Abstract
Background
In trigger-free anesthesia a volatile anesthetic concentration of 5 parts per million (ppm) should not be exceeded. According to European Malignant Hyperthermia Group (EMHG) guideline, this may be achieved by removing the vapor, changing the anesthetic breathing circuit and renewing the soda lime canister followed by flushing with O2 or air for a workstation specific time. Reduction of the fresh gas flow (FGF) or stand-by modes are known to cause rebound effects. In this study, simulated trigger-free pediatric and adult ventilation was carried out on test lungs including ventilation maneuvers commonly used in clinical practice. The goal of this study was to evaluate whether rebounds of sevoflurane develop during trigger-free anesthesia.
Methods
A Dräger® Primus® was contaminated with decreasing concentrations of sevoflurane for 120 min. Then, the machine was prepared for trigger-free anesthesia according to EMHG guideline by changing recommended parts and flushing the breathing circuits using 10 or 18 l⋅min− 1 FGF. The machine was neither switched off after preparation nor was FGF reduced. Simulated trigger-free ventilation was performed with volume-controlled ventilation (VCV) and pressure-controlled ventilation (PCV) including various ventilation maneuvers like pressure support ventilation (PSV), apnea, decreased lung compliance (DLC), recruitment maneuvers, prolonged expiration and manual ventilation (MV). A high-resolution ion mobility spectrometer with gas chromatographic pre-separation was used to measure sevoflurane in the ventilation gas mixture in a 20 s interval.
Results
Immediately after start of simulated anesthesia, there was an initial peak of 11–18 ppm sevoflurane in all experiments. The concentration dropped below 5 ppm after 2–3 min during adult and 4–18 min during pediatric ventilation. Other rebounds of sevoflurane > 5 ppm occurred after apnea, DLC and PSV. MV resulted in a decrease of sevoflurane < 5 ppm within 1 min.
Conclusion
This study shows that after guideline-compliant preparation for trigger-free ventilation anesthetic machines may develop rebounds of sevoflurane > 5 ppm during typical maneuvers used in clinical practice. The changes in rate and direction of internal gas flow during different ventilation modes and maneuvers are possible explanations. Therefore, manufacturers should provide machine-specific washout protocols or emphasize the use of active charcoal filters (ACF) for trigger-free anesthesia.
Similar content being viewed by others
Introduction
Malignant hyperthermia (MH) is a rare neuromuscular disorder, in which genetically predisposed individuals develop life-threatening metabolic crises [1] during general anesthesia caused by triggering substances like volatile anesthetics or succinylcholine. Patients with known or suspected increased risk of MH should therefore not be exposed to triggering substances. A trigger-free anesthesia is recommended instead. The Malignant Hyperthermia Association of the United States (MHAUS) [2] and the European Malignant Hyperthermia Group (EMHG) [3] both recommend that trace gas concentrations of any volatile anesthetic should not exceed 5 ppm during trigger-free anesthesia. But anesthetic machines continuously emit volatile anesthetics even after removal of the vaporizer because the substances are adsorbed and desorbed by rubber and plastic components inside the machine [4, 5]. Therefore, three different methods of preparation for trigger-free anesthesia are recommended by the EMHG and MHAUS: (1) Use of a dedicated machine that has never been contaminated with volatile anesthetics. (2) Use of active charcoal filters (ACF). (3) Washout method: Changing breathing circuits, soda lime canister and breathing system of the workstation with new uncontaminated components or autoclaved components and flushing the machine for a workstation specific time with O2 or air [3].
After preparation with the washout method the fresh gas flow (FGF) should not be reduced [6,7,8,9] and the machine should not be set to stand-by mode [6, 8, 10, 11]. In literature and expert opinion, it is widely assumed that after sufficient preparation of the anesthetic machines the concentration of volatile anesthetic would remain below 5 ppm during trigger-free anesthesia [7, 8, 12,13,14] but it was never tested. In this study, we prepared the anesthetic machines using the wash out method in accordance with EMHG guideline and subsequently simulated adult and pediatric trigger-free anesthesia including different ventilation maneuvers that are common in clinical practice. The goal of this laboratory study was to evaluate whether the anesthetic machine emits a sevoflurane concentration > 5 ppm during simulated trigger-free ventilation.
Methods
Contamination phase
A Dräger® Primus® was contaminated with sevoflurane for a total of 120 min as following: First, a test lung was ventilated with 8% sevoflurane using manual ventilation (MV) for 10 min in 4 l⋅min− 1 FGF, a tidal volume (VT) of 500 ml and a respiratory rate (RR) of 12 breaths min− 1. This was followed by 3% sevoflurane in 1 l⋅min− 1 FGF with continued MV for 20 min, pressure controlled ventilation (PCV) for 45 min and finally volume controlled ventilation (VCV) for 45 min. This contamination procedure was chosen to resemble the contamination during a typical course of a general anesthetic procedure with sevoflurane. Other settings during machine ventilation were: VT 500 ml, RR 12⋅breaths min− 1, positive end-expiratory pressure (PEEP) 5 mbar, inspired to expired time ratio (I:E) of 1:1.9. See Fig. 1 for an overview of the study protocol.
Trigger-free preparation with washout method
After contamination the anesthetic machine was prepared for trigger-free anesthesia according to the EMHG guideline [3]: The vapor was removed and new, uncontaminated components (CO2-line, soda lime canister, breathing hoses, breathing bag, test lung) as well as freshly autoclaved components (breathing system) were assembled. Afterwards the machine was flushed for 45 min at 18 l⋅min− 1 FGF or 90 min at 10 l⋅min− 1 FGF as recommended in the EMHG guideline [3]. Other settings were: VCV, VT 500 ml, RR 12 breaths⋅min− 1, PEEP 5 mbar, I:E 1:1.9. At the end of the preparation process sevoflurane concentration was measured for benchmark recording.
Adult protocol
A trigger-free anesthesia was simulated by ventilating a new test-lung using PCV, VCV, MV, and PSV:
First, the Y-piece of the breathing circuit was placed onto the circuit plug and the machine was set to spontaneous breathing mode with the adjustable pressure limiting valve (APL) open at 10 or 18 l⋅min− 1 FGF for 15 min respectively. This should simulate the time required for a patient to arrive in the operating theatre after preparation. Afterwards, the test lung was attached and the simulated ventilation started using PCV (VT 500 ml, RR 12 breaths⋅min− 1, PEEP 5 mbar, I:E 1:1.9) for 10 min. Various ventilation maneuvers followed: First, apnea phase for 2 min in spontaneous mode (apnea) to resemble breath-hold maneuvers needed during thoracic surgery or during magnet resonance scanning. Then, a manual recruitment maneuver was conducted. This was followed by simulating a decreased lung compliance (DLC) as seen in bronchospasm or insufficient depth of anesthesia. Therefore, a weight of 1600 g was put on the test lung and prolongation of expiration time. Between each ventilation maneuver the machine was set back to PCV for 2 min to normalize potential rebounds. Afterwards the protocol was repeated using the VCV mode with a pressure limit (Pmax) of 30 mbar starting at the 15 min interval simulating the wait for the patient’s arrival. To simulate the end of anesthesia with triggering of spontaneous breathing we used PSV with backup frequency of 3 breaths⋅min− 1 and a tidal volume of 250 ml for 5 min followed by manual ventilation with APL 12 mbar, RR 12 breaths⋅min− 1, VT 500 ml. Table 1 provides an overview and further information of the adult procedure protocol.
Pediatric protocol
To simulate pediatric anesthesia of a 1-year-old 10 kg patient, the following changes were made compared to the adult protocol: at PCV and VCV, RR was set to 30 breaths⋅min− 1, the inspiratory time to 0.7 s, and VT to 70 ml (7 ml·kg− 1 body weight). The weight, which was put on top of the test lung during the simulated decreased lung compliance, was reduced to 540 g. No recruitment maneuver was simulated as this is not considered as good clinical practice in pediatric anesthesia. At PSV, the backup frequency was set to 10 breaths⋅min− 1 and VT was set to 35 ml to simulate induce spontaneous breathing attempts at the end of anesthesia. Concerning manual ventilation, VT was set on 100 ml and RR on 20 breaths min− 1. Table 2 shows details of the pediatric procedure protocol.
Detecting method
Sevoflurane concentration was detected using a high-resolution (resolving power of RP = 90) ion mobility spectrometer (IMS) with gas chromatographic (GC) pre-separation, which was built at Leibniz University Hannover, and calibrated using a Vici® Dynacalibrator® Model 150 permeation oven and a gas mixing system for generating defined concentrations. The gas chromatographic pre-separation (GC temperature 50 °C, 10 m GC standard capillary column (Restek™, RTXTM-volatiles, inner diameter 530 μm, film thickness 2 μm)) separates a 10 µl gas sample into its components depending on their substance specific retention time. To increase sample rate, the method of multiple injections in a single experimental run (MISER) was applied to the GC pre-separation in which the subsequent sample is injected before all components of the previous injection elute from the separation column. This leads to the elution of the first analyte of the following chromatogram directly after the last analyte of the previous chromatogram [15,16,17,18]. Thereby, the sample frequency could be increased to 3 min− 1.
After pre-separation the analyte (sevoflurane) is ionized in the IMS and injected into the drift region where the ions move in an electric field in a defined drift gas and are separated by their ion mobility. When the ionized analytes reach the detector, a current results depending on the amount of ionized analytes. Plotting the current over time gives the ion mobility spectrum. The IMS was developed to detect concentrations at the range of parts per trillion (ppt) [19]. Therefore, we added a dilution stage to dilute the sample in real time when the maximum capacity of die IMS is reached. Sevoflurane concentrations could be detected between 1 and 50 ppm at basic dilution settings. The standard deviation for concentrations at 5 ppm was ± 0.172 ppm. The sevoflurane sample was taken directly from the heat and moisture exchanger (HME) of the breathing circuits (Fig. 2).
Experimental setup and gas flow diagram of Dräger® Primus®, modified from Drägerwerk AG & Co, Lübeck, Germany with kind permission. APL adjustable pressure limiting, HME heat and moisture exchanger, PEEP positive endexpiratory pressure, pmax maximum pressure, V gas flow sensor, P pressure sensor, IMS ion mobility spectrometer
Results
At the end of the washout preparation sevoflurane concentration in all experiments was 1.476 ppm ± 0.784 ppm. Minimum and Maximum sevoflurane concentrations during different maneuvers are shown in Table 3.
Adult protocol
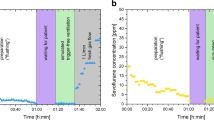
During the first minutes of trigger-free adult PCV the sevoflurane concentration showed a peak of 14 ppm (at 10 l⋅min− 1 FGF) and 15 ppm (at 18 l⋅min− 1 FGF) respectively. Afterwards the concentration dropped within 2–3 min below 5 ppm (see Fig. 3a, c). Peaks > 5 ppm also occurred during the first minutes of VCV as seen in Fig. 3b, d. During PSV a constant increase of sevoflurane concentration up to 4.5 ppm was noted without reaching a plateau within 5 min (see Fig. 3b, d). Manual Ventilation led to a sharp decrease in sevoflurane concentrations.
Results of adult experiments. ppm parts per million. a) 10 l·min− 1 fresh gas flow, pressure controlled ventilation b) 10 l·min− 1 fresh gas flow, volume controlled ventilation c) 18 l·min− 1 fresh gas flow, pressure controlled ventilation d) 18 l·min− 1 fresh gas flow, volume controlled ventilation
Pediatric protocol
Within the first minute of trigger-free pediatric PCV the sevoflurane concentration showed a peak of 18 ppm (at 10 l⋅min− 1 FGF) and 15 ppm (at 18 l⋅min− 1 FGF). During the experiment the concentration dropped below 5 ppm at ventilation with 18 l⋅min− 1 FGF after 5 min but remained > 5 ppm for 18 min at ventilation with 10 l⋅min− 1 FGF (see Fig. 4a, c). Other peaks > 5 ppm were recorded during VCV and decreased lung capacity. During PSV sevoflurane increased to a maximum of 6 ppm and no plateau was reached within experimental time. Manual ventilation led to a sharp decrease of sevoflurane in all experiments (see Fig. 4b, d).
Result of pediatric experiments. ppm parts per million. a) 10 l·min− 1 fresh gas flow, pressure controlled ventilation b) 10 l·min− 1 fresh gas flow, volume controlled ventilation c) 18 l·min− 1 fresh gas flow, pressure controlled ventilation d) 18 l·min− 1 fresh gas flow, volume controlled ventilation
Discussion
After preparation for trigger-free anesthesia using the washout method in accordance with EMHG recommendations the residual sevoflurane concentrations were 1.476 ppm ± 0.784 ppm. However, during simulated trigger-free anesthesia rebounds of sevoflurane > 5 ppm were detected during multiple ventilation modes and maneuvers, even though standby mode of the machine or FGF reduction were avoided after preparation. This can be explained by the different gas flow directions and rates inside the anesthetic machine resulting in different ventilation modes: The anesthetic machine used in this study operates a fresh gas decoupling valve to prevent dependency of tidal volume on FGF. Thereby the inspiratory part of the internal circuitry is flushed only intermittently during the respiratory cycle [8]. As a result, in our experiments the internal gas flow has been changed radically during simulated waiting for the patient: by placing the Y-piece of the breathing circuit onto the circuit plug, the FGF was directed through the absorber in direction of the scavenging system, leaving all parts from the fresh gas decoupling valve to the PEEP valve effectively unflushed (See Fig. 2). This may explain the initial rebounds observed. Other rebounds developed later during the simulated anesthesia. All rebound effects observed were higher during pediatric ventilation compared to adult ventilation and were detected even at maximum FGF (18 l⋅min− 1). Especially, the sevoflurane concentration in the pediatric 10 l⋅min− 1 FGF experiment was > 5 ppm during most of the experimental time.
Because of insufficient literature, EMHG and MHAUS guidelines do not differentiate between adult and pediatric trigger-free anesthesia. MHAUS generally recommends keeping the FGF at a minimum of 10 l⋅min− 1 at all times to avoid rebound effects. In this study the effort failed: even at 18 l⋅min− 1 FGF relevant sevoflurane rebounds occurred during simulated ventilation of a 10 kg pediatric patient. It should be highlighted, that a low FGF in pediatric anesthesia is strongly recommended along with breathing system filters to prevent loss of heat and airway moisture [20, 21], which leads to conflicting recommendations regarding trigger-free pediatric anesthesia.
Clinical relevance of rebounds
Are the detected rebounds clinically relevant? Unfortunately, it is still unknown which residual concentration of volatile anesthetics is safe for MH suspected patients to breathe in. First, MH suspected individuals are a heterogenous group affected by different genetic causes: Until now, there are 50 known mutations in the RYR1 and CACNA1S genes accepted as diagnostic mutations by the EMHG. More gene loci are currently under investigation (STAC3) [22]. Second, there are epigenetic effects that are not fully understood: a male predominance of clinical MH crises and diagnosis after muscle biopsy was repeatedly reported [23,24,25,26], other factors include pre-operative exercise, pyrexia [27] and body mass index [28]. Therefore, threshold levels of volatile anesthetics are thought to be highly individual.
Still, acute MH is believed to be a dose depending process; therefore, it may be unlikely that a short exposition to volatile anesthetic trace gas concentrations in the low ppm range would cause an acute MH crisis. However, there are numerous case reports in which MH suspected patients developed severe MH-like reactions even in the absence of general anesthesia [29,30,31]. Until now, it is not understood which MH suspected patients are at risk of such episodes and consequently EMHG and MHAUS both chose < 5 ppm of any volatile anesthetic concentration in trigger-free anesthesia for safety reasons. Using this definition, the tested anesthetic machine did not meet the requirements for trigger-free ventilation after preparation with the EMHG guideline compliant washout method. Until more data is available, it should be assumed that other anesthetic machines by other manufactures might show similar effects.
Limitations
There are some limitations to this study: First, the trigger-free anesthesia was performed on test lungs. Effects like heat, moisture, or emission of metabolic waste products from a patient were not simulated. Second, all experiments were performed on one sevoflurane contaminated anesthesia machine. Other machines of the same type may lead to different results, depending on long term contamination levels with volatile anesthetics, state of wear, age of plastic and rubber components. Third, all maneuvers during the simulated trigger-free anesthesia were performed in sequence during the experiment to resemble a real anesthetic procedure. Therefore, sevoflurane peaks during different maneuvers might be a result of a prior maneuver or ventilation mode.
Conclusion
Despite successful removal of sevoflurane concentration below 5 ppm using the washout method in accordance with EMHG recommendations, the anesthetic machine was in fact not clean but emitted continuously small amounts of sevoflurane. This led to relevant rebounds during simulated trigger-free ventilation even at maximal FGF (18 l⋅min− 1). Common features of the ventilation maneuvers that led to rebounds were low minute ventilation and low VT as well as no ventilation such as during the waiting period at the beginning of the maneuvers. In the tested anesthetic machine, a fraction of the FGF is directed through the manual ventilation bag, even during mechanical ventilation. It can be assumed that the rebounds observed in this experiment may be higher in anesthetic machines that separate the manual ventilation part from the FGF during mechanical ventilation. In Summary, standby mode should always be avoided, and flushing the machine by ventilating a test lung with air or O2 should be continued until the patient arrives in the operating theatre. We further emphasize the necessity for manufacturers to provide machine specific washout instructions which should specifically designed to prevent rebounds of volatile anesthetics, as recommended for the instruction for use of an anesthetic workstation in the EN ISO Standard 80601-2-13:2022. In the absence of such specific protocols, we prefer the use of ACF or uncontaminated anesthesia machines instead of the washout method. In pediatric trigger-free anesthesia the washout method should not be used at all because a necessary low-flow ventilation for conservation of humidity and temperature leads to prolonged rebounds of volatile anesthetics. In those cases the use of an uncontaminated anesthesia machine or the use of ACF, which have shown to be safe even when using a FGF of 1 l⋅min− 1[10], seems to be superior compared to the washout method.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACF:
-
activated charcoal filters
- APL:
-
adjustable pressure limiting valve
- DLC:
-
decreased lung compliance
- EMHG:
-
European Malignant Hyperthermia Group
- FGF:
-
fresh gas flow
- GC:
-
gas chromatographic pre-separation
- IMS:
-
ion mobility spectrometer
- I:E:
-
inspired to expired time ratio
- MAC:
-
minimum alveolar concentration
- MH:
-
malignant hyperthermia
- MHS:
-
malignant hyperthermia susceptible
- MHAUS:
-
Malignant Hyperthermia Association of the United States
- MISER:
-
multiple injections in a single experimental run
- MV:
-
manual ventilation
- PCV:
-
pressure control ventilation
- Pmax:
-
maximum pressure
- ppb:
-
parts per billion
- ppt:
-
parts per trillion
- PSV:
-
pressure support ventilation
- RR:
-
respiration rate
- Spont:
-
spontaneous breathing mode
- Tinsp:
-
inspiratory time
- Tplat:
-
time of plateau pressure
- VCV:
-
volume controlled ventilation
- VT:
-
tidal volume
References
Johannsen S, Schuster F. [Malignant hyperthermia - update on Pathophysiology, Diagnostics and Treatment]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2019;54(9):527–37.
preparation of anesthesia workstations. to anesthetize mh susceptible patients [http://www.mhaus.org/healthcare-professionals/mhaus-recommendations/preparation-of-anesthesia-workstations-to-anesthetize-mh-susceptible-patients/].
Rüffert H, Bastian B, Bendixen D, Girard T, Heiderich S, Hellblom A, Hopkins PM, Johannsen S, Snoeck MM, Urwyler A, et al. Consensus guidelines on perioperative management of malignant hyperthermia suspected or susceptible patients from the european malignant Hyperthermia Group. Br J Anaesth. 2021;126(1):120–30.
Crawford MW, Prinzhausen H, Petroz GC. Accelerating the washout of inhalational anesthetics from the Dräger Primus anesthetic workstation: effect of exchangeable internal components. Anesthesiology. 2007;106(2):289–94.
Targ AG, Yasuda N, Eger EI. Solubility of I-653, sevoflurane, isoflurane, and halothane in plastics and rubber composing a conventional anesthetic circuit. Anesth Analg. 1989;69(2):218–25.
Cottron N, Larcher C, Sommet A, Fesseau R, Alacoque X, Minville V, Fourcade O, Kern D. The sevoflurane washout profile of seven recent anesthesia workstations for malignant hyperthermia-susceptible adults and infants: a bench test study. Anesth Analg. 2014;119(1):67–75.
Shanahan H, O’Donoghue R, O’Kelly P, Synnott A, O’Rourke J. Preparation of the Drager Fabius CE and Drager Zeus anaesthetic machines for patients susceptible to malignant hyperthermia. Eur J Anaesthesiol. 2012;29(5):229–34.
Prinzhausen H, Crawford MW, O’Rourke J, Petroz GC. Preparation of the Dräger Primus anesthetic machine for malignant hyperthermia-susceptible patients. Can J Anaesth. 2006;53(9):885–90.
Heiderich S, Thoben C, Dennhardt N, Krauss T, Sumpelmann R, Zimmermann S, Reitz M, Ruffert H. Preparation of Drager Atlan A350 and General Electric Healthcare Carestation 650 anesthesia workstations for malignant hyperthermia susceptible patients. BMC Anesthesiol. 2021;21(1):315.
Thoben C, Dennhardt N, Krauß T, Sümpelmann R, Zimmermann S, Rüffert H, Heiderich S. Preparation of anaesthesia workstation for trigger-free anaesthesia: an observational laboratory study. Eur J Anaesthesiol. 2019;36(11):851–6.
Kim TW, Nemergut ME. Preparation of modern anesthesia workstations for malignant hyperthermia-susceptible patients: a review of past and present practice. Anesthesiology. 2011;114(1):205–12.
Beebe JJ, Sessler DI. Preparation of anesthesia machines for patients susceptible to malignant hyperthermia. Anesthesiology. 1988;69(3):395–400.
Kim TW, Wingate JR, Fernandez AM, Whitaker E, Tham RQ. Washout times of desflurane, sevoflurane and isoflurane from the GE Healthcare Aisys(R) and avance(R), Carestation(R), and Aestiva(R) anesthesia system. Paediatr Anaesth. 2013;23(12):1124–30.
Neira VM, Al Madhoun W, Ghaffari K, Barrowman N, Berrigan P, Splinter W. Efficacy of Malignant Hyperthermia Association of the United States-Recommended methods of Preparation for malignant hyperthermia-susceptible patients using Drager Zeus Anesthesia Workstations and Associated costs. Anesth Analg. 2019;129(1):74–83.
Wunsch MR, Lehnig R, Janke C, Trapp O. Online High Throughput measurements for fast catalytic reactions using Time-Division Multiplexing Gas Chromatography. Anal Chem. 2018;90(15):9256–63.
Welch CJ, Gong X, Schafer W, Pratt EC, Brkovic T, Pirzada Z, Cuff JF, Kosjek B. MISER chromatography (multiple injections in a single experimental run): the chromatogram is the graph. Tetrahedron: Asymmetry. 2010;21(13):1674–81.
Papp R, Andersson U, Cantin L-D. Evaluating MISER chromatography for a rapid formulation screen. J Pharm Biomed Anal. 2013;77:94–9.
Zawatzky K, Barhate CL, Regalado EL, Mann BF, Marshall N, Moore JC, Welch CJ. Overcoming “speed limits” in high throughput chromatographic analysis. J Chromatogr A. 2017;1499:211–6.
Speckbacher V, Zeilinger S, Zimmermann S, Mayhew CA, Wiesenhofer H, Ruzsanyi V. Monitoring the volatile language of fungi using gas chromatography-ion mobility spectrometry. Anal Bioanal Chem. 2021;413(11):3055–67.
Kramer A, Kranabetter R, Rathgeber J, Züchner K, Assadian O, Daeschlein G, Hübner NO, Dietlein E, Exner M, Gründling M et al. Infection prevention during anaesthesia ventilation by the use of breathing system filters (BSF): joint recommendation by german society of Hospital Hygiene (DGKH) and german society for Anaesthesiology and Intensive Care (DGAI). GMS Krankenhhyg Interdiszip 2010, 5(2).
Aldrete JA, Cubillos P, Sherrill D. Humidity and temperature changes during low flow and closed system anaesthesia. Acta Anaesthesiol Scand. 1981;25(4):312–4.
Zaharieva IT, Sarkozy A, Munot P, Manzur A, O’Grady G, Rendu J, Malfatti E, Amthor H, Servais L, Urtizberea JA, et al. STAC3 variants cause a congenital myopathy with distinctive dysmorphic features and malignant hyperthermia susceptibility. Hum Mutat. 2018;39(12):1980–94.
Klingler W, Heiderich S, Girard T, Gravino E, Heffron JJ, Johannsen S, Jurkat-Rott K, Ruffert H, Schuster F, Snoeck M, et al. Functional and genetic characterization of clinical malignant hyperthermia crises: a multi-centre study. Orphanet J Rare Dis. 2014;9:8.
Islander G, Rydenfelt K, Ranklev E, Bodelsson M. Male preponderance of patients testing positive for malignant hyperthermia susceptibility. Acta Anaesthesiol Scand. 2007;51(5):614–20.
Larach MG, Gronert GA, Allen GC, Brandom BW, Lehman EB. Clinical presentation, treatment, and complications of malignant hyperthermia in North America from 1987 to 2006. Anesth Analg. 2010;110(2):498–507.
Ibarra Moreno CA, Hu S, Kraeva N, Schuster F, Johannsen S, Rueffert H, Klingler W, Heytens L, Riazi S. An Assessment of Penetrance and Clinical expression of malignant hyperthermia in individuals carrying diagnostic ryanodine receptor 1 gene mutations. Anesthesiology. 2019;131(5):983–91.
Riazi S, Bersselaar L, Islander G, Heytens L, Snoeck MMJ, Bjorksten A, Gillies R, Dranitsaris G, Hellblom A, Treves S et al. Pre-operative exercise and pyrexia as modifying factors in malignant hyperthermia (MH). Neuromuscul Disord 2022.
Gonzalez A, Girard T, Dell-Kuster S, Urwyler A, Bandschapp O. BMI and malignant hyperthermia pathogenic ryanodine receptor type 1 sequence variants in Switzerland: a retrospective cohort analysis. Eur J Anaesthesiol. 2021;38(7):751–7.
Zvaritch E, Gillies R, Kraeva N, Richer M, Jungbluth H, Riazi S. Fatal awake malignant hyperthermia episodes in a family with malignant hyperthermia susceptibility: a case series. Can J Anaesth. 2019;66(5):540–5.
Lavezzi WA, Capacchione JF, Muldoon SM, Sambuughin N, Bina S, Steele D, Brandom BW. Case report: death in the emergency department: an unrecognized awake malignant hyperthermia-like reaction in a six-year-old. Anesth Analg. 2013;116(2):420–3.
Lehmann-Horn F, Klingler W, Jurkat-Rott K. Nonanesthetic malignant hyperthermia. Anesthesiology. 2011;115(5):915–7.
Acknowledgements
None.
Funding
No fundings to declare.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
SZS, SH, CT, ND, TK, SZ, RS and HR contributed to the study design, to collecting or analyzing data and drafting the manuscript. SH and SZS wrote the main manuscript text. CT and SZS prepared Figs. 1, 2, 3 and 4. CT and SZ adjusted the GC-IMS prototype to meet the requirements of this study. All authors have revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
As there were no humans or animals involved, no ethical approval was necessary for this laboratory technical study.
Consent for publication
Not applicable.
Competing interests
R. S. received fees for speeches and funds for research from B. Braun Melsungen AG, Melsungen, Germany. SH, CT, ND, TK, SZ, MR and HR have no conflicts of interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zumsande, S., Thoben, C., Dennhardt, N. et al. Rebounds of sevoflurane concentration during simulated trigger-free pediatric and adult anesthesia. BMC Anesthesiol 23, 196 (2023). https://doi.org/10.1186/s12871-023-02148-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-023-02148-3