Abstract
Background
Post-laparoscopic shoulder pain (PLSP) is a common complication following laparoscopic surgeries. This meta-analysis aimed to investigate whether pulmonary recruitment maneuver (PRM) was beneficial to alleviated shoulder pain after laparoscopic procedures.
Methods
We reviewed existing literature in the electronic database from the date of inception to January 31, 2022. The relevant RCTs were independently selected by two authors, after which data extraction, assessment of the risk of bias, and comparison of results.
Results
This meta-analysis included 14 studies involving 1504 patients, among which 607 patients were offered pulmonary recruitment maneuver (PRM) alone or in combination with intraperitoneal saline instillation (IPSI), while 573 patients were treated with passive abdominal compression. The administration of PRM significantly decreased the post-laparoscopic shoulder pain score at 12 h (MD (95%CI) − 1.12(–1.57, − 0.66), n = 801, P < 0.001, I2 = 88%); 24 h (MD (95%CI) − 1.45(–1.74, − 1.16), n = 1180, P < 0.001, I2 = 78%) and at 48 h (MD (95%CI) − 0.97(–1.57, − 0.36), n = 780, P < 0.001, I2 = 85%). We observed high heterogeneity in the study and analyzed the sensitivity but failed to identify the cause of the heterogeneity, which may have resulted from the different methodologies and clinical factors in the included studies.
Conclusion
This systematic review and meta-analysis indicate that PRM can reduce the intensity of PLSP. More studies may be needed to explore the usefulness of PRM in more laparoscopic operations besides gynecological surgeries and determine the optimal pressure of PRM or its appropriate combination with other measures. The results of this meta-analysis should be interpreted with caution owing to the high heterogeneity between the analyzed studies.
Similar content being viewed by others
Introduction
Laparoscopy is among the most used minimally invasive procedures that can reduce postoperative pain, lessen the duration of hospital stay and facilitate recovery earlier than laparotomy.Laparoscopy has been widely used in various abdominal surgeries, such as gastrectomy, cholecystectomy, appendectomy, hernia and gynecological surgery [1,2,3,4,5]. However, the post-laparoscopic shoulder pain (PLSP) is often occurs following laparoscopic surgeries, and its reported incidence varies from 35–80% [6–7]. The PLSP can even remain for up to three days and often upsets the patients [8]. Moreover, it can increase the costs of healthcare owing to an increased usage of analgesics, delayed discharge, and even re-admission [9]. Therefore, necessary measures should be taken to diminish the intensity of PLSP.
Although the exact mechanism of PLSP remains unclear, some studies have suggested that it is caused by the trapping of carbon dioxide (CO2) between the liver and the right diaphragm and subsequent conversion into carbonic acid, which irritates the diaphragm and subsequently generates referred shoulder pain (C4 dermatomal) [10,11,12]. Therefore, several studies have attempted to decrease the incidence or severity of PLSP by promoting the removal of remaining CO2 from the abdominal cavity. These efforts include drainage tube insertion, intraperitoneal saline instillation (IPSI), and the usage of intraperitoneal local anesthetic agents [13,14,15]. Moreover, the pulmonary recruitment maneuver (PRM) can also facilitate the removal of CO2 from the abdominal cavity by increasing positive airway pressure and intrathoracic pressure. PRM is more commonly used in clinical practice because it does not require drugs, specialized apparatus, or additional medical costs, unlike the other methods [16–17]. Several trials have described the advantages of PRM in patients undergoing laparoscopic operations compared to passive abdominal compression [18–20]. However, Kaloo et al. [9] reported no benefits of the PRM on postoperative patients suffering from PLSP. Thus, it remains unclear whether PRM is better than passive abdominal compression. Therefore, we systematically searched and analyzed the available studies to assess the efficacy and advantages of PRM over traditional abdominal compression in laparoscopic operations.
Methods
This systematic review and meta-analysis complied with the PRISMA statement [21]. This systematic review was registered on Prospero with the registration number CRD42022315025.
Eligibility criteria
This meta-analysis included randomized controlled trials (RCTs) irrespective of the language, year of publication, or sample size. Patients who had undergone any type of laparoscopic procedure were enrolled. In the control group, patients were subjected to abdominal compression to eliminate as much residual CO2 as possible, whereas, in the intervention groups, patients subjected to PRM alone with varying maximum inflation pressures or in combination with other interventions were included.
Search strategy and data extraction
A systematic literature research of electronic databases, including PubMed, Embase, Web of Science, and Cochrane Central Register of Controlled Trials (CENTRAL), was conducted from the date of inception to January 31, 2022. References were imported into EndNote™ X9 software (Clarivate™, London, UK) for deduplication.The following search terms were used for PubMed: (“laparoscopy” [MeSH Terms] OR“laparoscopy”[All Fields]) AND (“shoulder pain”[MeSH Terms] OR (“shoulder”[All Fields]) AND ((“lung”[MeSH Terms] OR “lung”[All Fields] OR “pulmonary”[All Fields]) AND (“recruit”[All Fields] OR “recruitment”[All Fields] OR “recruitments”[All Fields]) AND (“maneuver”[All Fields] OR “maneuvered”[All Fields] OR “maneuvering”[All Fields] OR “maneuverings” [All Fields] OR “maneuvers”[All Fields]).
The titles and abstracts of the articles were screened, and the full texts of relevant articles were studied further. DX and LH independently reviewed all resulting search entries against the inclusion and exclusion criteria and then extracted data from the included studies using a data extraction form. Data on the author’s name, year of publication, type of surgery, interventions used and relevant outcomes were collected from each study.
Assessment of the risk of bias
The online bias-assessment tool RoB-2 was used to assess the quality of included studies [22]. This tool evaluated the risk of bias in each included study based on the following aspects: (1) randomization process; (2) deviations from intended interventions; (3) missing outcome data; (4) measurement of the outcome; (5) selection; (6) selective reporting (reporting bias) and (7) other bias. The risk of bias in each item was categorized as low, high, and some concern.
Statistical analysis
Statistical meta-analysis was performed using the statistical software Rev Man version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark). Confidence intervals were set at 95%. The mean difference (MD) and 95%CI were the principal summary measures for pooled continuous and normally distributed outcomes. Zero-to-hundred pain scale scores for pain were converted to zero-to-ten scale scores to facilitate statistical analysis. The odds ratios (OR) and 95%CI were the principal summary measures for pooled dichotomous data. Summary measures were considered statistically significant if the 95% CI for the mean difference excluded zero and if the 95% CI or the odds ratios excluded 1.
The I2 statistic was used to quantify heterogeneity in the pooled results. Significant heterogeneity was defined as an I2 value of > 50%. The Der Simonian–Laird random-effects model was used if significant heterogeneity was detected in the methodologies of the included studies. The median and interquartile range (IQR) were transformed to mean and standard difference (SD) [23, 24].
Results
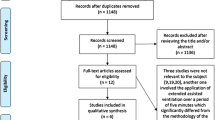
We searched the databases PubMed, EMBASE, Web of Science and Cochrane Central Register of Controlled Trials (CENTRAL) to obtain a total of 124 results. The full texts of 29 articles were examined in detail. Two researchers (DX and LH) reviewed all the full texts. Finally, we included 14 RCTs with a total of 1504 participants were included in the meta-analysis (Fig. 1).
Characteristics of the included studies
The details of included studies are presented in Table 1. Eleven studies compared the control group (passive abdominal compression) and PRM alone [16–17, 25,26,27,28,29,30,31,32,33,34,35,36]. Three studies compared passive abdominal compression in combination with intraperitoneal saline [33, 35−36].
Risk of bias in the included studies
Two authors (DX and LH) independently assessed the quality of the included studies using the online bias-assessment tool RoB-2 [22]. The risk of bias was classified as low, high, and some concern. Disagreements in risk assessment between the two authors were assessed and adjudicated by another independent reviewer (WYT). Figure 2 presents the risks of bias of the included references.
The intensity of shoulder pain
Compared with the control group, PRM can significantly decrease the visual analog scales (VAS) scores of shoulder pain at 12 h (MD (95%CI) − 1.12 (–1.57, − 0.66), n = 801, P < 0.001, I2 = 88%), at 24 h (MD (95%CI) − 1.45(–1.74, − 1.16), n = 1180, P < 0.001, I2 = 78%), and at 48 h (MD (95%CI) − 0.97(–1.57, − 0.36), n = 780, P < 0.001, I2 = 85%).
However, we noted a considerable heterogeneity among the studies at different follow-up times (I2 = 88%, 78%, and 85% at 12 h, 24 h, and 48 h, respectively). This high heterogeneity could not be eliminated when we performed sub-group analyses using different pressures of PRM or in combination with IPSI (Figs. 3, 4 and 5), which indicated that the high heterogeneity was not related to our subgroup analysis.
Sensitivity analysis
To further explore the possible cause of the high heterogeneity, we conducted a sensitivity analysis to assess the robustness of the synthesized results of repeat analyses by excluding one study at a time. We failed to find a difference in outcomes using this method. At 12 h after operation, the MD (95% CI) varied from − 1.42(–1.76, − 1.09) after excluding the study by Davari-Tanha et al. [25] to − 0.94(–1.58, − 0.31) after excluding the study by Güngördük et al.[26] At 24 h after operation, the MD (95% CI) varied from − 1.56 (–1.81, − 1.31) after excluding the study by Davari-Tanha et al. [25] to − 1.33 (–1.58, − 1.08) after excluding Güngördük et al. [26] At 48 h after operation, the MD (95% CI) varied from − 1.16 (–1.71, − 0.62) after excluding Güngördük et al. [26] to − 0.78 (–1.35, − 0.21) after excluding the study by Ryu et al. [35] (Tables 2, 3 and 4).
Other outcomes
PRM did not reduce the intensity of wound pain [MD (95% CI) − 0.16 (–0.45 to 0.12), n = 303, P = 0.26, I2 = 10%] or upper abdominal pain [MD (95% CI) -1.25 (–2.56 to 0.05), n = 450, P = 0.52, I2 = 98%] at 24 h postoperatively and the incidence of postoperative nausea and vomiting(PONV) [OR (95% CI) 0.84 (0.49–1.43), n = 714, P = 0.52, I2 = 61%] (Figs. 6, 7 and 8).
Discussion
Fourteen RCTs were included in our systematic review and meta-analysis comparing passive abdominal compression with PRM alone or in combination with IPSI. The results indicated that the application of PRM alone or in combination with IPSI could significantly decrease PLSP VAS scores at 12 h, 24 and 48 h postoperatively, compared with passive abdominal compression. However, this strategy was ineffective at reducing the intensity of postoperative wound pain, upper abdominal pain, and the incidence of PONV.
Although the mechanism of PLSP is not fully understood yet, it may involve the following hypotheses. First, carbonic acid that is converted from (CO2) by carbonic anhydrase on the surface of the diaphragm [16] can stimulate the phrenic nerve ending and transmits pain signals to the central nervous system (CNS) [37]. Moreover, the loss of suction from the liver and traction of the visceral ligament caused by residual gas in the enterocoeles can also directly cause pain [38]. It is suggested that residual CO2 in the abdominal cavity can remain for several days after laparoscopy [39–40] and postoperative shoulder pain may be correlated with the volume of CO2 under the right hemidiaphragm [12, 49]. The last hypothesis involves tissue trauma caused by the rapid insufflation of the pneumoperitoneum and the hyperdistention of the abdominal cavity, which results in overstretching of the diaphragmatic muscle fibers, traumatic straining of nerves, tearing of blood capillaries, and release of inflammatory mediators, which in turn elicits the referred pain to the shoulder [12, 41].
At the end of the surgery, PRM is often performed with manual positive-pressure ventilations, which not only inflate the lungs but also lower the diaphragm and increase intraperitoneal pressure. CO2 gas accumulated in the peritoneal cavity can be removed by increased intraperitoneal pressure, resulting in reduced irritation of the phrenic nerve or peritoneum and consequent shoulder pain. As indicated in our study, PRM could be easily performed and was an effective method for the prevention of PLSP. However, our study failed to show the benefit of PRM on the incision site and epigastric pain, as well as PONV. Pain at the wound and upper abdomen are mainly caused by surgical traumas such as skin incision and tissue excision, which are usually prevented and treated using oral analgesics, local infiltration, nerve block, and analgesic pump, and cannot be alleviated by reducing the residual CO2 gas in the cavity. As the incidence of PONV varies with several factors, including sex, history of PONV, smoking history, motion sickness, type of anesthetic and depth of anesthesia [55,56,57], the elimination of CO2 did not reduce the incidence of PONV.
It is worth noting that some other measures, including oral analgesics [42], intraperitoneal saline instillation (IPSI) [16], drain insertion [43], sodium bicarbonate sub-diaphragm irrigation [44], intraperitoneal anesthetic agents, and nerve-blocking agents [45,46,47,48] can also prevent PLSP. However, these methods not only require drugs and equipment but also involve additional medical costs. Moreover, they may even produce adverse effects. In contrast, the implementation of PRM is more convenient and simpler, which makes it worth popularizing. However, it should be noted that complications related to PRM, including barotrauma and hemodynamic deterioration, may occur when higher pressures are used [50,51,52,53]. Yilmaz et al. [54] suggested that a lower maximal inspiratory pressure of 15 cm H2O might be preferred to avoid the potential complications of PRM using higher pressures. Because of relatively fewer studies on the use of PRM at low pressures, we suggest that the optimal positive pressure of PRM, which minimizes the severity of PLSP and the incidence of adverse events, should be further explored further.
Compared with a previous study by Pergialiotis et al. [19], we included more types of laparoscopic surgeries besides gynecologic operations, such as cholecystectomy and hernia surgery. Moreover, our study analyzed more outcomes such as wound pain and the incidence of PONV. Therefore, our study provides more information and stronger evidence supporting the effect of PRM on PLSP.
This meta-analysis also have some limitations. First, despite the expansion of operation types, the final analysis only included two studies that were conducted on nongynecologic surgery patients. Further studies regarding to PLSP should investigate other types of laparoscopic operations in more detail. Second, there were high variations in medication for perioperative prophylactic analgesia in the included studies, which may affect the study results. Third, high heterogeneity was observed in our study, which may have resulted from different methodologies and clinical factors in the included studies, although we acknowledged this limitation and downgraded the quality of the evidence accordingly.
Conclusion
Our study suggested that PRM is a feasible preventive measure for reducing the intensity of PLSP. However, the results of this meta-analysis should be interpreted with caution owing to the high heterogeneity between the analyzed studies. Moreover, the usefulness of PRM in other types of laparoscopic operations besides gynecological operations should be further explored further.
Data Availability
materials.
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- CNS:
-
Central nerve system
- CO2:
-
Carbon dioxide
- IPSI:
-
Intraperitonial saline instillation
- IQR:
-
Inter quartile range
- LC:
-
Laparoscopic cholecystectomy
- MD:
-
Mean difference
- N/A:
-
Not applicable
- OR:
-
Odds ratio
- PLSP:
-
Post-laparoscopic shoulder pain
- PONV:
-
Postoperative nausea and vomiting
- PRM:
-
Pulmonary recruitment maneuver
- SD:
-
Standard difference
- SI:
-
Saline instillation
- VAS:
-
Visual analogue score
References
Tanaka T, Ueda S, Miyamoto S, et al. Comparison of prognosis between minimally invasive and abdominal radical hysterectomy for patients with early-stage cervical cancer. Curr Oncol. 2022;29:2272–83.
Luesma MJ, Fernando J, Cantarero I, Lucea P, Santander S. Surgical treatment of obesity. Special mention to roux-en-Y gastric bypass and vertical gastrectomy. Front Endocrinol (Lausanne). 2022;1:867838.
Demouron M, Selvy M, Dembinski J, et al. Feasibility and effectiveness of an enhanced recovery program after early cholecystectomy for acute calculous cholecystitis: a 2-step study. J Am Coll Surg. 2022;234:840–8.
Naidoo M, et al. Trends in adoption of laparoscopic appendicectomy in a developing country: closing the gap. World J Surg. 2022;46:1015–21.
Novik B, Sandblom G, Ansorge C, Thorell A. Association of mesh and fixation options with reoperation risk after laparoscopic groin hernia surgery: a swedish hernia registry study of 25,190 totally extraperitoneal and transabdominal preperitoneal repairs. J Am Coll Surg. 2022;234:311–25.
Alexander JI. Pain after laparoscopy. Br J Anaesth. 1997;79:369–78.
van Dijk JEW, Dedden SJ, Geomini PMAJ, et al. Postlaparoscopic reduction of pain by combining intraperitoneal normal saline and the pulmonary recruitment maneuver (POLAR BEAR trial). RCT to estimate reduction in pain after laparoscopic surgery when using a combination therapy of intraperitoneal normal saline and the pulmonary recruitment maneuver. BMC Womens Health. 2017;17:42.
Narchi P, Benhamou D, Fernandez H. Intraperitoneal local anaesthetic for shoulder pain after day-case laparoscopy. Lancet. 1991;338:1569–70.
Kaloo P, Armstrong S, Kaloo C, Jordan V, et al. Interventions to reduce shoulder pain following gynaecological laparoscopic procedures. Cochrane Database Syst Rev. 2019;1:CD011101.
Ryu KH, Lee SH, Cho EA, et al. Comparison of impacts of intraperitoneal saline instillation with and without pulmonary recruitment maneuver on post-laparoscopic shoulder pain prevention: a randomized controlled trial. Surg Endosc. 2019;33:870–8.
Pasquier EK, Andersson E. Pulmonary recruitment maneuver reduces pain after laparoscopic bariatric surgery: a randomized controlled clinical trial. Surg Obes Relat Dis. 2018;14:386–92.
Jackson SA, Laurence AS, Hill JC. Does post-laparoscopy pain relate to residual carbon dioxide? Anaesthesia.1996; 51:485–7.
Lepner U, Goroshina J, Samarutel J. Postoperative pain relief after laparoscopic cholecystectomy: a randomised prospective double-blind clinical trial. Scand J Surg. 2003;92:121–4.
Donatsky AM, Bjerrum F, Gögenur I. Intraperitoneal instillation of saline and local anesthesia for prevention of shoulder pain after laparoscopic cholecystectomy: a systematic review. Surg Endosc. 2013;27:2283–92.
Donatsky AM, Bjerrum F, Gögenur I. Surgical techniques to minimize shoulder pain after laparoscopic cholecystectomy. A systematic review. Surg Endosc. 2013;27:2275–82.
Kihlstedt PE, Andersson E. Pulmonary recruitment maneuver reduces shoulder pain and nausea after laparoscopic cholecystectomy: a randomized controlled trial. World J Surg. 2021;45:3575–83.
Phelps P, Cakmakkaya OS, Apfel CC, Radke OC. A simple clinical maneuver to reduce laparoscopy-induced shoulder pain: a randomized controlled trial. Obstet Gynecol. 2008;111:1155–60.
Tas B, Donatsky AM, Gogenur I. Techniques to reduce shoulder pain after laparoscopic surgery for benign gynaecological disease: a systematic review. Gynecol Surg. 2013;10:169–75.
Pergialiotis V, Vlachos DE, Kontzoglou K, Perrea D, Vlachos GD. Pulmonary recruitment maneuver to reduce pain after laparoscopy: a meta-analysis of randomized controlled trials. Surg Endosc. 2015;29:2101–8.
Kietpeerakool C, Rattanakanokchai S, Yantapant A, et al. Pulmonary recruitment maneuver for reducing shoulder pain after laparoscopic gynecologic surgery: a network meta-analysis of randomized controlled trials. Minim Invasive Surg. 2020;2020:7154612.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2009;366:l4898.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–805.
Davari-Tanha F, Samimi S, Khalaj Z, Bastanhagh E. Comparison of intraperitoneal normal saline infusion with pulmonary recruitment maneuver in reducing shoulder and upper abdomen pain following gynecologic laparoscopic procedures: a randomized, controlled, triple-blind trial. Anesth Pain Med. 2019;9:e92444.
Güngördük K, Aşıcıoğlu O, Özdemir İA. Effect of the pulmonary recruitment maneuver on pain after laparoscopic gynecological oncologic surgery: a prospective randomized trial. J Gynecol Oncol. 2018;29:e92.
Khanna A, Sezen E, Barlow A, Rayt H, Finch JG. Randomized clinical trial of a simple pulmonary recruitment manoeuvre to reduce pain after laparoscopy. Br J Surg. 2013;100:1290–4.
Lee J, Park C, Kim J, et al. Effect of low-pressure pulmonary recruitment maneuver on postlaparoscopic shoulder pain: randomized controlled trial. J Minim Invasive Gynecol. 2022;27:173–7.
Ryu KH, Lee SH, Cho EA, Kim JA, Lim GE, Song T. Comparison of impacts of intraperitoneal saline instillation with and without pulmonary recruitment maneuver on post-laparoscopic shoulder pain prevention: a randomized controlled trial. Surg Endosc. 2019;33:870–8.
Sharami SH, Sharami MB, Abdollahzadeh M, Keyvan A. Randomised clinical trial of the influence of pulmonary recruitment manoeuvre on reducing shoulder pain after laparoscopy. J Obstet Gynaecol. 2010;30:505–10.
Tsai HW, Chen YJ, Ho CM, et al. Maneuvers to decrease laparoscopy-induced shoulder and upper abdominal pain: a randomized controlled study. Arch Surg. 2011;146:1360–6.
Tsai HW, Yen MS, Ho CM, Hsen SS, Chao KC, Chen YJ. A randomized, controlled, single blind study comparing a pulmonary recruitment maneuver versus intraperitoneal infusion of normal saline to reduce shoulder tip pain after gynecologic laparoscopic surgery. J Minim Invasive Gynecol. 2010;17:15–S16.
van Dijk JEW, et al. Randomised controlled trial to estimate reduction in pain after laparoscopic surgery when using a combination therapy of intraperitoneal normal saline and the pulmonary recruitment manoeuvre. BJOG. 2018;125:1469–76.
Kiyak H, Yilmaz G, Ay N. Semi-fowler positioning in addition to the pulmonary recruitment manoeuvre reduces shoulder pain following gynaecologic laparoscopic surgery. Wideochir Inne Tech Maloinwazyjne. 2019;14:567–74.
Ryu K, Choi W, Shim J, et al. The impact of a pulmonary recruitment maneuver to reduce post-laparoscopic shoulder pain: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2017;208:55–60.
Tsai HW, Wang PH, Yen MS, Chao KC, Hsu TF, Chen YJ. Prevention of postlaparoscopic shoulder and upper abdominal pain: a randomized controlled trial. Obstet Gynecol. 2013;121:526–31.
Kandil TS, El Hefnawy E. Shoulder pain following laparoscopic cholecystectomy: factors affecting the incidence and severity. J Laparoendosc Adv Surg Tech A. 2010;20:677–82.
Tsimoyiannis EC, Siakas P, Tassis A, Lekkas ET, Tzourou H, Kambili M. Intraperitoneal normal saline infusion for postoperative pain after laparoscopic cholecystectomy. World J Surg. 1998;22:824–8.
Al Shehri MY. Radiological evaluation of patients after laparoscopic cholecystectomy. Saudi J Gastroenterol. 1999;5:23–6.
Draper K, Jefson R, Jongeward R Jr, McLeod M. Duration of postlaparoscopic pneumoperitoneum. Surg Endosc. 1997;11:809–11.
Wallace DH, Serpell MG, Baxter JN, O’Dwyer PJ. Randomized trial of different insufflation pressures for laparoscopic cholecystectomy. Br J Surg. 1997;84:455–8.
Nutthachote P, Sirayapiwat P, Wisawasukmongchol W, Charuluxananan SA. Randomized, double-blind, placebo-controlled trial of oral pregabalin for relief of shoulder pain after laparoscopic gynecologic surgery. J Minim Invasive Gynecol. 2014;21:669–73.
Yang SC, Chang KY, Wei LF, Shyr YM, Ho CM. To drain or not to drain: the association between residual intraperitoneal gas and post-laparoscopic shoulder pain for laparoscopic cholecystectomy. Sci Rep. 2021;11:7447.
Liu L, Xia T, Ji H, et al. Sodium bicarbonate sub-diaphragmatic irrigation relieves shoulder pain after total laparoscopic hysterectomy: a randomized controlled trial. J Pain Res. 2021;14:3615–22.
Gaballah KM, Habeeb RM, Abdallah SI. Efficacy of intraperitoneal bupivacaine, hydrocortisone, and magnesium sulfate in different combinations for pain relief after laparoscopic ovarian cystectomy: a double-blind randomized controlled trial. Minerva Anestesiol. 2020;86:14–22.
Asgari Z, Rezaeinejad M, Hosseini R, Nataj M, Razavi M, Sepidarkish M. Spinal anesthesia and spinal anesthesia with subdiaphragmatic lidocaine in shoulder pain reduction for gynecological laparoscopic surgery: a randomized clinical trial. Pain Res Manag. 2017;2017:1721460.
Raft J, Chin KJ, Richebé P. Erector spinae plane (ESP) block with a transverse in-plane approach for management of referred shoulder pain after laparoscopic cholecystectomy. J Clin Anesth. 2019;55:100–1.
Yi MS, Kim WJ, Kim MK, et al. Effect of ultrasound-guided phrenic nerve block on shoulder pain after laparoscopic cholecystectomy-a prospective, randomized controlled trial. Surg Endosc. 2017;31:3637–45.
Song T, Kim KH, Lee KW. The intensity of postlaparoscopic shoulder pain is positively correlated with the amount of residual pneumoperitoneum. J Minim Invasive Gynecol. 2017;24:984–9.
García-Fernández J. Pressure safety range of barotrauma with lung recruitment manoeuvres: a randomised experimental study in a healthy animal model. Eur J Anaesthesiol. 2013;30:567–74.
Lovas A, Szakmány T. Haemodynamic Effects of Lung Recruitment Manoeuvres. Biomed Res Int.2015; 2015:478970.
da Silva PS, de Aguiar VE, Fonseca MC. Iatrogenic pneumothorax in mechanically ventilated children: incidence, risk factors and other outcomes. Heart Lung. 2015;44:238–42.
Hsu CW, Sun SF. Iatrogenic pneumothorax related to mechanical ventilation. World J Crit Care Med. 2014;3:8–14.
Yilmaz G, Kiyak H, Akca A, Salihoglu Z. Low-pressure pulmonary recruitment maneuver: equal to or worse than moderate-pressure pulmonary recruitment maneuver in preventing postlaparoscopic shoulder pain? A randomized controlled trial of 72 patients. Wideochir Inne Tech Maloinwazyjne. 2020;15:519–25.
Doleman B, Leonardi-Bee J, Heinink TP, Bhattacharjee D, Lund JN, Williams JP. Pre-emptive and preventive opioids for postoperative pain in adults undergoing all types of surgery. Cochrane Database Syst Rev. 2018;12:CD012624.
Miller D, Lewis SR, Pritchard MW, et al. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery. Cochrane Database Syst Rev. 2018;8:CD012317.
Lewis SR, Pritchard MW, Fawcett LJ, Punjasawadwong Y. Bispectral index for improving intraoperative awareness and early postoperative recovery in adults. Cochrane Database Syst Rev. 2019;9:CD003843.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
Xiao Deng and Hao Li made equal substantial contributions and design the study; Xiao Deng and Hao Li designed the study, exacted data, conducted statistical analysis and wrote the manuscript; Yantong Wan analyzed data and revised the manuscript; Xuemei Lin designed the study and revised the manuscript. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Drs. Xiao Deng, Hao Li, Yantong Wan and Xuemei Lin have no conficts of interest or financial ties to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Deng, X., Li, H., Wan, Y. et al. Pulmonary recruitment maneuver reduces the intensity of post-laparoscopic shoulder pain: a systematic review and meta-analysis. BMC Anesthesiol 23, 155 (2023). https://doi.org/10.1186/s12871-023-02107-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-023-02107-y