Abstract
This case report highlights the effective use of intermittent hemodialysis (IHD) in warming a 71-year-old female patient with severe hypothermia who presented with a rectal temperature of 25 °C and signs of hemodynamic instability. The patient, found unconscious after prolonged exposure to cold exacerbated by alcohol consumption, initially showed some improvement in core temperature through active external rewarming methods. However, soon, her temperature plateaued at 27 °C. Patient was deemed unsuitable for extracorporeal membrane oxygenation (ECMO) or cardiopulmonary bypass (CPB) due to her age, and urgent IHD was initiated. This approach resulted in a stable increase in core temperature at approximately 2.0 °C/hr, along with normalization of lactic acidosis, creatinine phosphokinase, and correction of electrolyte imbalances, culminating in her full recovery and discharge after seven days in the hospital.
After reviewing this case alongside similar ones from before, this case report highlights the efficacy and safety of IHD as an efficient, readily available, and less invasive method for rewarming moderate to severe hypothermic patients who are hemodynamically unstable patients but do not have cardiac arrest or renal dysfunction. IHD is especially useful when less invasive cooling devices (Artic Sun/ CoolGard) are not available or more invasive extracorporeal life support options (ECMO/ CPB) are either not indicated or unavailable. IHD can also help improve concurrent electrolyte imbalances and/or toxin buildup. The report further emphasizes the necessity of monitoring for potential complications, such as post-dialysis hypophosphatemia and rebound hyperkalemia, following successful rewarming.
Similar content being viewed by others
Introduction
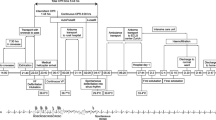
This case report details the successful treatment of a 71-year-old woman with severe hypothermia, with rectal temperature of 25 °C, using intermittent hemodialysis (IHD). Untreated severe hypothermia can lead to fatal arrhythmias and coma, with a higher mortality rate in rural areas compared to urban areas, as per recent data [1]. Our report highlights IHD as an effective strategy for managing severe hypothermia with hemodynamic instability in settings where advanced treatments like extracorporeal membrane oxygenation (ECMO) or cardiopulmonary bypass (CPB) are either not available, such as in smaller community hospitals or not indicated.
Case
A 71-year-old female with obesity was found unconscious outside her home after being exposed to the cold for about 10–12 h, following an evening of drinking alcohol. In the emergency department, patient had a Glasgow Coma Scale score of 3 (E1V1M1), rectal temperature of 24.5°C, heart rate of 58 beats per minute, blood pressure of 81/51mmHg, respiratory rate of 16 breaths per minute. Electrocardiogram showed atrial fibrillation with Osborne waves as shown in Fig. 1. Arterial blood gas analysis at 37°C revealed mixed respiratory and anion gap metabolic acidosis, attributed to hypoventilation and lactic acidosis, with high ethanol levels; other toxins were not elevated. Blood tests indicated hypoglycemia, elevated lactate, mild rhabdomyolysis, and significant hypokalemia, as shown in Tables 1 and 2. Serum cortisol levels were not ordered. Imaging excluded acute intracranial issues and fractures.
The patient was intubated for airway protection and received IV propofol, norepinephrine, antibiotics, and potassium (K +) of 60 mEq/L. Initial rewarming with forced-air warming blankets (Bair Hugger) and warm IV fluids over 1.5 h raised rectal temperature to 27 °C, which then plateaued. ECMO team deemed her unsuitable for treatment due to her age, leading to a consultation with a nephrologist for urgent IHD. A 16 cm, 12 French, double-lumen catheter was placed in the right internal jugular vein for dialysis purposes. The patient underwent IHD using a Fresenius 2008 T machine and an F-180 dialyzer. The dialysate temperature was set at 37 °C, with a dialysate flow rate of 400 ml/min and blood flow rate of 200 ml/min. The potassium, calcium, and bicarbonate levels in the bath were 3 mEq/L, 2.5 mEq/L, and 35 mEq/L respectively. No ultrafiltration was performed, and the total duration of the dialysis session was 2.5 h. The patient’s core temperature improved from 27.5 °C to 32.1 °C at approximately 2.0 °C/hr, while maintaining stable vitals. After dialysis, repeat levels of serum lactic acid, creatine phosphokinase (CPK), potassium and phosphate levels remained within normal limits. Bair Hugger was restarted, and patient's rectal temperature increased to 37 °C in the next 7–8 h. By the next day, her mental status improved. She was weaned off support and extubated, with her atrial fibrillation converting to sinus rhythm. After a 7-day hospital stay, she recovered fully and was discharged home.
Discussion
Compared to a hot environment, the body has limited ability to respond to cold. Hypothermia is clinically staged using “Alert Verbal Painful Unconsciousness scale and Revised Swiss Staging, focusing on consciousness and vital signs, and which is not applicable to cases with comorbidities like trauma or sepsis [2]. Our patient had stage 3 hypothermia (unconscious, not shivering and vitals present) based on Swiss staging system of hypothermia.
Certain factors have been identified as independent prognostic indicators for in-hospital mortality in patients with moderate-to-severe illness. These include age ≥ 75 years, need for assistance with activities of daily living, hemodynamic instability, and hyperkalemia. Recognizing these indicators can help determine the necessity for intensive care, invasive treatment, or the withdrawal of aggressive interventions [3].
Active external rewarming such as forced-air warming blankets (Bair Hugger, Maplewood, Minnesota), and warm fluids have limited effectiveness for moderate to severe hypothermia and should be used to complement active internal rewarming procedures.
Minimally invasive techniques, including adhesive hydrogel devices (Artic Sun) and intravascular temperature cooling catheters (CoolGard, CoolLine, and Icy) [4] are recommended to manage persistent moderate to severe hypothermia without cardiac arrest. If these devices are unavailable, peritoneal and/ or pleural irrigation with warmed isotonic fluid can be considered.
If severe hypothermia is accompanied by cardiovascular instability or cardiac arrest, extracorporeal life support options such as veno-venous rewarming circuit without ECMO, CPB, and veno-arterial ECMO should be considered [5,6,7]. The ECMO circuit can warm the patient up to a rate of 7–10 °C per hour [8, 9]. In cases where minimally invasive cooling catheters are unavailable, and the patient has cardiopulmonary instability but not cardiac arrest, with ECMO either contraindicated or unavailable, IHD / peritoneal dialysis can be efficiently utilized [5,6,7]. Important contraindications to ECMO treatment include terminal illness, decompensated chronic conditions, prolonged cardiac arrest, severe coagulopathy [10].
IHD is safe, readily accessible, less invasive, and requires a hemodialysis catheter, making it easy to set up, unlike peritoneal dialysis, which would require an expert surgeon or nephrologist to place a peritoneal dialysis catheter. IHD can increase the core temperature by 2–3 °C per hour [9, 11, 12]. In patients with hypothermia-associated severe lactic acidosis, renal dysfunction, and/ or rhabdomyolysis, IHD can be used as a first-line treatment for severe hypothermia when primary or continuous warming methods are not readily available. This is particularly important in low socioeconomic regions where access to primary or continuous warming techniques is frequently restricted. In a hypothermic patient without any overt metabolic abnormalities, the balance between the possible advantages of intermittent hemodialysis and the risks of procedure-related complications is uncertain (for example, at what temperature should hemodialysis be performed below). IHD treatment in patients without kidney dysfunction can cause hypophosphatemia due to the absence of phosphate in the dialysate. Therefore, it is essential to check serum phosphate levels before and after the treatment and provide phosphate repletion as needed.
When using IHD to treat patients for hypothermia or poisoning caused by dialyzable toxins in patients without renal dysfunction and/ or hyperkalemia, there is no need for any net potassium removal. In such situations, it is appropriate to use a dialysate potassium (K) concentration of 4 mEq/L, which is within the normal range, if available. In our case, the patient received potassium of 60 mEq in the emergency department, and at the time of IHD, a 4 K bath was unavailable, so a 3 K bath was utilized.
It is essential to be aware of the risk of hazardous core temperature after-drop when actively rewarming a hypothermic patient [7, 13]. This complication may occur when both the patient's extremities and trunk are warmed simultaneously [14]. The problem is that the cold, acidic blood that has pooled in the narrowed blood vessels of the extremities returns to the core circulation, causing the temperature and pH to drop further. Additionally, the transition from the cold environment to a warmer one causes the peripheral blood vessels to dilate, leading to a sudden drop in blood pressure, inadequate blood supply to the heart, and even ventricular fibrillation [15]. This may explain why some patients experience fatal heart rhythms during rewarming. However, combining active core rewarming techniques with active external rewarming can help minimize the risk of rewarming shock and after-drop in patients with severe hypothermia. In the event of an after drop, pressor support can be used to maintain appropriate blood pressure while continuing with the warming process.
We conducted an analysis incorporating our case with eight previously reported case of severe hypothermia without renal dysfunction treated via hemodialysis [11, 12, 16,17,18,19,20], detailed in Table 3. The observed average rewarming rate during hemodialysis was 1.88 °C per hour, which is consistent with the results reported by Mendrala et al. [9]. An important prognostic factor identified is that delays in the rewarming process are closely associated with increased mortality rates [9]. D.F. Danzl et al. performed a retrospective study of hypothermia outcomes over 25 years ago, factors linked to death within 24 h included prehospital cardiac arrest, low or absent blood pressure upon arrival, high blood urea nitrogen levels, and the requirement for endotracheal intubation [21].
Our case report has certain limitations, including its single-case nature, the absence of significant cardiopulmonary comorbidities, and the lack of baseline cortisol level measurements during the hospital stay.
Conclusion
-
1.
IHD is a safe, readily available, and effective method to rewarm severe hypothermic patients with hemodynamic instability without cardiac arrest, especially when ECMO or CPB are not available or not indicated.
-
2.
The use of IHD has an added advantage of correcting associated electrolyte imbalance, toxins burden, and rhabdomyolysis.
-
3.
IHD without ultrafiltration and dialysate temperature of 37 °C can be used to effectively manage severe hypothermia with favorable outcome.
-
4.
After dialysis, it is essential to closely monitor for hypophosphatemia and rebound hyperkalemia/ hypoglycemia.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
QuickStats: Death Rates Attributed to Excessive Cold or Hypothermia, by Urbanization Level and Sex — National Vital Statistics System, 2018–2020. MMWR Morb Mortal Wkly Rep 2022;71:282. https://doi.org/10.15585/mmwr.mm7107a6external icon.
Musi ME, Sheets A, Zafren K, et al. Clinical staging of accidental hypothermia: The Revised Swiss System. Resuscitation. 2021;162:182–7. https://doi.org/10.1016/j.resuscitation.2021.02.038.
Okada Y, Matsuyama T, Morita S, et al. Prognostic factors for patients with accidental hypothermia: A multi-institutional retrospective cohort study. Am J Emerg Med. 2019;37(4):565–70. https://doi.org/10.1016/j.ajem.2018.06.025.
Morita S, Inokuchi S, Yamagiwa T, et al. Efficacy of portable and percutaneous cardiopulmonary bypass rewarming versus that of conventional internal rewarming for patients with accidental deep hypothermia*. Crit Care Med. 2011;39(5):1064–8. https://doi.org/10.1097/CCM.0b013e31820edd04.
Saczkowski RS, Brown DJA, Abu-Laban RB, Fradet G, Schulze CJ, Kuzak ND. Prediction and risk stratification of survival in accidental hypothermia requiring extracorporeal life support: An individual patient data meta-analysis. Resuscitation. 2018;127:51–7. https://doi.org/10.1016/j.resuscitation.2018.03.028.
Splittgerber FH, Talbert JG, Sweezer WP, Wilson RF. Partial cardiopulmonary bypass for core rewarming in profound accidental hypothermia. Am Surg. 1986;52(8):407–12.
Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992;10(2):311–27.
Bonnemain J, Rusca M, Liaudet L. ECMO for Accidental Hypothermia and Cardiorespiratory Arrest. In: Maybauer MO, ed. Extracorporeal Membrane Oxygenation. Oxford University Press; 2022:501–512. https://doi.org/10.1093/med/9780197521304.003.0049
Mendrala K, Kosiński S, Podsiadło P, et al. The Efficacy of Renal Replacement Therapy for Rewarming of Patients in Severe Accidental Hypothermia—Systematic Review of the Literature. Int J Environ Res Public Health. 2021;18(18):9638. https://doi.org/10.3390/ijerph18189638.
Lu SY, Ortoleva J, Colon K, et al. Association Between Body Mass Index and Outcomes in Venoarterial Extracorporeal Membrane Oxygenation. Anesth Analg. 2022;134(2):341–7. https://doi.org/10.1213/ANE.0000000000005689.
Caluwé R, Vanholder R, Dhondt A. Hemodialysis as a Treatment of Severe Accidental Hypothermia. Artif Organs. 2010;34(3):237–9. https://doi.org/10.1111/j.1525-1594.2009.00837.x.
Murakami T, Yoshida T, Kurokochi A, et al. Accidental Hypothermia Treated by Hemodialysis in the Acute Phase: Three Case Reports and a Review of the Literature. Intern Med. 2019;58(18):2743–8. https://doi.org/10.2169/internalmedicine.1945-18.
Danzl DF, Pozos RS. Accidental hypothermia. N Engl J Med. 1994;331(26):1756–60. https://doi.org/10.1056/NEJM199412293312607.
Giesbrecht GG. Cold stress, near drowning and accidental hypothermia: a review. Aviat Space Environ Med. 2000;71(7):733–52.
Sterba JA. Efficacy and safety of prehospital rewarming techniques to treat accidental hypothermia. Ann Emerg Med. 1991;20(8):896–901. https://doi.org/10.1016/s0196-0644(05)81434-9.
Sultan N, Theakston KD, Butler R, Suri RS. Treatment of severe accidental hypothermia with intermittent hemodialysis. CJEM. 2009;11(2):174–7. https://doi.org/10.1017/s1481803500011167.
Rahman S, Rubinstein S, Singh J, Samih M, Balsam L. Early use of hemodialysis for active rewarming in severe hypothermia: a case report and review of literature. Ren Fail. 2012;34(6):784–8. https://doi.org/10.3109/0886022X.2012.673466.
Carr ME, Wolfert AI. Rewarming by hemodialysis for hypothermia: Failure of heparin to prevent DIC. J Emerg Med. 1988;6(4):277–80. https://doi.org/10.1016/0736-4679(88)90362-9.
Hernandez E, Praga M, Alcazar JM, et al. Hemodialysis for Treatment of Accidental Hypothermia. Nephron. 1993;63(2):214–6. https://doi.org/10.1159/000187185.
Singh T, Hallows KR. Hemodialysis for the Treatment of Severe Accidental Hypothermia. Semin Dial. 2014;27(3):295–7. https://doi.org/10.1111/sdi.12156.
Danzl DF, Hedges JR, Pozos RS. Hypothermia outcome score. Crit Care Med. 1989;17(3):227–31. https://doi.org/10.1097/00003246-198903000-00005.
Funding
No funding of any sort provided by any individual or institution while writing this case report.
Author information
Authors and Affiliations
Contributions
S.U, J.D, M.K and S.H wrote the manuscript. H.P and S.M collected lab results and made the tables. S.U and D.P reviewed the original manuscript and improved on the revised version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written consent was obtained from the patient prior to writing this case report which can be provided upon request.
Consent for publication
Written informed consent was obtained from the patient for the publication of identifying information/images in an online open access publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Usman, S., Daloya, J., Khan, M.J. et al. Intermittent hemodialysis as a rewarming strategy for severe hypothermia in patients without renal failure: a case report. BMC Anesthesiol 24, 284 (2024). https://doi.org/10.1186/s12871-024-02664-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-024-02664-w