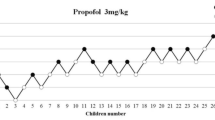Abstract
Background
This study aimed to investigate the effect of esketamine on the dose–effect relationship between remifentanil and the cardiovascular response to endotracheal intubation during target-controlled infusion (TCI) of propofol.
Methods
Patients underwent elective gynecological laparoscopic surgery under general anesthesia with endotracheal intubation, aged 18–65 years, American Society of Anesthesiologists class I or II, 18 kg/m2 ≤ body mass index ≤ 30 kg/m2, were randomly divided into the control (group C) and esketamine groups (group E). Before anesthesia induction, group E received an intravenous injection of 0.3 mg/kg of esketamine, while group C received an equal dose of physiological saline. TCI of propofol to the effect-site concentration (EC) of 3.0 μg/mL, and then TCI of remifentanil to the effect room and intravenous injection of rocuronium 0.6 mg/kg after MOAA/S was 0. Endotracheal intubation was performed after 2 min. Dixon’s modified sequential method was used, and the initial EC of remifentanil was 3.0 ng/mL. The EC of remifentanil was determined according to the intubation response of the previous patient, with an adjacent concentration gradient of 0.3 ng/mL. The EC50 and EC95 values and their 95% confidence intervals (CIs) were determined using probit regression analysis.
Results
The EC50 for cardiovascular response inhibition to endotracheal intubation using remifentanil was 3.91 ng/mL (95% CI: 3.59–4.33 ng/mL) and EC95 was 4.66 ng/mL (95% CI: 4.27–6.23 ng/mL) with TCI of propofol 3.0 μg/mL. After intravenous administration of 0.3 mg/kg of esketamine, the EC50 of remifentanil was 3.56 ng/mL (95% CI: 3.22–3.99 ng/mL) and EC95 was 4.31 ng/mL (95% CI: 3.91–5.88 ng/mL).
Conclusions
Combined with TCI of propofol 3.0 μg/mL for anesthesia induction, esketamine significantly reduced the EC50 and EC95 of remifentanil to inhibit the cardiovascular response to endotracheal intubation.
Trial registration
The trial was registered in the Chinese Clinical Trials Registry (www.chictr.org.cn; registration number: ChiCTR2200064932; date of registration:24/10/2022).
Similar content being viewed by others
Background
Endotracheal intubation produces strong airway stimulation, which is no less intense than the stress produced by surgical skin incision [1]. The dramatic fluctuations in hemodynamics caused by sympathetic nerve excitation and the release of catecholamines may lead to cardiovascular accidents during induction of general anesthesia [2]. Remifentanil is a short-acting μ-receptor agonist with fast metabolism. The use of larger doses can reduce the response to endotracheal intubation and cardiovascular risk due to the induction of anesthesia and endotracheal intubation [3]. Ahonen et al. reported that induction of anesthesia with remifentanil 2 μg/kg and propofol, maintained at 0.25 or 0.5 μg/(kg·min), can provide appropriate anesthesia and allow patients to quickly recover and extubate [4]. However, large doses of remifentanil may be accompanied by adverse reactions such as coughing, bradycardia, or hypotension [5, 6].
Esketamine is an intravenous anesthetic with potent analgesic effects and act on N-methyl-D-aspartate receptors (NMDAR) and opioid and monoaminergic receptors in the brain and spinal cord to produce dose-related sedative-hypnotic, analgesic, and amnestic effects [7,8,9]. Esketamine does not inhibit respiration and maintains hemodynamic stability. Under sedative concentration, it can effectively maintain patient's spontaneous breathing and reduce the incidence of hypoxemia [10]. Ledowski et al. reported that the combined use of 0.5 mg/kg esketamine during general anesthesia induction can improve tracheal intubation conditions [11]. During induction of general anesthesia, esketamine can significantly reduce the dosage of opioid analgesic drugs [12], improve the analgesic effect, reduce the incidence of opioid side effects [13, 14], and have protective effects on the respiratory systems [15]. For the circulatory system, esketamine increases cardiac output in a dose-dependent manner, to some extent counteracting the inhibitory effects of other anesthetic drugs on circulation, thereby maintaining the stability of patient circulation during anesthesia induction [16, 17]. However, the excitatory effect of this cardiovascular system is not always beneficial, and esketamine are known to increase myocardial VO2 and increase MAP, which may pose certain risks when applied to patients with cardiovascular diseases such as hypertension and coronary heart disease.
Propofol combined with remifentanil is the most common target-controlled infusion (TCI) regimen for anesthesia induction [18]. However, the concentration of remifentanil required to reduce endotracheal intubation stress when combined with esketamine is unclear. This trial aimed to determine the effect of esketamine on the quantitative-effect relationship of remifentanil in suppressing the cardiovascular response to endotracheal intubation under propofol anesthesia and to provide a reference for clinical application.
Methods
Ethical statements and study design
This prospective, double-blind, dose-finding clinical trial was approved by the Ethics Committee of Chongqing University Fuling Hospital, Chongqing, China in 2022 (approval number: 2022CQSFLZXYYEC-057) and registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2200064932). All patients signed an informed consent form before the trial.
Female patients who underwent underwent elective gynecological laparoscopic surgery under general anesthesia with endotracheal intubation between November 2022 and April 2023 were included. The following inclusion criteria were applied: patients aged 18–65 years, American Society of Anesthesiologists classification I or II, and 18 kg/m2 ≤ body mass index ≤ 30 kg/m2. The exclusion criteria were as follows: patients anticipating airway difficulties; those with a history of intolerance or allergy to experimental medications; those with a history of severe cardiovascular disease, hypertension or screening systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg, hyperthyroidism, or asthma; and those who were pregnant or lactating.
Introduction and anesthetic management
All patients fasted for at least 8 h, abstained from drinking for 2 h before surgery, and did not receive any preoperative medications. After admission, patients were divided into the saline control group (group C) or esketamine group (group E) using a randomized numerical table method. Electrocardiography, noninvasive blood pressure (BP), heart rate (HR), and pulse oxygen saturation were monitored for all patients (N15 Anesthesia Monitor, Mindray, China). A venous catheter of 20- or 22-gauge was inserted in one of the arms, and 6–8 mL/kg of multiple electrolytes injection was infused as a balanced salt solution for compensatory intravascular volum expansion before anesthesia induction. During surgery, infusion pathways may be increased according to the patient's actual situation if necessary. A mask oxygen dose of 6 L/min was administered for 5 min and anesthesia was induced. Patients in group E were given esketamine 0.3 mg/kg, while patients in group C were administered an equal dose of saline. Patients in both groups were treated using a TCI-I injection pump (Beijing Oriental Chengyitong Technology Co., Ltd.) for TCI of propofol with an effect-site concentration (EC) of 3.0 μg/mL (Marsh model), followed by TCI of remifentanil (20A03171, Yichang Humanwell Pharmaceutical Co., Ltd., Minto model) at a Modified Observer's Assessment of Alertness/Sedation (MOAA/S: No response after painful trapezius squeeze is 0. Responds only after painful trapezius squeeze is 1. Responds only after mild prodding or shaking is 2. Responds only after loud and/or repeated demand is 3. Lethargic response to demand in normal tone is 4. Responds readily to demand in normal tone is 5) of 0 [19, 20]. Rocuronium 0.6 mg/kg was administered after stabilizing the drug concentration. After 2 min, a skilled anesthesiologist judged the loss of blinking reflex and performed tracheal intubation under the guidance of a videolaryngoscopy, and the procedure was completed within 30 s.
During anesthesia induction, if the MAP was < 60 mmHg or 30% below the preoperative basal value, 10 mg of ephedrine was administered intravenously. For HR < 50 beats/min, atropine 0.3 mg was given intravenously. Patients with HR < 50 beats per minute or SBP < 80 mmHg were recorded as adverse events after symptomatic treatment, and their data were not included in the calculation of drug EC50 and EC95, as the changes in blood pressure and heart rate after symptomatic treatment cannot be distinguished as being caused by drug factors or tracheal intubation stimulation. Patients in whom the first endotracheal intubation failed or those who were intubated for more than 30 s were excluded. Participants who did not complete the study or violated the study protocol were also excluded. Due to prolonged or repeated tracheal intubation, there is greater stimulation and more significant changes in heart rate and blood pressure compared to a single smooth tracheal intubation.
Dixon’s up-and-down and sample size
The EC50 of remifentanil was calculated using a modified Dixon up-and-down method. The initial EC of remifentanil was set at 3.0 ng/mL with a concentration gradient of 0.3 ng/mL. If there was a positive response to endotracheal intubation, the target concentration was adjusted upward by one gradient; otherwise, the concentration was decreased by one gradient. According to the simulation study conducted by Stylianou and Flournoy, it is preliminarily estimated that the sample size of 20–40 patients can provide a stable target dose [21]. After obtaining 6 inflection points from positive to negative endotracheal intubation reactions, patient recruitment was terminated. A positive response to endotracheal intubation was defined as SBP 15% above the basal level, HR 15% above the basal level, or the presence of a lacrimal response within 2 min of endotracheal intubation [22].
Blinding
All patients were anesthetized by one investigator, and another investigator assessed them for the presence of a positive response to endotracheal intubation. Neither the anesthesiologist performing endotracheal intubation nor the patient was aware of the anesthesia medication regimen.
Statistical analysis
All statistical analyses were performed using SPSS 21.0 software. The Shapiro–Wilk test was used to assess the normality of continuous variables. Normally distributed measurements were expressed as mean ± standard deviation (x ± S) using an independent samples t-test, and counts were expressed as cases (%) using Fisher’s exact test. EC50, EC95, and the corresponding 95% confidence intervals (CIs) of the drugs were calculated using probit regression analysis. An independent sample t-test was used for comparison between groups of remifentanil EC50 and EC95. A two-sided P< 0.05 was considered statistically significant.
Results
Basic information
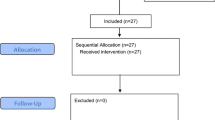
According to Dixon's sequential design requirements, recruitment of patients should be stopped when all groups reach the intersection point of positive and negative results in the 6th endotracheal intubation test. Overall, 42 female patients who underwent elective gynecological laparoscopic surgery under general anesthesia with endotracheal intubation were recruited for this study: 22 in group C and 18 in group E. The difference in the general condition of the patients in the two groups was not statistically significant (P > 0.05) (Table 1). There was no statistically significant difference in the general condition of patients between the positive and negative endotracheal intubation groups (P > 0.05) (Table 2).
EC 50 and EC 95 of remifentanil
Sequential plots of the inhibition of cardiovascular responses to endotracheal intubation in groups E and C are shown in Fig. 1. Dose response curves are shown in Fig. 2. When propofol 3.0 μg/mL was administered by TCI, the EC50 of remifentanil inhibiting the cardiovascular response to endotracheal intubation in group C was 3.91 ng/mL (95% CI, 3.59–4.33 ng/mL) and the EC95 was 4.66 ng/mL (95% CI, 4.27–6.23 ng/mL). In group E, the EC50 of remifentanil was 3.56 ng/mL (95% CI, 3.22–3.99 ng/mL), and the EC95 was 4.31 ng/mL (95% CI, 3.91–5.88 ng/mL). The EC50 and EC95 values of group E were significantly lower than those of group C (P < 0.05).
During the induction of anesthesia, one patient in group C was intubated at a concentration of 3.6 ng/mL, and two at concentrations of 3.9 ng/mL were intubated after receiving additional remifentanil for 5 min due to delayed loss of consciousness [23]. The endotracheal intubation reaction was positive in all three patients. No adverse effects such as hypotension, bradycardia, cough, chest wall rigidity, or postoperative delirium were observed in any patient.
Discussion
Using the improved Dixon up and down method, this study demonstrated that the EC50 of remifentanil inhibiting cardiovascular response to tracheal intubation was 3.91 ng/mL when the target controlled infusion of propofol effect chamber concentration was 3.0 µg/mL. After intravenous injection of 0.3 mg/kg esketamine, the EC50 decreased to 3.56 ng/mL.
As a more precise mode of drug delivery, TCI can rapidly and accurately achieve the set drug EC and maintain the desired depth of anesthesia [24]. Most previous studies have focused on the inhibition of the endotracheal intubation response at half of the plasma drug concentration; however, there are some differences between the EC and plasma drug concentration (Cp). As the plasma is not the anatomical site of action of anaesthetic drugs, a delay (hysteresis) is observed between the time course of the Cp and the drug effect [25]. The reduction in the endotracheal intubation stress response to the concentration of remifentanil when combined with esketamine remains unclear. In the present study, we determined the effect of esketamine on the quantitative relationship of remifentanil in suppressing the cardiovascular response to endotracheal intubation under propofol anesthesia.
EC50 is located at the midpoint of the drug’s quantitative effect curve and best reflects the potency of the drug. In this study, Dixon’s modified sequential method was used, and a small number of samples reflected the potency of the drug, suitable for the study of drugs with a rapid onset of action and short-term evaluation of the effect. The drugs used in the study were all fast-acting clinical anesthetics, and both groups had six crossings; that is, the thresholds crossed the median, which satisfied the conditions of the study design.
Remifentanil, an opioid analgesic, has been proved to effectively blunt the hemodynamic response to endotracheal intubation when administered via injection or infusion. However, the effect of small doses of remifentanil is not obvious, while large doses can cause adverse reactions such as hypotension and bradycardia [26]. Many studies have evaluated the efficacy of remifentanil in TCI for the cardiovascular response to endotracheal intubation. Mustola and Toivonen proposed that the EC50 and EC95 values for remifentanil are 3.17 ng/mL and 3.79 ng/mL, respectively, when propofol is administered to maintain a BIS value between 40 and 60 in patients [27]. Kim et al. used Ce of propofol 3.5 µg/mL for laryngeal mask airway (LMA) insertion requiring EC50 of remifentanil 3.04 ± 0.49 ng/mL [28]. The majority of the aforementioned research centers on identifying the necessary effect chamber concentration of propofol-induced remifentanil for suppressing related stress responses. Nevertheless, there remains a scarcity of studies on esketamine. Consequently, we can only tentatively establish an initial effective chamber concentration of remifentanil at 3.0 ng/mL, relying solely on the available research reports.
In this study, TCI of propofol 3.0 μg/mL for anesthesia induction combined with esketamine can significantly reduce the EC50 and EC95 of endotracheal intubation cardiovascular response inhibition by remifentanil. The combined use of esketamine successfully decreased the required dosage of remifentanil, potentially mitigating its side effects, although this study did not explicitly evaluate this aspect. The primary objective of this investigation was not to assess the incidence of side effects, hence the study's sample size was insufficient to produce statistically significant results regarding this matter. This may be because esketamine can act on NMDAR and opioid receptors to produce analgesia together with remifentanil [29], which reduces the intensity of patients’ stress response to endotracheal intubation and better maintains hemodynamic stability during anesthesia-induced endotracheal intubation and extubation [16]. It is widely recognized that the mitigation of tracheal intubation stress response heavily relies on analgesic agents. When esketamine is administered in combination with remifentanil, the required concentration of remifentanil to suppress tracheal intubation response decreases, primarily due to the synergistic analgesic effect between these two agents. This observation also explains why the measured concentrations of remifentanil in the control group were similar to those reported by Bouillon et al. [18].
There is still controversy over the optimal PK parameter model for propofol [30,31,32]. Yang, X. Y. et al. suggested that the Marsh model induces sedation faster than that using the Schnider model, with no statistically significant differences in hemodynamic changes [33]. Shunsheng C et al. suggested that in the TCI of propofol in Chinese gynecological surgery patients, the accuracy of the Marsh model is higher than that of the Schnider model [34]. Hence, the Marsh model for propofol potentially offers superior advantages among the Chinese population, prompting its selection as the preferred TCI model for propofol in this study. Okuyama et al. suggested that the predicted EC of propofol alone at which 50% of patients did not move with laryngeal mask airway insertion was 3.59 μg/mL [35]. The combined use of remifentanil can reduce the required concentration of propofol, and the use of a combination of low-dose esketamine reduces the propofol dosage [18, 36]. In addition, with reference to the propofol effect chamber concentration adopted in previous studies [28, 35], the target-controlled effector room concentration of propofol was set at 3.0 μg/mL in the study.
Three patients in group C showed delayed loss of consciousness, suggesting that there may be individual differences in the synergistic sedative effect of remifentanil and propofol. To patients experiencing delayed consciousness loss, the use of remifentanil instead of propofol was based on the study's prerequisite of a propofol effect chamber concentration of 3.0 μg/mL. Remifentanil exhibits a synergistic effect with propofol, ensuring smooth induction of anesthesia in patients. During the clinical trail, the patient did indeed enter a state of anesthesia following the administration of remifentanil, and no patient was excluded for severe hypotension or bradycardia after anesthesia induction as specified in the methods section, which may be because esketamine can act on receptors, such as NMDAR, opioid, and monoaminergic, to produce sedative-hypnotic effects and reduce propofol dosage. In addition, the stimulating effect of esketamine on the circulatory system effectively reduces the occurrence of hypotension. Previous studies indicated that low doses of esketamine can produce safe and effective anesthesia [37].
None of the patients included in this study experienced significant adverse effects such as hypotension, bradycardia, or cough, probably because remifentanil itself has less effect on the circulatory system [38] and esketamine excites the sympathetic nerves, dose-dependently increases cardiac output, and better maintains intraoperative hemodynamic stabilization when applied in combination with opioids [16, 39]. Additionally, esketamine has bronchodilating and hyperventilating effects, reduces the incidence and intensity of choking in patients, improves the body’s sensitivity to CO2 [15], and is more suitable for use in patients at risk of respiratory complications.
This study has some limitations. In this study, the depth of anesthesia was meticulously monitored using the MOAA/S score. Intravenous muscle relaxants are administered when the patient's MOAA/S score reached 0, indicatingan absence of response to stimulation, which corresponds to a Richmond Agitation-Sedation Scale (RASS) score of -5, followed by tracheal intubation 2 min later. This stringent protocol ensured that the patient was maintained at an appropriate level of anesthesia throughout the procedure. Sang Yun Cho et al. suggested that increased effect-site concentration of propofol might reduce EC50 and EC95 of remifentanil during endotracheal intubation [3]. There is a close relationship between the response to tracheal intubation and the depth of anesthesia, and this study did not quantitatively monitor the depth of anesthesia due to insufficient funding. Ensuring anesthesia depth solely by ensuring that the patient's MOAA/s score is 0 may have some impact on the research results, which may have a certain impact on the research results. Animal and human studies have shown sex differences in opioid-induced analgesia and related adverse events [40, 41], and differences in gene expression between sexes may be involved in the regulatory mechanisms involved [42], resulting in lower sensitivity to opioids [43] and higher opioid dosage in men than that in women [44]. In addition, esketamine are known to increase myocardial VO2 and increase MAP, which may pose certain risks when applied to patients with cardiovascular diseases. Therefore, patients with cardiovascular diseases were excluded from the clinical trail. Although ketamine has a bronchodilatory effect, its application in asthma patients is somewhat controversial [45]. The focus of this study is on the concentration requirements for drug inhibition of cardiovascular response to tracheal intubation. Asthma patients have higher airway reactivity than that of non-asthma patients. Patients with hyperthyroidism have strong excitability in the nervous system, and generally, their heart rate and blood pressure fluctuate more than those of ordinary patients. The concentration of remifentanil required to suppress cardiovascular response to tracheal intubation in the above two types of patients may be higher. Therefore, they were excluded from the study. In order to exclude the influence of gender and related disease factors, this study included female patients with ASA I/II grade and aged 18–65. However, the pharmacokinetics and pharmacodynamics of the drug are also influenced by other factors such as obesity, internal environmental status, and drug interactions. Therefore, the EC50 of remifentanil required to inhibit the response to endotracheal intubation in male, pediatric, and elderly patients, and those with serious comorbidities need to be further investigated because of their characteristics and changes in pharmacokinetics.
Conclusions
In conclusion, TCI of propofol 3.0 μg/mL for anesthesia induction combined with esketamine can significantly reduce the EC50 and EC95 of endotracheal intubation cardiovascular response inhibition by remifentanil. Reducing the dosage of remifentanil may reduce related drug side effects, while ensuring stable circulation during anesthesia induction in patients. This can provide some reference for anesthesia induction medication in female patients undergoing general anesthesia who require tracheal intubation.
Availability of data and materials
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TCI:
-
Target-controlled infusion
- EC:
-
Effect-site concentration
- EC50 :
-
50% Effective concentration
- EC95 :
-
95% Effective concentration
- CI:
-
Confidence intervals
- NMDAR:
-
N-methyl-D-aspartate receptors
- SBP:
-
Systolic blood pressure
- HR:
-
Heart rate
References
Karanth H, Raveendra US, Shetty RB, Shetty P, Thalanjeri P. Comparative Evaluation between Sevoflurane and Propofol for Endotracheal Intubation without Muscle Relaxants in Pediatric Cleft Surgeries. Anesth Essays Res. 2018;12(2):434–9.
Zou Y, Kong G, Wei L, Ling Y, Tang Y, Zhang L, Huang Q. The effect of intravenous lidocaine on hemodynamic response to endotracheal intubation during sufentanil-based induction of anaesthesia. Anaesthesiol Intensive Ther. 2020;52(4):287–91.
Sang Yun Cho, Chang Wook Lee, Hyung Joon Park, Seong Ho Park, Woo Jae Jeon: Increased Effect-Site Concentration of Propofol Reduces EC50 of Remifentanil for Successful Intubation Using the Shikani Optical Stylet without Neuromuscular Blockade. Soonchunhyang Med Sci 2019; 25(2): 104-109
Ahonen J, Olkkola KT, Verkkala K, Heikkinen L, Järvinen A, Salmenperä M. A comparison of remifentanil and alfentanil for use with propofol in patients undergoing minimally invasive coronary artery bypass surgery. Anesth Analg. 2000;90(6):1269–74.
Kazmaier S, Hanekop GG, Buhre W, Weyland A, Busch T, Radke OC, Zoelffel R, Sonntag H. Myocardial consequences of remifentanil in patients with coronary artery disease. Br J Anaesth. 2000;84(5):578–83.
Dai M, Dou X, Chen M, Yang J, Long J, Lin Y: Strong opioids-induced cardiac, neurologic, and respiratory disorders: a real-world study from 2004 to 2023 based on FAERS. Naunyn-Schmiedeberg's Arch Pharmacol 2023.
Olofsen E, Sigtermans M, Noppers I, Niesters M, Mooren R, Bauer M, Aarts L, Sarton E, Dahan A. The dose-dependent effect of S(+)-ketamine on cardiac output in healthy volunteers and complex regional pain syndrome type 1 chronic pain patients. Anesth Analg. 2012;115(3):536–46.
Olofsen E, Noppers I, Niesters M, Kharasch E, Aarts L, Sarton E, Dahan A. Estimation of the contribution of norketamine to ketamine-induced acute pain relief and neurocognitive impairment in healthy volunteers. Anesthesiology. 2012;117(2):353–64.
Dahan A, Olofsen E, Sigtermans M, Noppers I, Niesters M, Aarts L, Bauer M, Sarton E. Population pharmacokinetic-pharmacodynamic modeling of ketamine-induced pain relief of chronic pain. Eur J Pain. 2011;15(3):258–67.
Huang X, Ai P, Wei C, Sun Y, Wu A: Comparison of the Effects of Esketamine/Propofol and Sufentanil/Propofol on the Incidence of Intraoperative Hypoxemia during Bronchoscopy: Protocol for a Randomized, Prospective, Parallel-Group Trial. J Clin Med 2022, 11(15).
Ledowski T, Wulf H. The influence of fentanyl vs. s-ketamine on intubating conditions during induction of anaesthesia with etomidate and rocuronium. Eur J Anaesthesiol. 2001;18(8):519–23.
Fanta S, Kinnunen M, Backman JT, Kalso E. Population pharmacokinetics of S-ketamine and norketamine in healthy volunteers after intravenous and oral dosing. Eur J Clin Pharmacol. 2015;71(4):441–7.
Wang X, Lin C, Lan L, Liu J. Perioperative intravenous S-ketamine for acute postoperative pain in adults: A systematic review and meta-analysis. J Clin Anesth. 2021;68:110071.
Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet (London, England). 2019;393(10180):1558–68.
Jonkman K, van Rijnsoever E, Olofsen E, Aarts L, Sarton E, van Velzen M, Niesters M, Dahan A. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. 2018;120(5):1117–27.
Kamp J, van Velzen M, Aarts L, Niesters M, Dahan A, Olofsen E. Stereoselective ketamine effect on cardiac output: a population pharmacokinetic/pharmacodynamic modelling study in healthy volunteers. Br J Anaesth. 2021;127(1):23–31.
Yang T, Mudabbar MS, Xu M, Xiang Q, Liu B, Fu Q. The effects of esketamine on blood pressure and hypotension incidence during induction of bariatric surgery: A randomized controlled trial. Medicine. 2023;102(51):e36754.
Bouillon TW, Bruhn J, Radulescu L, Andresen C, Shafer TJ, Cohane C, Shafer SL. Pharmacodynamic interaction between propofol and remifentanil regarding hypnosis, tolerance of laryngoscopy, bispectral index, and electroencephalographic approximate entropy. Anesthesiology. 2004;100(6):1353–72.
Minto CF, Schnider TW, Egan TD, Youngs E, Lemmens HJ, Gambus PL, Billard V, Hoke JF, Moore KH, Hermann DJ, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I Model development Anesthesiology. 1997;86(1):10–23.
Yamada J, Hazama D, Tachihara M, Kawanami Y, Kawaguchi A, Yatani A, Sato H, Mimura C, Katsurada N, Yamamoto M, et al. The utility of bispectral index monitoring in flexible bronchoscopy: A single-center, retrospective observational study. Thoracic cancer. 2022;13(21):3052–7.
Stylianou M, Proschan M, Flournoy N. Estimating the probability of toxicity at the target dose following an up-and-down design. Stat Med. 2003;22(4):535–43.
Albertin A, Casati A, Federica L, Roberto V, Travaglini V, Bergonzi P, Torri G. The effect-site concentration of remifentanil blunting cardiovascular responses to tracheal intubation and skin incision during bispectral index-guided propofol anesthesia. Anesth Analg. 2005;101(1):125–30 (table of contents).
Zhang X WX: Effective target plasma concentration of remifentanil required to prevent tracheal intubation response in 50% of patients anesthetized with propofol by TCI. Chin J Anesthesiol 2006;26(3):204-206.
Gray JM, Kenny GN. Development of the technology for “Diprifusor” TCI systems. Anaesthesia. 1998;53(Suppl 1):22–7.
Abad-Torrent A, Martínez-Vázquez P, Somma J, Hsu YW, Izquierdo E. Remifentanil pharmacodynamics during conscious sedation using algometry: a more clinically relevant pharmacodynamical model. Br J Anaesth. 2022;129(6):868–78.
O’Hare R, McAtamney D, Mirakhur RK, Hughes D, Carabine U. Bolus dose remifentanil for control of haemodynamic response to tracheal intubation during rapid sequence induction of anaesthesia. Br J Anaesth. 1999;82(2):283–5.
Mustola S, Toivonen J. Effect-site concentration of remifentanil attenuating surgical stress index responses to intubation of the trachea. Anaesthesia. 2010;65(6):581–5.
Kim MK, Lee JW, Jang DJ, Shin OY, Nam SB. Effect-site concentration of remifentanil for laryngeal mask airway insertion during target-controlled infusion of propofol. Anaesthesia. 2009;64(2):136–40.
Mion G, Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther. 2013;19(6):370–80.
Barakat AR, Sutcliffe N, Schwab M. Effect site concentration during propofol TCI sedation: a comparison of sedation score with two pharmacokinetic models. Anaesthesia. 2007;62(7):661–6.
Coetzee JF. Allometric or lean body mass scaling of propofol pharmacokinetics: towards simplifying parameter sets for target-controlled infusions. Clin Pharmacokinet. 2012;51(3):137–45.
Masui K, Upton RN, Doufas AG, Coetzee JF, Kazama T, Mortier EP, Struys MM. The performance of compartmental and physiologically based recirculatory pharmacokinetic models for propofol: a comparison using bolus, continuous, and target-controlled infusion data. Anesth Analg. 2010;111(2):368–79.
Yang XY, Zhou ZB, Yang L, Zhou X, Niu LJ, Feng X. Hemodynamic responses during induction: comparison of Marsh and Schnider pharmacokinetic models. Int J Clin Pharmacol Ther. 2015;53(1):32–40.
Shunsheng C, Weiwei L, Changlian W, Caizhu L, Cuihong L. Comparison of accuracy of Marsh model versus Schnider model for propofol target-controlled infusion system. Chinese Journal of Anesthesiology. 2015;12:1466–9.
Okuyama K, Inomata S, Okubo N, Watanabe I. Pretreatment with small-dose ketamine reduces predicted effect-site concentration of propofol required for loss of consciousness and Laryngeal Mask Airway insertion in women. J Clin Anesth. 2011;23(2):113–8.
Chen Miao SX, Dong Gang, Wang Yong, Han Xueping, Yang Jianjun. The effectiveness of a low-dose esketamine versus alfentanil adjunct to propofol sedation during hepatocellular carcinoma radiofrequency ablation in elderly patients. Int J Anesth Resus 2021, 42(12):1255-1259.
Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, Mazzucco C, Hough D, Thase ME, Shelton RC, et al. Efficacy and Safety of Flexibly Dosed Esketamine Nasal Spray Combined With a Newly Initiated Oral Antidepressant in Treatment-Resistant Depression: A Randomized Double-Blind Active-Controlled Study. Am J Psychiatry. 2019;176(6):428–38.
Bolliger D, Seeberger MD, Kasper J, Skarvan K, Seeberger E, Lurati Buse G, Buser P, Filipovic M. Remifentanil does not impair left ventricular systolic and diastolic function in young healthy patients. Br J Anaesth. 2011;106(4):573–9.
Li J, Wang Z, Wang A, Wang Z. Clinical effects of low-dose esketamine for anaesthesia induction in the elderly: A randomized controlled trial. J Clin Pharm Ther. 2022;47(6):759–66.
Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8(5):397–411.
Nasser SA, Afify EA. Sex differences in pain and opioid mediated antinociception: Modulatory role of gonadal hormones. Life Sci. 2019;237:116926.
Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KV, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, et al. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci U S A. 2003;100(8):4867–72.
Madla CM, Gavins FKH, Merchant HA, Orlu M, Murdan S, Basit AW. Let’s talk about sex: Differences in drug therapy in males and females. Adv Drug Deliv Rev. 2021;175:113804.
Wang L, Wu Q, Wang M, Ding M, Cao Y. Gender differences in the effective dose of alfentanil in painless bronchoscopy. J Thorac Dis. 2023;15(1):216–8.
La Via L, Sanfilippo F, Cuttone G, Dezio V, Falcone M, Brancati S, Crimi C, Astuto M. Use of ketamine in patients with refractory severe asthma exacerbations: systematic review of prospective studies. Eur J Clin Pharmacol. 2022;78(10):1613–22.
Acknowledgements
Not applicable.
Funding
This work was supported by the Hospital level cultivation project of Chongqing University Fuling Hospital [grant numbers: Flyyyjkypy2022001] and the Sichuan Province Science and Technology Support Program [grant numbers: 2022YFS0632].
Author information
Authors and Affiliations
Contributions
FZQ and HKY participated in the design of the study, data acquisition, the statistical analysis, the data interpretation, revised and drafted the manuscript. BYP participated in the design of study, the data interpretation and revised the manuscript. XY participated in the data acquisition, the data interpretation, revised and drafted the manuscript. LL revised and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Chongqing University Fuling Hospital, Chongqing, China (approval number: 2022CQSFLZXYYEC-057). All patients signed an informed consent form before the trial.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ziqiang, F., Keyu, H., Yun, X. et al. Effect of esketamine on the EC50 of remifentanil for blunting cardiovascular responses to endotracheal intubation in female patients under general anesthesia: a sequential allocation dose-finding study. BMC Anesthesiol 24, 67 (2024). https://doi.org/10.1186/s12871-024-02454-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-024-02454-4