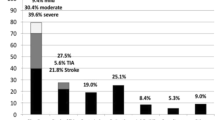
Abstract
Background
Implantation of left atrial appendage (LAA) occlusion devices was shown to be a feasible and effective alternative to oral anticoagulation in patients with non-valvular atrial fibrillation. However, only few data about in-hospital and peri-procedural data are currently available. This study aims to report about echocardiographic, procedural and in-hospital data of patients receiving LAA occlusion devices.
Methods
This single-center, prospective and observational study includes consecutively patients being eligible for percutaneous implantation of LAA occlusion devices (either Watchman™ or Amplatzer™ Cardiac Plug 2). Data on pre- and peri-procedural transesophageal echocardiography (TEE), implantation and procedure related in-hospital complications were collected. The primary efficacy outcome measure was a successful device implantation without relevant peri-device leaks (i.e., < 5 mm).
Results
In total, 37 patients were included, 22 receiving the Watchman™ and 15 ACP 2 device. Baseline characteristics did not differ significantly in both patient groups. The primary efficacy outcome measure was reached in 91.9 % of patients (90.9 % for the Watchman™, 93.3 % for the ACP 2 group). One device embolization (Watchman™ group) with successful retrieval occurred (2.7 % of patients). No thromboembolism or device thrombosis were present. The majority of bleedings was caused by access site bleedings (88.3 % of all bleedings), consisting mostly of mild hematomas corresponding to a BARC type 1 bleeding (80.0 % of all access-site complications). One patient died due to septic shock (non-procedure related).
Conclusions
In daily real-life practice, percutaneous treatment with LAA occlusion devices appears to be an effective and safe.
Similar content being viewed by others
Background
Atrial fibrillation (AF) represents the most common cardiac arrhythmia with a current age-dependent prevalence of 1–2 % in the Western population, while being assumed to rise significantly within the next couple of years [1]. Cerebral ischemic stroke represents the most important fatal complication of AF deteriorating the prognosis of each individual patient. In the presence of AF, the risk of stroke increases about 5-times [2], being accompanied by a stroke-related mortality rate of about 20 % [3, 4] and a high percentage of patients remaining disabled. The so called CHA2DS2-VASc score assesses the patient’s individual annual stroke risk and allows a specific risk-adapted anticoagulant treatment [5].
Oral anticoagulation (OAC) with vitamin K antagonists or with new direct oral anticoagulants (DOACs) prevents effectively thromboembolic events in patients with at least one risk factor [6]. In contrast, major bleedings-particularly cerebral bleedings-still represent a most crucial complication of OAC, despite the fact that DOACs were shown to reduce the occurrence of intracranial hemorrhage [7]. The individual bleeding risk as assessed by the HAS-BLED score [5], contraindications against OAC and the patient’s preference to deny OAC, need to be considered before initiating OAC. In daily clinical practice, half of patients with an increased risk for thromboembolic complications and without any contraindication against OAC does not receive this treatment [8].
The percutaneous implantation of left atrial appendage (LAA) occlusion devices such as the Watchman™ device (Boston Scientific, Natick, MA) and the Amplatzer™ Cardiac Plug (ACP) replaced by the Amplatzer™ Amulet™ (also known as ACP 2, both St. Jude Medical, St. Paul, MN) was evaluated as an effective alternative to OAC in patients with non-valvular AF and concomitant high bleeding risk or in patients being unwilling to take lifelong OAC. The non-inferiority to warfarin therapy in patients being eligible for OAC was proven in the PROTECT-AF trial for the Watchman™ device [9]. Here, warfarin was prescribed for at least 45 days after successful device implantation. In subsequent studies dual antiplatelet therapy (DAPT) for at least 6 months after implantation was shown to be safe in patients with contraindications for OAC [10]. With regard to the ACP device, a non-inferiority study has not yet been investigated. However, DAPT was shown to be safe and effective for this device [11]. First clinical experience with the ACP 2 did not show significant differences compared to the original ACP with regard to peri- and post-procedural complications [12].
Although the implantation of LAA occlusion devices is well established, there is still a great lack of in-hospital and peri-procedural data addressing the safety and efficacy of these devices [12–19]. Specifically, peri-procedural data have been shown to vary tremendously between individual centers. Today, an increase of and peri-procedural safety has developed compared to early implantation periods [13, 14, 20]. Therefore, this study aims to comparatively evaluate in-hospital single-center registry data of patients scheduled for LAA occlusion device implantation (i.e., Watchman™ versus ACP 2).
Methods
Enrollment
The present study is a single-center, prospective, observational, descriptional and non-randomized registry including consecutively 37 patients with all three types of non-valvular AF, a CHA2DS2-VASc score ≥ 2 and, therefore, indication for OAC. Patient enrollment started in June 2014. Further inclusion criteria were age ≥ 18 years, any relative or absolute contraindication for standard OAC - i.e., major bleeding with tendency to recidivity, HAS-BLED score ≥ 3 and neurological symptoms during treatment with OAC or intolerance to OAC. Written informed consent was obtained from all study patients. Patients were excluded if at least one of the following criteria were evident: single episode of AF or treatable reason for AF, catheter ablation of AF within 30 days prior to or after potential LAA occluder implantation, electrical cardioversion within 30 days after potential occluder implantation, congestive heart failure corresponding to functional class NYHA IV, myocardial infarction within the last 3 months, atrial septum defect or interventional/surgical occlusion of ASD, mechanical heart valve, status after heart transplant, symptomatic carotid artery stenosis, transient ischemic attack (TIA) or stroke within last 30 days, intracerebral bleeding within the last 3 months, acute infection, existing or planned pregnancy, existing thrombus. The study was carried out according to the principles of the declaration of Helsinki and was approved by the medical ethics committee II of the Faculty of Medicine Mannheim, University of Heidelberg, Germany.
Procedure
An electrocardiogram (ECG), standard blood analyses and transesophageal echocardiography (TEE) were performed for pre-procedural planning. LAA occlusion device implantation was performed by experienced interventional cardiologists (≥50 LAA closure device implantations each prior to the study). Patients were treated with conscious sedation using intravenous 2,6-di(propan-2-yl)phenol and midazolam. One arterial access sheath (5 French, F) for arterial blood pressure monitoring and one venous access sheath for interventional device implantation were used. Transseptal puncture was performed with a SL1 sheath and BRK1 Brockenbrough needle (St. Jude Medical, St. Paul, MN). Prior transseptal puncture heparin was administered to achieve an active clotting time of at least 250 s. A stiff guide wire (Cook Medical, Bloomington, IN) was placed in the left upper pulmonary vein (LUPV) and the transseptal sheath was removed and replaced by the Watchman™ (14 F), respectively ACP 2 (12 or 14 F) delivery sheath. Device allocation algorithm was performed according to latest consensus recommendations [21]. All patients underwent pre-procedural 2D/3D TEE to assess initially the anatomy, size and absence of thrombus or sludge on all recommended TEE views. Accordingly, peri-procedural imaging was based on fluoroscopy and 2D/3D TEE imaging in order to re-assess prior findings of the LAA and to decide which device size was appropriate in the individual patient. Fluoroscopy was performed using a 5 F pigtail catheter to visualize the LAA in at least 2 standard angulations (RAO 30°/10° cranial, RAO 30°/10° caudal). Regarding TEE, the following parameters of the LAA were assessed for appropriate sizing, as recommended for both devices [21]: LAA ostium, landing zone, angle of the LAA, depth of the LAA, differentiation of main lobes versus smaller side lobes. The device size was chosen at least 20 % larger than the measured diameters at the landing zone [22]. Post-implantation the compression of the devices was measured by 2D TEE and a device compression of at least 10 % was defined as valuable, next to lacking of relevant peri-device leaks (>5 mm), and no compression or attachment of neighboring structures, such as the circumflex coronary artery, mitral annulus or pulmonary veins. A tug test was performed repetitively before final liberation of the device as recommended. Access site was closed with an Angio-Seal™ Evolution™ (St. Jude Medical, St. Paul, MN) for the arterial access and with two ProGlide™ (Abbott Vascular, Santa Clara, CA) for the venous access and followed by a pressure band for 6 hours.
Post-procedural measures
In accordance with our institutional protocol, acetylsalicylic acid (ASA) 100 mg/d was administered lifelong, clopidogrel for at least 6 months commencing the day of implantation with a loading dose of 250 mg, respectively 600 mg, if not taken before. The day after the procedure, a transthoracic echocardiogram (TTE) and a chest X-ray were performed to rule out device dislodgement and pericardial effusion, an ECG was performed to rule out a new bundle branch block (BBB) or atrioventricular (AV) block and repetitive thorough clinical examinations were carried out to rule out other clinical disorders.
Outcome measures
The primary efficacy outcome measure of our in-hospital register was defined as technical success with a successful device implantation without relevant peri-device leaks (i.e., < 5 mm). Primary safety outcome measure was defined as the occurrence of bleeding events classified according to the BARC definition [23], pericardial effusion, device embolization, peri-procedural stroke and peri-procedural death. Events resulting in death, aggravated morbidity or prolonged hospitalization being associated with the procedure were defined as complications. Any events not being associated with the procedure were termed adverse events.
Statistics
Statistical analyses were performed with SPSS Statistics (IBM, Armonk, NY). Descriptive statistics are given as medians (25th and 75th percentiles) or as total numbers with group-related percentages. Normal distribution of data was tested with the Kolmogorov-Smirnov test. In case of normal distribution, the t-test was applied to compare scaled data. Scaled variables not normally distributed were compared using the Mann-Whitney U test. Categorical variables were compared using the chi-squared test. Level of significance was set at p < 0.05 (two-tailed).
Results
Baseline characteristics
Baseline demographic and clinical characteristics of the study population including indications for implantation of LAA occlusion devices and risk-stratification according to CHA2DS2-VASc and HAS-BLED scores are shown in Table 1. Of 37 patients, 22 patients received the Watchman™ and 15 patients received the ACP 2 device. The most common indication was a prior history of bleedings (78.4 %) under treatment with OAC. 51.4 % of all patients were not treated with OAC, whereas patients with OAC were mostly treated with DOAC (66.7 %). Table 2 shows two-dimensional (2D) and functional echocardiographic measurements. As assessed by angiography, the majority of patients revealed a chicken-wing shaped LAA (67.6 %).
Procedural data
Procedural data related to the implantation of LAA occlusion devices are summarized in Table 3. The primary efficacy outcome measure in the Watchman™ group was 90.9 %, whereas it was 93.3 % in the ACP 2 group (p = 0.791). Only the Watchman™ device device could not be implanted in two patients (9.1 %) either due to an anatomical mismatch or due to an incomplete occlusion of the LAA landing zone. In contrast, the ACP 2 device was implanted in all patients with one patient with incomplete ostial occlusion (6.7 %). More than one transseptal puncture was needed only in only one patient (2.7 %) due to a transseptal retrieval of an intra-atrial embolized Watchman™ occluder during the procedure. Pericardial effusion and subsequent intermittent circulatory failure with rapid hemodynamic stabilization without the need for cardiopulmonary resuscitation occurred in one patient of the Watchman™ group (2.7 %). All these results showed no statistically significant difference between the Watchman™ and the ACP 2 group.
In-hospital outcome and peri-procedural safety events
Table 4 shows all relevant data related to in-hospital outcome and peri-procedural safety events. None of the patients died due to the implantation of LAA occlusion devices. However, one patient with a preexisting highly reduced left ventricular function (ejection fraction 28 %) died from an acute heart failure linked to a severe urosepis 20 days after the implantation (i.e., adverse event). In another patient with non-ST elevation myocardial infarction (NSTEMI) and percutaneous coronary intervention (PCI) with stent implantation known clopidogrel non-response, DAPT with ASA and ticagrelor instead of clopidogrel was continued as an individual treatment attempt.
Severe conduction blocks, such as BBB or AV blocks, did not occur. Access site complications, such as groin bruise and bleedings at the access sites represented 75.0 % of all complications. Most of them were mild, corresponding to a BARC type 1 bleeding (76.5 % of all bleeding complications) and only 1 case (2.7 % of all patients) needed transfusion without the need for vascular surgery. The patient with a pericardial effusion (3 mm) in the Watchman™ group could be treated conservatively. Notably, neither peri-procedural nor in-hospital transient ischemic attack, stroke or device thrombosis occurred. Patients could have been discharged with a median of 4 days after the intervention. None of the surviving patients developed persistent neurological or heart-failure related disability.
Discussion
AF is one of the main causes leading to ischemic stroke and persistent neurological disability. In almost 25 % of patients developing an ischemic stroke AF can be documented by ECG recording [3]. Therefore effective prevention of cerebral embolization represents the most important aspect for an optimal treatment of patients with AF. Besides the established vitamin K antagonists such as warfarin, innovative DOACs emerged as reliable treatment alternatives. In a current meta-analysis these drugs were shown to reduce significantly the risk of major cerebral bleedings and hemorrhagic stroke when compared to warfarin [7, 24]. Specific subsets of patients of patients are prone to develop severe bleedings as a consequence of OAC, for instance patients with malignoma, major gastrointestinal bleedings and geriatric patients with an increasing risk to fall in everyday life. Accordingly, most of the patients within the presented study revealed a history of severe bleedings leading to the decision to implant an LAA occlusion device. These patients revealed an increased median HAS-BLED score of 4 points and still revealed an increasing risk of developing stroke, as indicated by an increased median CHA2DS2-VASc score of 5 points corresponding to an estimated annual risk of thromboembolic stroke of about 6.7 % [4].
Relative contraindications against the use of OAC have been reported in about 20 % of patients with AF [25, 26]. Even patients without any contraindication against OAC are often not treated by the optimal antithrombotic drug regimes [27, 28]. Within the present study, half of the study patients were not anticoagulated previously due to relevant contraindications. In these patients exclusion of the LAA has become the therapy of choice in order to prevent fatal thromboembolic events apart from OAC [9, 29, 30]. Since we only enclosed patients with a high bleeding risk (a median HAS-BLED score of 4 points indicates “high risk” [6]) or refusal for OAC and, therefore, relative or absolute contraindication for OAC, DAPT with ASA and clopidogrel following implantation procedure was thought to be appropriate for these patients. The duration of DAPT lasted 6 months as previously been shown to be an effective and safe antithrombotic treatment [10].
Success rates of 95.5–100 %, respectively, were comparable to prior studies using the Watchman™ device [9, 10] or were even higher for the ACP 2 [12, 14]. Since complete coverage of the LAA ostium is crucial for preventing from embolization from the LAA, this was defined as a primary efficacy outcome measure, which was also beyond 90 % for both devices.
The documented high efficacy appears to be associated with a careful and appropriate selection of device sizes according to the pre- and peri-procedural measurements of LAA dimensions being assessed by a multi-modal imaging approach with angiography and TEE imaging [14, 22] allowed a safe and accurate peri-procedural guiding for an optimal positioning and adaption. Diameters of the LAA ostium, landing zone, angulation, deepness and volume appeared most similar in recommended angulations (0, 45, 90 and 135°) with a tendency for larger orifice diameters being measured at 135° of angulation [31]. The median size of the devices was about 40 % larger than the median landing as assessed by TEE, surpassing the 20 % range of recommended oversizing [22].
One patient revealed a flat tub-shaped LAA morphology, which made implantation of both devices impossible, whereas all other typical LAA morphologies were able to be accessed by LAA occlusion devices. Even the relatively frequent occurrence of the chicken-wing morphology, described as often being a challenging morphology for transcatheter occlusion device implantation [32], was not seen to decrease the high success rates of both devices. Time of fluoroscopy was comparable to prior studies [18, 19] and did not differ significantly between both groups despite the fact that decision making algorithm in our study regarding accurate measurements of the LAA and selection of the adequate device size was based on peri-procedural 2D TEE in combination with angiography. 3D TEE has been described as an additional imaging technique for optimal visualization of cardiac anatomy during percutaneous cardiac interventions [22, 33]. However, efficacy data evaluating TEE (either 2D or 3D) versus sole or combined angiography during implantation of LAA occlusion devices are not available at present. Since the procedures were performed by experienced interventional cardiologists, well practiced in LAA closure device implantation, we could not find a certain learning curve neither concerning procedural time nor related to peri-procedural complications [34].
Major peri-procedural complications were rare (8.1 %) and did not reveal persistent disability. At 40.5 %, groin hematomas and groin bleedings occurred more frequently compared to recently published data [10, 18, 20]. However, the majority of access site bleedings were minor hematomas (80.0 % of all access-site complications corresponded to a BARC type 1 bleeding). The majority of patients suffering from access-site complications was affected by combined arterial and venous access-site bleedings (21.6 % of all patients). As must be expected, the arterial access-site was the second most common bleeding site (16.2 % of all patients). Since an arterial access during LAA closure device implantation procedure is not mandatory [35], a certain number of access-site bleedings may be avoidable if this access way would be abandoned. Notably, the arterial access allows a safer performance [35] at the expense of some additional mild hematomas. Only one patient was in need of blood transfusion, whereas vascular surgery was never needed. With respect to major complications, one case of circulatory failure was linked to a post-procedural detected pericardial effusion (3 mm), which could be treated conservatively. Early device thrombosis followed by TIA or stroke did not occur in our population suggesting that DAPT initiated by a loading dose might be effective to prevent thromboembolism at the intraluminal device site prior to neo-endothelialization. One patient with intra-procedural device dislodgement in the left atrium (Watchman™ group) which could be successfully retrieved by a veno-venous double lasso interventional technique.
Limitations of the study
This study is based on observational registry data concerning a relative small real-life patient population and was not intended to reliable calculate significant differences between both device types. Comparison results of both devices are for informative reasons only. The aim was to demonstrate essential peri-interventional efficacy and safety data concerning LAA occlusion device implantation in general. A subsequent enlarged set of this registry data might be able to evaluate further differences between both devices.
As this observational study focused on the peri-procedural time, the follow-up period was limited to the discharge from hospital.
The application of any of the two devices was not randomly assessed and was based on the operator’s discretion being mainly based on LAA anatomic considerations. In contrast, this more individualized and clinically driven decision algorithm might have strongly influenced the high achievement of primary efficacy outcome measure, which realistically reflects clinical and interventional practice in experienced centers.
Conclusions
Transcatheter implantation of LAA occlusion devices appears to be a feasible and safe percutaneous cardiac intervention with a rare occurrence of major complications and a high success rate in a real-life patient population.
Ethics, consent and permissions
Written informed consent was obtained from all study patients. The study was carried out according to the principles of the declaration of Helsinki and was approved by the medical ethics committee II of the Faculty of Medicine Mannheim, University of Heidelberg, Germany.
Availability of supporting data
Sharing of additional data is not necessary, because all relevant patient data are presented in this article.
References
Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–5.
Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–70.
Marini C, De Santis F, Sacco S, Russo T, Olivieri L, Totaro R, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke. 2005;36(6):1115–9.
European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery, Camm AJ, Kirchhof P, Lip GY, Schotten U, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–429.
Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126(7):860–5.
Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. Guidelines ESCCfP: 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–47.
Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–62.
Waldo AL, Becker RC, Tapson VF, Colgan KJ, Committee NS. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J Am Coll Cardiol. 2005;46(9):1729–36.
Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374(9689):534–42.
Reddy VY, Mobius-Winkler S, Miller MA, Neuzil P, Schuler G, Wiebe J, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix feasibility study with watchman left atrial appendage closure technology). J Am Coll Cardiol. 2013;61(25):2551–6.
Urena M, Rodes-Cabau J, Freixa X, Saw J, Webb JG, Freeman M, et al. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol. 2013;62(2):96–102.
Gloekler S, Shakir S, Doblies J, Khattab AA, Praz F, Guerios E, et al. Early results of first versus second generation Amplatzer occluders for left atrial appendage closure in patients with atrial fibrillation. Clin Res Cardiol. 2015.
Pison L, Potpara TS, Chen J, Larsen TB, Bongiorni MG, Blomstrom-Lundqvist C. Scientific Initiative Committee EHRA: Left atrial appendage closure-indications, techniques, and outcomes: results of the European Heart Rhythm Association Survey. Europace. 2015;17(4):642–6.
Freixa X, Abualsaud A, Chan J, Nosair M, Tzikas A, Garceau P, et al. Left atrial appendage occlusion: initial experience with the Amplatzer Amulet. Int J Cardiol. 2014;174(3):492–6.
Meerkin D, Butnaru A, Dratva D, Bertrand OF, Tzivoni D. Early safety of the Amplatzer Cardiac Plug for left atrial appendage occlusion. Int J Cardiol. 2013;168(4):3920–5.
Streb W, Szymala M, Kukulski T, Nowak J, Lekston A, Sokal A, et al. Percutaneous closure of the left atrial appendage using the Amplatzer Cardiac Plug in patients with atrial fibrillation: evaluation of safety and feasibility. Kardiol Pol. 2013;71(1):8–16.
Lam YY, Yip GW, Yu CM, Chan WW, Cheng BC, Yan BP, et al. Left atrial appendage closure with AMPLATZER cardiac plug for stroke prevention in atrial fibrillation: initial Asia-Pacific experience. Catheter Cardiovasc Interv. 2012;79(5):794–800.
Chun KR, Bordignon S, Urban V, Perrotta L, Dugo D, Furnkranz A, et al. Left atrial appendage closure followed by 6 weeks of antithrombotic therapy: a prospective single-center experience. Heart Rhythm. 2013;10(12):1792–9.
Gafoor S, Franke J, Bertog S, Boehm P, Heuer L, Gonzaga M, et al. Left atrial appendage occlusion in octogenarians: short-term and 1-year follow-up. Catheter Cardiovasc Interv. 2014;83(5):805–10.
Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011;123(4):417–24.
Meier B, Blaauw Y, Khattab AA, Lewalter T, Sievert H, Tondo C, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. EuroIntervention. 2015;10(9):1109–25.
Wunderlich NC, Beigel R, Swaans MJ, Ho SY, Siegel RJ. Percutaneous Interventions for left atrial appendage exclusion: options, assessment, and imaging Using 2D and 3D Echocardiography. JACC Cardiovasc Imaging. 2015;8(4):472–88.
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736–47.
Greenspon AJ. A review of oral anticoagulants in patients with atrial fibrillation. Postgrad Med. 2012;124(6):7–16.
O’Brien EC, Holmes DN, Ansell JE, Allen LA, Hylek E, Kowey PR, et al. Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am Heart J. 2014;167(4):601–9. e601.
Bradley BC, Perdue KS, Tisdel KA, Gilligan DM. Frequency of anticoagulation for atrial fibrillation and reasons for its non-use at a Veterans Affairs medical center. Am J Cardiol. 2000;85(5):568–72.
Jorge E, Pereira FS, Baptista R, Monteiro P, Santos L, Fonseca I, et al. Anticoagulation in elderly patients with atrial fibrillation: from the guidelines to the daily medical practice. Acta Med Port. 2011;24 Suppl 2:293–300.
Diez-Manglano J, Gomes-Martin J, Al-Cheikh-Felices P, Perez SI, Diez-Angulo R, Clemente-Sarasa C. Adherence to guidelines and mortality in atrial fibrillation. Int J Cardiol. 2014;176(2):430–6.
Madden JL. Resection of the left auricular appendix; a prophylaxis for recurrent arterial emboli. JAMA. 1949;140(9):769–72.
Bayard YL, Omran H, Neuzil P, Thuesen L, Pichler M, Rowland E, et al. PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) for prevention of cardioembolic stroke in non-anticoagulation eligible atrial fibrillation patients: results from the European PLAATO study. EuroIntervention. 2010;6(2):220–6.
Chan SK, Kannam JP, Douglas PS, Manning WJ. Multiplane transesophageal echocardiographic assessment of left atrial appendage anatomy and function. Am J Cardiol. 1995;76(7):528–30.
Freixa X, Tzikas A, Basmadjian A, Garceau P, Ibrahim R. The chicken-wing morphology: an anatomical challenge for left atrial appendage occlusion. J Interv Cardiol. 2013;26(5):509–14.
Kretzschmar D, Lauten A, Goebel B, Doenst T, Poerner TC, Ferrari M, et al. Optimal prosthesis sizing in transcatheter aortic valve implantation by exclusive use of three-dimensional transoesophageal echocardiography. Clin Physiol Funct Imaging. 2014.
Cruz-Gonzalez I, Perez-Rivera A, Lopez-Jimenez R, Rodriguez-Collado J, Martin-Moreiras J, Cascon M, et al. Significance of the learning curve in left atrial appendage occlusion with two different devices. Catheter Cardiovasc Interv. 2014;83(4):642–6.
Mobius-Winkler S, Majunke N, Sandri M, Mangner N, Linke A, Stone GW, et al. Percutaneous left atrial appendage closure: Technical aspects and prevention of periprocedural complications with the watchman device. World J Cardiol. 2015;7(2):65–75.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CF conceived the study, participated in its design and coordination, participated in data analysis and interpretation and helped to draft and revise the manuscript for important intellectual content. MBe conceived the study, participated in its design and coordination, participated in data analysis and interpretation and helped to draft and revise the manuscript for important intellectual content. BS participated in the study design and coordination, data acquisition and analysis and helped to draft the manuscript for important intellectual content. MY participated in the study design and coordination, as well as data analysis and revised the manuscript. KM participated in the study design and coordination, as well as data analysis and revised the manuscript. IE-B participated in the study design and coordination, participated in data analysis and interpretation and helped to draft and revise the manuscript for important intellectual content. RL participated in the study design and coordination, as well as data analysis and revised the manuscript. SB participated in the study design and coordination, as well as data analysis and revised the manuscript. TB participated in the study design and coordination, as well as data analysis and revised the manuscript. MBo participated in the study design and coordination, as well as data acquisition and revised the manuscript for important intellectual content. IA conceived the study, participated in its design and coordination, participated in data analysis and interpretation and helped to draft and revise the manuscript for important intellectual content. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fastner, C., Behnes, M., Sartorius, B. et al. Left atrial appendage morphology, echocardiographic characterization, procedural data and in-hospital outcome of patients receiving left atrial appendage occlusion device implantation: a prospective observational study. BMC Cardiovasc Disord 16, 25 (2016). https://doi.org/10.1186/s12872-016-0200-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-016-0200-z