated cardiomyopathy, decreased MFR obtained by thermodilution was associated with decreased myocardial capillary density measured in endomyocardial biopsies. These results support that angiogenic therapies with intramyocardial delivery of different kinds of vascular endothelial growth factors could be considered for future treatment of heart failure [24]. In contrast, a review article by Linzbach et al. described eccentric cardiomyopathy as a structural dilation with a rearrangement of muscle fibers and sufficient capillary supply but a retarded growth of the coronary arteries and coronary ostia with more frequent atherosclerotic lesions [27]. Another explanation may be a myogenic response from coronary arterioles related to oxygen consumption [6, 28].
In the current study we found a significant positive association between CVR during stress and ESVI and EDVI that remained significant in a multivariable analysis. One explanation could be that the lower MFR is simply due to a higher CVR at stress in these patients, perhaps caused by higher myocardial tissue pressure in diastole [29]. Median global rest MBF was within the normal range, although in the high end, while stress MBF on the other hand was slightly below the normal range. In addition, a decrease in stress MBF was associated with increasing ESVI univariable and multivariable and increasing EDVI in a univariable analysis (although this association became borderline significant in a multivariable analysis). This suggests that the correlation between impaired MFR with increasing ESVI and EDVI may primarily be explained by decreased stress MBF and increased CVR during stress.
It is well known that impaired MFR is associated with increased CACS both in patients with intermediate risk of coronary artery disease and in asymptomatic adults [30, 31]. Also in our population we found a significant association between MFR and CACS in the multivariable analyses. However, this association did not change the associations between MFR and ESVI and EDVI, indicating LV volume indexes are independently associated with impaired perfusion in patients with non-ischemic systolic heart failure.
It remains uncertain whether impaired myocardial perfusion causes dilatation or dilatation leads to impaired perfusion. If the first scenario is true, treatment focused on the impaired perfusion may prevent further dilatation. To investigate the second scenario further, it would be interesting to measure MFR before patients initiate treatment for heart failure and when they are fully up titrated to observe if MFR improves with reversibility in dilatation.
Limitations
This study was conducted in a group of patients with non-ischemic systolic heart failure from a larger randomized clinical trial [15]. The patients in our study population may have been healthier than the total study population of the main study (lower NT-proBNP, higher eGFR and fewer with diabetes mellitus). This could possibly be caused by death or exclusion of the sickest patients before the sub study inclusion started leaving a healthier population for inclusion to 82Rb-PET/CT scan. We adjusted for various potential risk factors in the multivariable adjustment and the association between MFR and ESVI as well as between MFR and EDVI stayed significant. We chose not to include LV mass in the multivariable adjustment, because of a very strong correlation between ESVI and LV mass (r = 0.92) and EDVI and LV mass (r = 0.90), respectively. However, if LV mass was included in the model, the association between both MFR and ESVI and MFR and EDVI remained significant, while LV mass was not independently correlated with MFR. Patients could be included in our study with some extent of coronary arteriosclerosis. However, this was not the cause of their heart failure and CACS did not explain the association between MFR and ESVI and EDVI, respectively. Additionally, at the time of 82Rb-PET/CT, no patients complained of angina, none had received interventional treatment and only 2.6% of patients received calcium antagonists and 2.0% received long lasting nitrates.
Conclusion
In patients with non-ischemic systolic heart failure, impaired MFR was significantly associated with increased left ventricular ESV and EDV. This is the first study using 82Rb-PET/CT with a quantitative measure of myocardial perfusion to show a linear association between myocardial perfusion and end-systolic- and end-diastolic volumes in patients with non-ischemic systolic heart failure. Whether microvascular dysfunction plays a role in heart failure development remains uncertain. Our results imply that myocardial perfusion measured as MFR may be a useful tool to elucidate this. Clinical trials investigating changes in MFR and its relation to treatment responses may clarify the clinical use of MFR in patients with non-ischemic systolic heart failure.
Abbreviations
- 82Rb-PET/CT:
-
Rubidium-82 positron emission tomography / computed tomography
- CACS:
-
Coronary artery calcium score
- CRT:
-
Cardiac resynchronization therapy
- CVR:
-
Coronary vascular resistance
- EDVI:
-
End-diastolic volume index
- ESVI:
-
End-systolic volume index
- MBF:
-
Myocardial blood flow
- MFR:
-
Myocardial flow reserve
- MPI:
-
Myocardial perfusion imaging
- RPP:
-
Rate pressure product
- SDS:
-
Summed difference score
- SRS:
-
Summed rest score
- SSS:
-
Summed stress score
References
Saraste A, Kajander S, Han C, Nesterov SV, Knuuti J. PET: is myocardial flow quantification a clinical reality? J Nucl Cardiol. 2012;19:1044–59.
Johnson NP, Gould KL, Di Carli MF, Taqueti VR. Invasive FFR and noninvasive CFR in?The?Evaluation of ischemia. J Am Coll Cardiol. 2016;67:2772–88.
Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3:623–40.
Neglia D, Michelassi C, Trivieri MG, Sambuceti G, Giorgetti A, Pratali L, et al. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation. 2002;105:186–93.
Majmudar MD, Murthy VL, Shah RV, Kolli S, Mousavi N, Foster CR, et al. Quantification of coronary flow reserve in patients with ischaemic and non-ischaemic cardiomyopathy and its association with clinical outcomes. Eur Heart J Cardiovasc Imaging. 2015;16:900–9.
Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–40.
De Boer RA, Pinto YM, Van Veldhuisen DJ. The imbalance between oxygen demand and supply as a potential mechanism in the pathophysiology of heart failure: the role of microvascular growth and abnormalities. Microcirculation. 2003;10:113–26.
Ylä-Herttuala S, Bridges C, Katz MG, Korpisalo P. Angiogenic gene therapy in cardiovascular diseases: dream or vision? Eur Heart J. 2017;38:1365–71.
Saraste A, Knuuti J. PET imaging in heart failure: the role of new tracers. Heart Fail Rev. 2017;22:501–11.
Lortie M, Beanlands RSB, Yoshinaga K, Klein R, DaSilva JN, deKemp RA. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging. 2007;34:1765–74.
Diaz RA, Obasohan A, Oakley CM. Prediction of outcome in dilated cardiomyopathy. Br Heart J. 1987;58:393–9.
Inoue T, Sakai Y, Morooka S, Hayashi T, Takayanagi K, Yamanaka T, et al. Coronary flow reserve in patients with dilated cardiomyopathy. Am Heart J. 1993;125:93–8.
Santagata P, Rigo F, Gherardi S, Pratali L, Drozdz J, Varga A, et al. Clinical and functional determinants of coronary flow reserve in non-ischemic dilated cardiomyopathy. Int J Cardiol. 2005;105:46–52.
Shiroodi MK, Shafiei B, Baharfard N, Gheidari ME, Nazari B, Pirayesh E, et al. 99 mTc-MIBI washout as a complementary factor in the evaluation of idiopathic dilated cardiomyopathy (IDCM) using myocardial perfusion imaging. Int J Cardiovasc Imaging. 2012;28:211–7.
Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016. https://doi.org/10.1056/NEJMoa1608029.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32.
Armstrong IS, Tonge CM, Arumugam P. Impact of point spread function modeling and time-of-flight on myocardial blood flow and myocardial flow reserve measurements for rubidium-82 cardiac PET. J Nucl Cardiol. 2014;21:467–74.
Czernin J, Muller P, Chan S, Brunken RC, Porenta G, Krivokapich J, et al. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation. 1993;88:62–9.
Renaud JM, DaSilva JN, Beanlands RSB, deKemp RA. Characterizing the normal range of myocardial blood flow with 82rubidium and 13N-ammonia PET imaging. J Nucl Cardiol. 2013;20:578–91.
Cerqueira MD. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the cardiac imaging Committee of the Council on clinical cardiology of the American Heart Association. Circulation. 2002;105:539–42.
Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23:1187–226.
Bravo PE, Chien D, Javadi M, Merrill J, Bengel FM. Reference ranges for LVEF and LV volumes from electrocardiographically gated 82Rb cardiac PET/CT using commercially available software. J Nucl Med. 2010;51:898–905.
Chander A, Brenner M, Lautamaki R, Voicu C, Merrill J, Bengel FM. Comparison of measures of left ventricular function from electrocardiographically gated 82Rb PET with contrast-enhanced CT Ventriculography: a hybrid PET/CT analysis. J Nucl Med. 2008;49:1643–50.
Tsagalou EP, Anastasiou-Nana M, Agapitos E, Gika A, Drakos SG, Terrovitis JV, et al. Depressed coronary flow reserve is associated with decreased myocardial capillary density in patients with heart failure due to idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2008;52:1391–8.
Parodi O, De Maria R, Oltrona L, Testa R, Sambuceti G, Roghi A, et al. Myocardial blood flow distribution in patients with ischemic heart disease or dilated cardiomyopathy undergoing heart transplantation. Circulation. 1993;88:509–22.
Knaapen P, Götte MJW, Paulus WJ, Zwanenburg JJM, Dijkmans PA, Boellaard R, et al. Does myocardial fibrosis hinder contractile function and perfusion in idiopathic dilated cardiomyopathy? PET and MR imaging study. Radiology. 2006;240:380–8.
Linzbach AJ. Heart failure from the point of view of quantitative anatomy. Am J Cardiol. 1960;5:370–82.
Kuo L, Chilian WM, Davis MJ. Coronary arteriolar myogenic response is independent of endothelium. Circ Res. 1990;66:860–6.
Hoffman JI. Determinants and prediction of transmural myocardial perfusion. Circulation. 1978;58(3 Pt 1):381–91.
Kim J, Bravo PE, Gholamrezanezhad A, Sohn S, Rafique A, Travis A, et al. Coronary artery and thoracic aorta calcification is inversely related to coronary flow reserve as measured by 82Rb PET/CT in intermediate risk patients. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol. 2013;20:375–84.
Wang L, Jerosch-Herold M, Jacobs DR, Shahar E, Detrano R, Folsom AR. Coronary artery calcification and myocardial perfusion in asymptomatic adults. J Am Coll Cardiol. 2006;48:1018–26.
Acknowledgements
Not applicable.
Funding
Unrestricted grants from: Københavns Universitet (Copenhagen, DK), Hjerteforeningen (Copenhagen, DK), Arvid Nielssons Fond (Copenhagen, DK), Grosserer Valdemar Foersom og hustru Thyra Foersoms Fond (Copenhagen, DK), Snedkermester Sophus Jacobsen og hustru Astrid Jacobsens Fond (Copenhagen, DK), and Eva og Henry Frænkels Mindefond (Holte, DK) supported the salary of Christina Byrne. TrygFonden (Copenhagen, DK), Medtronic (US) and St. Jude Medical (US) supported the completion of the DANISH study.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
CB included the patients in the study, collected the data, performed the statistical analysis and was a major contributor in designing the study and writing the manuscript. PH interpreted the scans and was a major contributor in performing the scans as well as in designing the study and revising the manuscript. AK provided the possibility of the study setup and was a major contributor in designing the study and revising the manuscript. JJT provided statistical guidance and was a major contributor in designing the study and revising the manuscript. LK suggested the study and was a major contributor in designing the study and revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patients had given informed oral and written consents, and the Scientific Ethics Committee of the Capital Region of Denmark and the Danish Data Protection Agency approved the protocol. (protocol number H-15000346).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1. Multivariable analysis with myocardial flow reserve as the dependent variable including end-diastolic volume index, sex, age, hypertension, diabetes, NT-pro-BNP, LV bundle branch block, LV ejection fraction, atrial fibrillation during scan, increases in heart rate from rest to stress and coronary calcium score as possible explanatory variables. (DOCX 19 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Byrne, C., Hasbak, P., Kjaer, A. et al. Impaired myocardial perfusion is associated with increasing end-systolic- and end-diastolic volumes in patients with non-ischemic systolic heart failure: a cross-sectional study using Rubidium-82 PET/CT. BMC Cardiovasc Disord 19, 68 (2019). https://doi.org/10.1186/s12872-019-1047-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-019-1047-x