Abstract
Background
Residual neuromuscular block after using neuromuscular blocking agents is a common and potentially harmful complication of general anesthesia. Neostigmine is a widely used antagonist, but its optimal dose for elderly patients is unclear.
Objectives
To compare the optimal dosage and safety of neostigmine for reversing shallow residual block in elderly patients after cisatracurium-induced neuromuscular block.
Methods
A randomized controlled trial was conducted in 196 elderly patients undergoing non-cardiac surgery under general anesthesia with cisatracurium. Patients were assigned to receive either no neostigmine (control group) or neostigmine at 20 µg/kg, 40 µg/kg or 50 µg/kg when train-of-four (TOF) ratio reached 0.2 at the end of surgery. The primary outcome was the time to reach TOF ratio of 0.9 after administration. Secondary outcomes included TOF ratio at 10 min after administration, postoperative nausea and vomiting, postoperative cognitive impairment and post-anesthesia care unit (PACU) stay time.
Results
The time to reach TOF ratio of 0.9 in the 20 µg/kg, 40 µg/kg and 50 µg/kg groups was significantly shorter than the control group (H = 104.257, P < 0.01), and the time of 40 µg/kg group and 50 µg/kg group was significantly shorter than the 20 µg/kg group (P < 0.001). There was no significant difference between 40 µg/kg and 50 µg/kg groups (P = 0.249). The TOF ratio at 10 min after administration showed similar results. There were no significant differences among groups in postoperative nausea and vomiting, postoperative cognitive impairment or post-operation hospital stay.
Conclusions
Timely use of neostigmine after general anesthesia in elderly patients can significantly shorten time of TOF value reaching 0.9, among which 40 µg/kg dosage may be a more optimized choice.
Trial registration
this study was registered on chictr.org.cn (ChiCTR2100054685, 24/12/2021).
Similar content being viewed by others
Background
Neuromuscular blocking agents (NMBAs) are widely used to provide muscle relaxation for endotracheal intubation, certain modes of mechanical ventilation and surgical procedures. However, with the widespread application of neuromuscular blocking agents in general anesthesia, the residual effects have become one of the important factors of postoperative complications [1,2,3]. At present, the most widely used muscle relaxation antagonists include acetylcholinesterase inhibitors and sugammadex. These intermediate-acting NMBAs, such as cisatracurium, can be metabolized quickly and consequently reduce the incidence of postoperative residual neuromuscular block [4]. However, the pooled rate of residual blockade, defined as a TOF ratio less than 0.90, was 41% with Confidence Interval (25-58%) when studies using intermediate-acting NMBDs were analyzed [5], leading to significant respiratory events (e.g. severe hypoxemia, airway obstruction), pharyngeal function impairment, and even increased mortality [4, 6,7,8,9].
Previous studies have shown that the optimal dose of neostigmine for reversal of minimal NMB (TOFr = 0.5) in adults is 40 µg/kg [10]. Nevertheless, compared to the adult, the elderly changes physiologically, including a reduced glomerular filtration rate, increased body fat, decrease in lean muscle mass, and decrease in total body water [11, 12], which may alter the pharmacokinetics and metabolism of drugs. A larger dose may be required to antagonize the residual block of muscle relaxants in the elderly [13]. However, a larger dose of neostigmine may increase the occurrence of side effects such as bradycardia and increased secretions. Thus, it is urgent to recommend the optimal dose of neostigmine for antagonizing the neuromuscular blocking effect of cisatracurium in the elderly with general anesthesia. Accordingly, we aimed to explore the optimal dosage and safety of neostigmine to reverse shallow residual block from a TOF ratio of 0.2 in elderly patients.
Methods
Study design and patient selection
This study was approved by the Ethics Committee of the Third Xiangya Hospital. Written informed consent was obtained from all patients. Inclusion criteria were: aged 60 to 85 years, American Society of Anesthesiology (ASA) physical status 1 to 3, and scheduled for elective surgery under general anesthesia with cisatracurium for tracheal intubation. Exclusion criteria were: BMI < 18.5 kg/m2 or BMI ≥ 28 kg/m2, significant hepatic or renal dysfunction (glutamic-pyruvic transaminase/glutamic oxaloacetic transaminase > 80 U/L, creatinine > 104 umol/L), family history of malignant hyperthermia, known allergy to one of the drugs used in this protocol, or recent use of sedatives, anti-depressants.
Patients were randomly assigned into four groups according to the dose of neostigmine used, using a computer generated list of random numbers. Neuromuscular block was induced with cisatracurium and neostigmine 0 µg/kg (Normal Saline, NS), 20 µg/kg, 40 µg/kg or 50 µg/kg was administered at a TOF ratio of 0.2 to reverse the block. Anaesthesia nurses who were not involved in the care of the patients helped prepare the study drug according to randomisation.
Procedure
On arrival at the operating room, an intravenous cannula was inserted in the forearm vein of the patient, and standard anesthesia monitoring (noninvasive blood pressure, electrocardiogram, and oxygen saturation) were established and anesthesia depth was monitored using Bispectral Index (BIS, Medtronic, Minneapolis, MN, USA). Anesthesia was induced with sufentanil (0.5 µg/kg) and etomidate (0.2–0.3 mg/kg), cisatracurium (0.15–0.2 mg/kg). Anaesthesia was maintained by a continuous inhalation of 1% sevoflurane and infusion of propofol (3–4 mg/kg/h) and remifentanil (0.5-1.0 µg/kg/min); the infusion speed was adjusted to maintain the BIS between 40 and 60. Cisatracurium was added as required to facilitate the completion of the surgery. After tracheal intubation, ventilation was controlled to maintain arterial oxygen saturation at 96% or higher and normocapnia. Body temperature was maintained at 36.0 °C or higher. Sevoflurane was stopped about 1 h before the end of the surgery and the flow of fresh gas was increased to ensure no residual of inhalational anesthetics at the end of the surgery. Ondansetron 4 mg was administered intravenously 0.5 h before the end of the surgery to prevent nausea and vomiting. An additional 10–15 µg of sufentanil was administered at the end of surgery for postoperative pain management.
Neuromuscular function was evaluated by TOF-Watch SX acceleration muscle relaxation monitor (Organon, Dublin, Ireland). After the skin had been cleaned carefully, two surface skin electrodes were attached over the ulnar nerve proximal to the wrist at a distance of 3–6 cm. After immobilising the forearm, the acceleration transducer was fixed firmly to the volar side of the distal phalanx of the thumb on a small elastic hand adapter (TOF-Watch Handadapter; Schering-Plough, Swords, Ireland). The monitoring was initiated after induction, but before cisatracurium administration. The device was calibrated automatically after a 50-Hz tetanic stimulation for 5 s and a stable baseline (< 5% change in TOF ratio) was recorded. Normalized TOF values were calculated and recorded according to baseline TOF values. TOF stimulus was applied every 60 s until a TOF ratio greater than or equal to 0.9. When a TOF ratio of 0.2 was reached two times consecutively, a pre-determined dose of neostigmine provided by Sine Jinzhu Pharmaceutical company (Shanghai, China) was administered according to the patient group (NS, 20 µg/kg, 40 µg/kg or 50 µg/kg), accompanied by glycopyrrolate in an 1:5 ratio. The time from the administration of neostigmine to a TOF ratio of 0.9 was recorded. The proportion of patients with TOF value ≥ 0.9 10 min after neostigmine administration; the incidence of nausea and vomiting in patients on postoperative day 1-day 3; the proportion of patients with Mini-Mental State Examination (MMSE) score decrease ≥ 3 points on postoperative day 3; and PACU stay time after neostigmine administration were followed up.
Statistical analysis
A sample size was calculated based on the primary outcome variable (the time from the administration of neostigmine to a TOF ratio of 0.9). To achieve a power of 0.8 with an alpha level of 0.05 and an effect size of f = 0.25, and considering a 10% drop out rate, a total sample size of N = 200 was needed. SPSS 22.0 software was used for statistical analysis. Count data were expressed as composition ratio; normally distributed measures were expressed as mean ± standard deviation, and t-test was used for comparison between two groups and one-way ANOVA for comparison between multiple groups; non-normally distributed measures were expressed as median (interquartile range), and Kruskal-Wallis H rank sum test was used for comparison between multiple groups; while categorical data were expressed as numbers with percentages and were compared using the Chi-square test. P < 0.05 was considered to indicate statistical difference.
Results
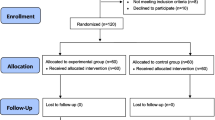
From January 2022 to November 2022, 240 ASA I-III patients aged from 60 to 85 who were scheduled for elective non-cardiac surgery under general anesthesia were enrolled, of which 40 patients were excluded due to contraindications (n = 18), allergies (n = 12) or declined to participate (n = 10). 200 patients completed randomization and participated in the study. 4 patients were excluded due to protocol violation (3 in NS group and 1 in 20 µg/kg group B). The number of patients included in the final statistical analysis was 196 (Fig. 1).

(adapted from http://www.consort-statement.org/)
CONSORT 2010 flow diagram.
Comparison of general data and perioperative data between the groups
Table 1 summarized baseline characteristics and perioperative data of patients. There were no significant differences in the age, gender, BMI between four groups (P > 0.05, Table 1), which means baseline characteristics were generally consistent between groups. As for the perioperative data, there was no significant difference in the amount of muscle relaxant cisatracurium, surgery duration, anesthesia duration among the four groups (P > 0.05, Table 1). Intergroup comparison suggests that patients from 40 µg/kg group had more blood loss than those from control group (P < 0.05). The type of surgery is comparable among the groups.
Comparison of results of postoperative muscle relaxation monitoring
As listed in Table 2, the time to reach TOF value of 0.9 after administration of neostigmine, TOF value at 10 min after administration of neostigmine and PACU stay time were significantly different among the four groups of patients (H = 104.257, P < 0.01). In detail, intergroup comparison showed that the time to reach TOF value of 0.9 in 20 µg, 40 and 50 µg groups was significantly shorter than that in NS group after administration of neostigmine (all P < 0.01); meanwhile, the time to reach TOF value of 0.9 in 40 and 50 µg groups were further significantly reduced compared with that in 20 µg group (P = 0.032 and P < 0.001, respectively), but there was no significant difference between 40 and 50 µg groups (P = 0.249) (Fig. 2).
Similar to the results of time to reach TOF value of 0.9, TOF value at 10 min after administration in 20 µg, 40 and 50 µg groups was significantly increased compared with that in control group (H = 93.351, P < 0.01); meanwhile, TOF value at 10 min after administration in 40 and 50 µg groups was further significantly increased compared with that in 20 µg group (P = 0.049 and P < 0.001,respectively), but there was no significant difference between 40 and 50 µg groups (P = 0.345) (Fig. 3).
In addition, we evaluated the postoperative outcomes of each group. As shown in the Table 2, PACU stay time after neostigmine was different among the four groups (H = 10.671, P < 0.01). Intergroup analysis suggests that 50 µg/kg may shortern PACU stay time compared with normal saline (P = 0.024). We found no difference among the groups in the incidence of cognitive decline, postoperative nausea and vomiting; the length of postoperative hospital stay showed no difference either.
Analysis of time of TOF ≥ 90% after neostigmine (min). Time of 20 µg, 40 and 50 µg groups was significantly shorter than that in NS group after administration of neostigmine (all P < 0.001); Time of 40 and 50 µg groups was further significantly reduced compared with that in 20 µg (P = 0.032 and P < 0.001,respectively);yet the time between 40 and 50 µg was comparable (P = 0.249); *P < 0.05
Analysis of TOF value 10 min after neostigmine (%). TOF value of 20 µg, 40 and 50 µg groups was significantly higher than that in NS group after administration of neostigmine (P < 0.001); value of 40 and 50 µg groups was further significantly increased compared with that in 20 µg (P = 0.049 and P < 0.001,respectively);yet the difference between 40 and 50 µg was not significant (P = 0.345);*P < 0.05
Discussion
This study explored the optimal dose of neostigmine in elderly patients to reverse the TOF value to 0.9. The results indicated that the dose of 40 µg/kg may be an optimized choice and did not increase the incidence of postoperative cognitive impairment or PONV.
Incomplete neuromuscular recovery after general anesthesia is a common complication in patients after general anesthesia, with an incidence ranging from 31 to 64% [5, 14,15,16]. Neostigmine, as an acetylcholinesterase inhibitor, can bind to acetylcholinesterase like acetylcholine, but the binding is firm and hydrolysis is slow, which makes acetylcholinesterase lose its activity. The released neurotransmitter acetylcholine accumulates in the synaptic gap, thereby exciting skeletal muscle and achieving the effect of antagonizing non-depolarizing muscle relaxants.
Elderly patients are prone to residual muscle relaxation due to organ function degeneration and physiological changes that lead to slow metabolism of neuromuscular blockers [13]. Related guidelines and consensus also recommend intravenous administration of neostigmine for antagonism under the premise of no contraindications; however, the recommended dose is no different from that of normal adults, while large doses may lead to an increase in adverse effects of neostigmine (e.g. cause nausea, vomiting, convulsions, coma, slurred speech, anxiety, bradycardia and other symptoms). Therefore, clinical anesthesiologists maybe reduce or even avoid using neostigmine from a safety reason.
The results of this study show that neostigmine was used for antagonism at the end of surgery, and even a small dose (20 µg/kg) can significantly accelerate muscle function recovery; but 40 µg/kg can achieve the purpose of rapid reversal of muscle blockade effect, further increasing the dose (50 µg/kg) cannot significantly shorten the time for muscle recovery. It suggested that a dose of 40 µg/kg of neostigmine may be an optimized choice to reverse the effects of muscle relaxants quickly, without increasing postoperative complications.
Previous studies have explored the dose of neostigmine required for reversal of neuromuscular blockade. Preault A et al.[17] concluded that the neostigmine 20 µg/kg was sufficient to successfully reverse the TOF ratio from 0.4 to 0.9 within 10 min [9]. Similar result was also observed in another study conducted by Fuchs-Buder T [18]. However, these studies did not evaluate the postoperative outcome after the use of neostigmine, the conclusions still need to be further verified. Furthermore, consistent with our study, the results of E. S. Choi et al.[10] also showed that 40 µg/kg dosage of neostigmine may be a better choice. Nevertheless, the population of their study were adults aged 20–70 years (while our study was 60–85 years), the results may not be applicable to older patients. Meanwhile, our study also shows that in balanced anesthesia, 40 µg/kg of neostigmine is still the appropriate dosage to reverse neuromuscular blockade after adequate sevoflurane clearance.
Additionally, our study found that the use of neostigmine to antagonize muscle relaxation at the end of surgery did not significantly shorten the postoperative PACU stay time of patients, which may be affected by confounding factors such as surgical type, analgesic sedative drugs and wound drainage at the end of surgery. Subsequent further trials should limit and standardize trial conditions, reduce related confounding factors and increase sample size to clarify the role of neostigmine in shortening PACU stay.
Conclusion
Timely use of neostigmine after general anesthesia in elderly patients can significantly shorten time of TOF value reaching 0.9, among which 40 µg/kg dosage may be a more optimized choice.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kopman AF, Yee PS, Neuman GG. Relationship of the train-of-four fade ratio to clinical signs and symptoms of residual paralysis in awake volunteers. Anesthesiology. 1997;86(4):765–71.
Sundman E, Witt H, Olsson R, Ekberg O, Kuylenstierna R, Eriksson LI. The incidence and mechanisms of pharyngeal and upper esophageal dysfunction in partially paralyzed humans: pharyngeal videoradiography and simultaneous manometry after atracurium. Anesthesiology. 2000;92(4):977–84.
Murphy GS, Szokol JW, Marymont JH, Greenberg SB, Avram MJ, Vender JS. Residual neuromuscular blockade and critical respiratory events in the postanesthesia care unit. Anesth Analg. 2008;107(1):130–7.
Bronsert MR, Henderson WG, Monk TG, Richman JS, Nguyen JD, Sum-Ping JT, Mangione MP, Higley B, Hammermeister KE. Intermediate-Acting Nondepolarizing Neuromuscular blocking agents and risk of postoperative 30-Day morbidity and mortality, and long-term survival. Anesth Analg. 2017;124(5):1476–83.
Naguib M, Kopman AF, Ensor JE. Neuromuscular monitoring and postoperative residual curarisation: a meta-analysis. Br J Anaesth. 2007;98(3):302–16.
Raval AD, Uyei J, Karabis A, Bash LD, Brull SJ. Incidence of residual neuromuscular blockade and use of neuromuscular blocking agents with or without antagonists: a systematic review and meta-analysis of randomized controlled trials. J Clin Anesth. 2020;64:109818.
Xará D, Santos A, Abelha F. Adverse respiratory events in a post-anesthesia care unit. Arch Bronconeumol. 2015;51(2):69–75. English, Spanish.
Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Shear TD, Vender JS, Parikh KN, Patel SS, Patel A. Residual neuromuscular block in the Elderly: incidence and clinical implications. Anesthesiology. 2015;123(6):1322–36.
Broens SJL, Boon M, Martini CH, Niesters M, van Velzen M, Aarts LPHJ, Dahan A. Reversal of partial neuromuscular block and the ventilatory response to Hypoxia: a randomized controlled trial in healthy volunteers. Anesthesiology. 2019;131(3):467–76.
Choi ES, Oh AY, Seo KS, Hwang JW, Ryu JH, Koo BW, Kim BG. Optimum dose of neostigmine to reverse shallow neuromuscular blockade with rocuronium and cisatracurium. Anaesthesia. 2016;71(4):443–9.
McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev. 2004;56(2):163–84.
Thürmann PA. Pharmacodynamics and pharmacokinetics in older adults. Curr Opin Anaesthesiol. 2020;33(1):109–13.
McCarthy GJ, Cooper R, Stanley JC, Mirakhur RK. Dose-response relationships for neostigmine antagonism of vecuronium-induced neuromuscular block in adults and the elderly. Br J Anaesth. 1992;69(3):281–3.
Stewart PA, Liang SS, Li QS, Huang ML, Bilgin AB, Kim D, Phillips S. The impact of residual neuromuscular blockade, oversedation, and hypothermia on adverse respiratory events in a Postanesthetic Care Unit: a prospective study of prevalence, predictors, and outcomes. Anesth Analg. 2016;123(4):859–68.
Yu B, Ouyang B, Ge S, Luo Y, Li J, Ni D, Hu S, Xu H, Liu J, Min S, Li L, Ma Z, Xie K, Miao C, Wu X. RECITE–China investigators. Incidence of postoperative residual neuromuscular blockade after general anesthesia: a prospective, multicenter, anesthetist-blind, observational study. Curr Med Res Opin. 2016;32(1):1–9.
Thilen SR, Weigel WA, Todd MM, et al. 2023 American Society of Anesthesiologists practice guidelines for monitoring and antagonism of neuromuscular blockade: a report by the American Society of Anesthesiologists Task Force on Neuromuscular Blockade. Anesthesiology. 2023;138(1):13–41.
Fuchs-Buder T, Meistelman C, Alla F, Grandjean A, Wuthrich Y, Donati F. Antagonism of low degrees of atracurium-induced neuromuscular blockade: dose-effect relationship for neostigmine. Anesthesiology. 2010;112(1):34–40.
Preault A, Capron F, Chantereau C, Donati F, Dimet J. Under sevoflurane anaesthesia, a reduced dose of neostigmine can antagonize a shallow neuromuscular block: a double-blind, randomised study. Anaesth Crit Care Pain Med. 2016;35(4):269–73.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Yan Liao and Jianbin Tong designed the study and collected the data. Mengya Cao, Yangwen Ou analyzed the data and Huifan Huang, Yan Liao drafted the manuscript. Mengya Cao and Jianbin Tong provided critical feedback on the study design, data analysis, and manuscript revisions.All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of the Ethics Committee of the Third Xiangya Hospital (reference number [R22008]) and written informed consent was obtained from all participants. The authors declare that all experiments were performed in accordance with relevant guidelines and regulations (such as the Declaration of Helsinki).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cao, M., Huang, H., Tong, J. et al. Optimal dose of neostigmine antagonizing cisatracurium-induced shallow neuromuscular block in elderly patients: a randomized control study. BMC Anesthesiol 23, 269 (2023). https://doi.org/10.1186/s12871-023-02233-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-023-02233-7