Abstract
Purpose
Carbon dioxide (CO2) increases cerebral perfusion. The effect of CO2 on apnea tolerance, such as after anesthesia induction, is unknown. This study aimed to assess if cerebral apnea tolerance can be improved in obese patients under general anesthesia when comparing O2/Air (95%O2) to O2/CO2 (95%O2/5%CO2).
Methods
In this single-center, single-blinded, randomized crossover trial, 30 patients 18–65 years, with body mass index > 35 kg/m2, requiring general anesthesia for bariatric surgery, underwent two apneas that were preceded by ventilation with either O2/Air or O2/CO2 in random order. After anesthesia induction, intubation, and ventilation with O2/Air or O2/CO2 for 10 min, apnea was performed until the cerebral tissue oxygenation index (TOI) dropped by a relative 20% from baseline (primary endpoint) or oxygen saturation (SpO2) reached 80% (safety abortion criterion). The intervention was then repeated with the second substance.
Results
The safety criterion was reached in all patients before cerebral TOI decreased by 20%. The time until SpO2 dropped to 80% was similar in the two groups (+ 6 s with O2/CO2, 95%CI -7 to 19 s, p = 0.37). Cerebral TOI and PaO2 were higher after O2/CO2 (+ 1.5%; 95%CI: from 0.3 to 2.6; p = 0.02 and + 0.6 kPa; 95%CI: 0.1 to 1.1; p = 0.02).
Conclusion
O2/CO2 improves cerebral TOI and PaO2 in anesthetized bariatric patients. Better apnea tolerance could not be confirmed.
Similar content being viewed by others
Introduction
Before general anesthesia induction, patients are administered 100% oxygen (O2) to prevent hypoxia and brain damage should ventilation fail. In the blood, oxygen is transported predominantly (≥ 98%) bound to hemoglobin within red blood cells [1]. Increased carbon dioxide partial pressure (PCO2), temperature, and decreased pH, lower O2 hemoglobin affinity [2]. In the systemic circulation, high CO2 and low pH promote vasodilatation. Metabolically active tissue is; therefore, better perfused, and oxygen release is facilitated. Hypercarbia and acidemia are essential components of cerebral autoregulation [3]. Hypoxia stimulates vasodilation. This effect is amplified by the more potent vasodilator CO2; thus, cerebral blood flow increases more than with hypoxia alone [4]. Cerebral oxygenation and task performance of rhesus monkeys subjected to hypoxia improved under the influence of an admixture of CO2 [5], which may be a result of the above-mentioned mechanisms.
To date, functional effects of CO2 have been primarily described for hypobaric hypoxic conditions, such as in high altitude, aviation, and aerospace medicine [6,7,8].
Improved task performance with an increased CO2 partial pressure has also been described under normobaric hypoxia [8]. A higher CO2 partial pressure increases cerebral blood flow [9], which results in a higher TOI [10]. Whether higher CO2 concentrations improve cerebral oxygenation in anesthetized humans and protect the brain from impending hypoxia, respectively, prolonging the time for a defined TOI drop is unclear. Obese patients have a decreased oxygen reserve due to a low functional residual lung capacity (FRC), are therefore at risk for hypoxia, and might benefit from CO2 application. Low TOI may be associated with delirium [11], but no TOI and time thresholds have been established yet. Clinical studies on apnea tolerance during anesthesia induction are almost nonexistent [12], let alone in obese patients. One study reported that the incidence or a relevant drop of peripheral oxygen saturation (SpO2) to < 80% could be observed in 8% of obese patients undergoing bariatric surgery [13]. This is all the more relevant as obesity is a risk factor for difficult mask ventilation [14].
This current study aimed to assess if cerebral apnea tolerance can be improved with the use of O2/CO2 compared to O2/Air in obese patients under general anesthesia. We hypothesized that ventilation with O2/CO2 would extend apnea time until cerebral tissue oxygenation index (TOI = regional oxygen saturation, SrO2) decreased by a relative 20% from baseline.
Materials and methods
Trial design
This is a single-center, single-blinded, proof-of-concept randomized crossover trial.
Participants, eligibility criteria, setting
This study was approved by the local ethics committee (Kantonale Ethikkommission Zurich, Zurich, Switzerland, number 2017 – 01,790) as well as by the national authorization and supervisory authority for drugs and medical products (Swissmedic Bern, Switzerland, notification number 2017DR2183).
Inclusion criteria were defined as patients between 18 and 65 years old being scheduled for primary bariatric surgery with a BMI > 35 kg/m2 and signed informed consent to participate in this study. Exclusion criteria were severe end-organ damage (chronic obstructive pulmonary disease GOLD III and IV or other chronic respiratory diseases; known hepatic insufficiency or liver enzymes > 50% over the upper reference value of the University Hospital Zurich; renal creatinine clearance < 30 ml/min; diagnosed pulmonary hypertension (mean pulmonary arterial pressure ≥ 25 mmHg); severe cardiovascular disease (New York Heart Association, NYHA classification III and IV); history of cerebrovascular disease; drug- or alcohol abuse; and pregnancy). In patients at risk for cardiovascular disease (age > 50 years, history of atherosclerosis, or any related diseases), significant vascular stenosis (> 50%) of the carotid arteries was excluded by duplex examination.
All participants of the study provided written and informed consent to participate in this trial. It was registered before patient enrollment on www.clinicaltrials.gov (NCT 03,338,907; date of registration: 09/11/2017) and is reported according to the consolidated standards of reporting trials (CONSORT) checklist [15]. The study was monitored by the clinical trials center of the University Hospital Zurich, Zurich, Switzerland. The trial was performed at the University Hospital Zurich between January and November 2018.
Randomization and blinding
Patients were randomized on the day of their scheduled surgery using a web-based randomizer (www.randomizer.at) which allowed allocation concealment. Patients were randomized (1:1) using simple randomization to the intervention sequence O2/Air—O2/CO2 or to O2/CO2—O2/Air. The allocation sequence was generated electronically. The parameters to generate the allocation sequence have been determined by the biostatistician. The participants were enrolled by one of the investigators and were assigned to the intervention sequence by the web-based randomizer.
Participant preparation for anesthesia and anesthesia induction
Upon arrival in the operating theater, patients were monitored by the study team with peripheral oxygen saturation (SpO2) monitoring, electrocardiogram (ECG), venous and arterial catheters, bispectral index (BIS), and near-infrared spectroscopy (NIRS, Sensmart X-100, Nonin Medical, Plymouth, MN, USA) for tissue oxygen index (TOI) measurement. The NIRS and BIS sensors were placed on the patient’s left and right foreheads, respectively.
Patients were preoxygenated with 100% O2 until an expiratory fraction of oxygen of ≥ 90% was reached, after which rapid sequence induction according to departmental guidelines was performed with intravenous fentanyl 2–3 µg/kg, propofol 2 mg/kg, and rocuronium 0.9 mg/kg. The airway was secured by an endotracheal tube placed with a C-MAC video laryngoscope (Storz, Tuttlingen, Germany). General anesthesia was then maintained with propofol 4–10 mg/kg/h, and BIS monitoring was used to ensure a constant depth of anesthesia throughout the study intervention (BIS target range 30–50).
Study intervention, definition of the time-points T0-T4
T0
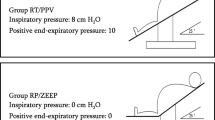
An overview of the study intervention is given in Fig. 1. After induction, patients were ventilated (Dräger Primus Infinity Empowered, Dräger, Lübeck, Germany) in a volume-controlled mode with an inspiratory oxygen fraction (FiO2) of 0.8. Tidal volume was set at 6–8 ml/kg ideal body weight. At peak pressure above 35 cmH2O, tidal volume was reduced so that this value was not exceeded. Peak inspiratory pressure was 15–35 cmH2O, and the respiratory rate was 10–18 breaths per minute to obtain normocapnic end-tidal CO2 (EtCO2) values of 4.5–5.5 kPa for ≥ 2 min. This steady state was defined as time-point 0 (T0), and baseline values and ventilator settings were recorded. The ventilator setting established at T0 was maintained for each patient throughout the experiment.
T1 and T2
Patients were then first connected either to O2/Air (administered by a Dräger Primus Infinity Empowered ventilator, connected to the hospital’s O2 and compressed air wall supply) or to O2/CO2 (administered by a Dräger Evita Infinity V500 ventilator, connected to an O2/CO2 gas bottle, Linde Group, Dublin, Ireland).
Patients were ventilated for ≥ 10 min with O2/Air or O2/CO2; the fraction of expired O2 (FeO2) had to reach ≥ 80% and be stable for more than one minute. To initiate apnea, the endotracheal tube was disconnected from the ventilator. T1 was the initiation of the first apnea phase. Ventilation was resumed when TOI had dropped 20% from baseline (primary endpoint). The 20% drop was defined as a relative drop from the baseline and not an absolute 20% drop. This value was chosen according to institutional standards and expert recommendations for carotid endarterectomy [16]. For safety reasons, the apnea phase was terminated early if a SpO2 of 80% was reached (safety endpoint).
T2 was defined as the time point at the end of the first apnea phase (when the primary or the safety endpoint was reached). Ventilation was performed with the ventilator settings used at T0 to allow the patient to return to its steady state for ≥ 2 min. Then the crossover ventilation was initiated for 10 min.
T3 and T4
T3 corresponds to the beginning of the second apnea phase, and T4 to the end of the second apnea phase.
End of study intervention and follow-up
After T4, upon reaching a steady state, the study intervention was completed, and bariatric surgery was started. To exclude study-related adverse (AE) or serious adverse events (SAE), each patient was followed-up after 24 h. In the case of an AE or SAE, the patient was followed-up until the AE or SAE was resolved.
Outcomes
The primary outcome of this study was the length of the apnea phase until TOI decreased by 20% from baseline (assessed by NIRS). Differences between the start and stop of apnea phases were measured for PaO2, PaCO2, SpO2, and TOI (also if the primary endpoint could not be reached), BIS values, heart rate, and mean arterial pressure (MAP) were defined as secondary endpoints.
At all time points (T0 to T4), blood gas samples were obtained and measured using the ABL835 Flex blood gas analyzer from Radiometer Medical (Copenhagen, Denmark).
Sample size calculation
For the power calculation, data variability has been estimated using data from Eichhorn et al. [17] on the primary outcome (length of the apnea phase in s until TOI decreased by 20% from baseline). Assuming a within-subject correlation of ρ = 0.3, a standard deviation of 45.04 s was calculated [18]. We determined that a mean difference of 30 s in the primary outcome would be clinically relevant. Using the formula published by Senn [19], a total of 28 patients would be needed to show an average difference of 30 s between the two treatments. Assuming a dropout rate of 5%, the target sample size of 30 was determined.
Blinding
Patients were blinded to the intervention. The biostatistician was blinded for the analysis. The investigators were not blinded.
Statistical analyses
Continuous data were summarized as median and interquartile range (IQR), and categorical data were summarized as numbers (n) and proportions of the total (%). As specified in the study protocol, linear mixed-effects models were used to estimate the average effect of O2/CO2 adjusted for potential period effects while including the patient ID as a random effect. Other models were additionally adjusted for baseline measurements or the presence of obstructive sleep apnea. As baseline correction did not impact the study results, all data are presented without baseline correction in the final analyses.
Linear mixed models with and without adjustment for additional covariables, model diagnostics, as well as visualization methods adhere to the recommendations published by Senn [19]. Pearson correlation was used to quantify the linear association between study outcomes at T2 and T4. As presumed in the trial protocol, missing data did not exceed 5% in any of the primary or secondary outcomes. While no imputation methods were considered, the models included all patients, even if missing values were present in one of the two intervention periods. All analyses were performed in the R programming language (R Core Team, 2017) (R version 3.5.2 (2018–12-20)). Linear mixed-effects models were fit using the lme4 package [20], with p-values computed using the lmerTest package [21].
Results
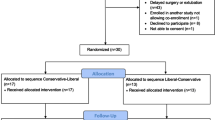
Between January 24 and October 26, 2018, 104 patients scheduled for bariatric surgery at the University Hospital Zurich were screened for eligibility, of which 30 were enrolled in the study. Seventeen patients were randomized to receive O2/Air followed by O2/CO2, and 13 patients to receive O2/CO2 followed by O2/Air. The patient flow is depicted in Fig. 2.
Data collection and presentation
Two patients, one in the O2/Air—O2/CO2 group and one in the O2/CO2—O2/Air group, could not undergo the second intervention due to bronchospasm during the first re-ventilation phase. Two patients started to breathe spontaneously during the apnea phase, and in one patient, a blood sample was drawn not at the end of the apnea phase but when re-ventilation had already started.
Patient characteristics and baseline parameters
Patient characteristics, including age, sex, BMI, as well as comorbidities (arterial hypertension, diabetes, NYHA class,) and laboratory parameters, were balanced between the two randomization sequences, except for the incidence of sleep apnea, which was higher in the O2/Air—O2/CO2 than in the O2/CO2—O2/Air sequence. All patient characteristics are presented in Table 1.
At baseline (T0), TOI, BIS values, MAP, HR, SpO2, EtCO2, respiratory rate, airway pressures, PaCO2, and PaO2 were comparable between the two randomization sequences. At the same time, the O2/Air-first group had a higher FiO2 and minute ventilation than the O2/CO2—O2/Air group. Detailed information regarding the neurological activity, vital parameters, and ventilator settings at T0 are provided in Table 2.
Primary and secondary outcomes
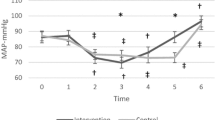
The primary outcome, time until TOI dropped by 20%, was not a feasible target. The safety criterion for early termination of the apnea—SpO2 dropping to 80%—was reached first in all patients (n = 30). The primary outcome, time until TOI dropped by 20%, could thus not be measured. The time until SpO2 dropped to 80% was similar after ventilation with O2/CO2 vs. O2/Air (+ 6 s; 95%CI: from -7 to 19; p = 0.37; Fig. 3A).
Values at the end of the apnea. The time until the peripheral oxygen saturation (SpO2) reached 80% (A), cerebral tissue oxygenation index (TOI) (B), arterial oxygen pressure (PaO2) (C), and arterial carbon dioxide pressure (PaCO2) (D) at the end of the apnea phase are displayed. The time until SpO2 reached 80% was similar after the two treatments, while TOI (p = 0.02), PaO2 (p = 0.02), and PaCO2 (p < 0.001) increased under O2/CO2 administration
At the end of the apnea phase (T2 and T4, respectively), cerebral TOI was significantly higher after O2/CO2 compared to O2/Air, with a mean difference of + 1.5% (95%CI: from 0.3 to 2.6; p = 0.02; Figs. 3B and 4). Similarly, O2/CO2 was also associated with a higher PaO2 (at T2 and T4, respectively, mean difference + 0.6 kPa; 95%CI: from 0.1 to 1.1; p = 0.02; Fig. 3C) and higher PaCO2 at the end of the apnea phase (mean difference + 1.06 kPa; 95%CI: from 0.76 to 1.36; p < 0.001; Fig. 3D).
BIS, MAP, and HR were comparable after ventilation with O2/CO2 (vs. O2/Air) (BIS: + 1%; 95%CI: from -3 to 5; p = 0.7; MAP: -3 mmHg MAP; 95%CI: from -9 to 3; p = 0.3; HR: + 1/min; 95%CI: from -1 to 4; p = 0.3). Details about the treatment effects on all primary and secondary outcomes are reported in Table 3.
Additionally, the lowest TOI value for both interventions was defined in each subject, and the time to reach it after apnea initiation was determined. The time to the lowest TOI was similar for O2/CO2 vs. O2/Air (mean: + 9 s; 95%CI: from -2 to 20; p = 0.1). Details are available in the supporting information S1.
Adverse events
Two patients had bronchospasm after the first apnea phase and were not subjected to a second apnea phase. Two individuals complained of postoperative thoracic pain and one of epigastric pain. Cardiac involvement could be excluded by electrocardiogram and troponin T measurement in all patients.
Discussion
This study assessed the effect of O2/CO2 to improve cerebral oxygenation and apnea tolerance in obese patients under general anesthesia. While the primary endpoint could not be reached due to safety reasons, our results indicate improved cerebral TOI and a higher PaO2 after ventilation with O2/CO2. However, the time until SpO2 dropped to the safety threshold of 80%, as well as BIS, MAP, and HR values, were similar after O2/Air and O2/CO2 application. Ventilation with O2/CO2 did not affect the time to the lowest TOI measured compared to O2/Air.
The benefits of CO2 on brain oxygenation and functional outcomes, such as task performance, have mainly been the subject of high-altitude or aviation research. These outcome changes might result from a right shift of the oxygen dissociation curve [22], a reduction in pulmonary shunting [23], or an increase in cardiac output [24] in response to a higher CO2 partial pressure. Most important, however, is probably cerebral tissue oxygenation, which is affected by CO2-dependent vasodilation [25].
Obese patients represent a population particularly vulnerable to hypoxia as they have a reduced oxygen reserve due to a lower FRC. Indeed, even after pre-oxygenation, obese patients present with a clinically relevant desaturation to 90% in less than three minutes, which is much faster compared to non-obese patients [26, 27]. As these patients have such a low tolerance for apnea, even a slight advantage provided by CO2 could benefit and help to prevent neurological sequelae if scenarios such as “cannot ventilate, cannot intubate” occur.
A strength of our study was the crossover study design, which increases the precision of the treatment effect measurement and minimizes confounding, as each patient acts as their control.
Our study, however, has some limitations. The primary outcome, the length of the apnea phase until TOI decreased by a relative 20% from baseline, could not be reached due to safety reasons. Changing a primary endpoint after the start of a clinical trial is only justifiable if the reasons for doing so are based on new information unrelated to the study conducted, for example, from another independent study [28]. Since this was not the case, the primary endpoint could not be changed. Nevertheless, all data from clinical trials should be published. Our data could lay the ground for studies with a more accessible primary endpoint. Furthermore, the crossover design of this proof-of-concept study allowed for a smaller sample size in a single-center trial. A further limitation of this study was that only patients and the biostatistician were blinded, while the investigators were not blinded.
For this study, we defined our primary endpoint as a relative 20% cerebral TOI reduction from baseline. A larger decrease has a negative predictive value in carotid surgery [16]. Experimental studies in piglets suggest that immediate damage is unavoidable if TOI drops below 30–45%. Above this value, which has not been established nor confirmed in humans, the onset of injury appears to be time-dependent [29]. The benefit of CO2 could, therefore, be more significant at different thresholds or under more severe conditions of hypoxia.
The safety abortion criterion was chosen so that a clinically relevant level of hypoxia was reached while ensuring the patient’s safety, based on previous data. While data on anesthetized patients are scarce, hypoxia studies in awake probands exposed to SaO2 levels of < 80% for 15 min [29] and hypoxia to SaO2 levels of 50–70% for 10 to 30 min were well tolerated and not associated with adverse outcomes in healthy adults [30]. Data from healthy subjects cannot be extrapolated to multimorbid patients, such as morbidly obese patients. A recent systematic review in sedated ICU patients suggests that low TOI is associated with a higher incidence of delirium in critically ill patients [11]. The duration of low TOI is likely to be important. Because the duration of measurement has not been reported in many studies and TOI values are variable [11]. a safety threshold has not yet been established. A relative decrease in TOI of 20% from baseline, established in patients diagnosed with cerebrovascular disease and undergoing carotid endarterectomy, was proposed as the primary endpoint of this study [16]. Because the authors decided to resume ventilation immediately after the primary endpoint or safety endpoint was met, and because patients with a cerebrovascular disease were excluded from study participation, it is unlikely that patients were exposed to excessive health risk.
Conclusions
O2/CO2 improves cerebral TOI and PaO2 in anesthetized bariatric patients. Better apnea tolerance could not be confirmed.
Availability of data and materials
De-identified data of the measurements are available from the corresponding author upon reasonable request.
References
Boron WF, Boulpaep EL: Medical physiology : a cellular and molecular approach, Updated second edition. edn. Philadelphia, PA: Saunders/Elsevier; 2012.
Brzecka A. Role of hypercapnia in brain oxygenation in sleep-disordered breathing. Acta Neurobiol Exp (Wars). 2007;67(2):197–206.
Przybylowski T, Bangash MF, Reichmuth K, Morgan BJ, Skatrud JB, Dempsey JA. Mechanisms of the cerebrovascular response to apnoea in humans. J Physiol. 2003;548(Pt 1):323–32.
Ainslie PN, Poulin MJ. Ventilatory, cerebrovascular, and cardiovascular interactions in acute hypoxia: regulation by carbon dioxide. J Appl Physiol (1985). 2004;97(1):149–59.
Karl AA, McMillan GR, Ward SL, Kissen AT, Souder ME. Effects of increased ambient CO2 on brain tissue oxygenation and performance in the hypoxic rhesus. Aviat Space Environ Med. 1978;49(8):984–9.
Harvey TC, Raichle ME, Winterborn MH, Jensen J, Lassen NA, Richardson NV, Bradwell AR. Effect of carbon dioxide in acute mountain sickness: a rediscovery. Lancet. 1988;2(8612):639–41.
Van Dorp E, Los M, Dirven P, Sarton E, Valk P, Teppema L, Stienstra R, Dahan A. Inspired carbon dioxide during hypoxia: effects on task performance and cerebral oxygen saturation. Aviat Space Environ Med. 2007;78(7):666–72.
Alberto A. Terapia del male degli aviatori. La Ipobaropatia Giorn di Med Mil. 1918;66:183–91.
Godoy DA, Seifi A, Garza D, Lubillo-Montenegro S, Murillo-Cabezas F: Hyperventilation therapy for control of posttraumatic intracranial hypertension. Frontiers in Neurology 2017, 8.
Silvera F, Gagliardi T, Vollono P, Fernández C, García-Bayce A, Berardi A, Badía M, Beltrán B, Cabral T, Abella P, et al. Study of the relationship between regional cerebral saturation and pCO2 changes during mechanical ventilation to evaluate modifications in cerebral perfusion in a newborn piglet model. Braz J Med Biol Res. 2022;55:e11543.
Bendahan N, Neal O, Ross-White A, Muscedere J, Boyd JG. Relationship between near-infrared spectroscopy-derived cerebral oxygenation and delirium in critically ill patients: a systematic review. J Intensive Care Med. 2018;34(6):514–20.
Naguib M, Brewer L, LaPierre C, Kopman AF, Johnson KB. The Myth of Rescue Reversal in “Can’t Intubate, Can’t Ventilate” Scenarios. Anesth Analg. 2016;123(1):82–92.
Eikermann M, Garzon-Serrano J, Kwo J, Grosse-Sundrup M, Schmidt U, Bigatello L. Do patients with obstructive sleep apnea have an increased risk of desaturation during induction of anesthesia for weight loss surgery? Open Respir Med J. 2010;4:58–62.
El-Orbany M, Woehlck HJ. Difficult mask ventilation. Anesth Analg. 2009;109(6):1870–80.
Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340: c332.
Mille T, Tachimiri ME, Klersy C, Ticozzelli G, Bellinzona G, Blangetti I, Pirrelli S, Lovotti M, Odero A. Near infrared spectroscopy monitoring during carotid endarterectomy: which threshold value is critical? Eur J Vasc Endovasc Surg. 2004;27(6):646–50.
Eichhorn L, Erdfelder F, Kessler F, Doerner J, Thudium MO, Meyer R, Ellerkmann RK. Evaluation of near-infrared spectroscopy under apnea-dependent hypoxia in humans. J Clin Monit Comput. 2015;29(6):749–57.
Julious SA. Sample sizes for clinical trials with normal data. Stat Med. 2004;23(12):1921–86.
Senn S: Cross-over trials in clinical research, 2nd edn. Chichester, Eng. ; New York: J. Wiley; 2002.
Bates D, Machler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48.
Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Softw. 2017;82(13):1–26.
Boning D, Schwiegart U, Tibes U, Hemmer B. Influences of exercise and endurance training on the oxygen dissociation curve of blood under in vivo and in vitro conditions. Eur J Appl Physiol Occup Physiol. 1975;34(1):1–10.
Michenfelder JD, Fowler WS, Theye RA. CO2 levels and pulmonary shunting in anesthetized man. J Appl Physiol. 1966;21(5):1471–6.
Lamia B, Monnet X, Teboul JL. Meaning of arterio-venous PCO2 difference in circulatory shock. Minerva Anestesiol. 2006;72(6):597–604.
Calderon PGB, Habib M, Kappel F. de Los Reyes AAt: Control aspects of the human cardiovascular-respiratory system under a nonconstant workload. Math Biosci. 2017;289:142–52.
Altermatt FR, Munoz HR, Delfino AE, Cortinez LI. Pre-oxygenation in the obese patient: effects of position on tolerance to apnoea. Br J Anaesth. 2005;95(5):706–9.
Jense HG, Dubin SA, Silverstein PI, Olearyescolas U. Effect of obesity on safe duration of apnea in anesthetized humans. Anesth Analg. 1991;72(1):89–93.
Kurth CD, McCann JC, Wu J, Miles L, Loepke AW. Cerebral oxygen saturation-time threshold for hypoxic-ischemic injury in piglets. Anesth Analg. 2009;108(4):1268–77.
Bickler PE, Feiner JR, Lipnick MS, Batchelder P, MacLeod DB, Severinghaus JW. Effects of acute, profound hypoxia on healthy humans: implications for safety of tests evaluating pulse oximetry or tissue oximetry performance. Anesth Analg. 2017;124(1):146–53.
Grocott MP, Martin DS, Levett DZ, McMorrow R, Windsor J, Montgomery HE. Caudwell xtreme everest research G: arterial blood gases and oxygen content in climbers on mount everest. N Engl J Med. 2009;360(2):140–9.
Acknowledgements
The authors would like to thank Sabine Kern (study nurse), Rolf Schüpbach (MD), Simona Bergamin (MD), Konstantin Dirscherl (MD), and Mattia Müller (MD) for their help with recruiting patients and conducting the study intervention.
Funding
Financial support was provided by a grant of the Swiss National Airforce. The Near Infrared Spectrometer SenSmart X-100, Nonin Medical, was made available for this clinical trial free of charge by MK-Med AG Medizintechnik, Raron, Switzerland.
Author information
Authors and Affiliations
Contributions
Study design: StM, KA, SS, BJM, BM, HSR, BSB, SchM; data collection: SMT, BSB, SchM; data analysis: KL, HSR, SchM; manuscript preparation: SMT, PA, BSB, SchM; manuscript revision: SMT, StM, KA, SS, BJM, BM, KL, HSR, PA, BSB, and SchM; approval of the final manuscript: SMT, StM, KA, SS, BJM, BM, KL, HSR, PA, BSB, and SchM; agreement to be accountable for all aspects of the work: SMT, StM, KA, SS, BJM, BM, KL, HSR, PA, BSB, and SchM. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the local ethics committee (Kantonale Ethikkommission Zurich, Zurich, Switzerland, number 2017 – 01790) as well as by the national authorization and supervisory authority for drugs and medical products (Swissmedic Bern, Switzerland, notification number 2017DR2183). All patients provided written and informed consent before the study intervention. All study interventions were conducted following the national and international guidelines and in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
Sandro Studer, Andres Kunz, Marc Schmidt, Sarah Haile, Lucas Kook, Andreas Pregernig, and Marco Bueter declare no conflicts of interest.
Beatrice Beck-Schimmer: BBS received a grant from Baxter AG (not related to this work). BBS was a participant in an Advisory Board Meeting of Baxter AG (not related to this topic). BBS has a patent 04/10/14 – 20140100278: Injectable formulation for treatment and protection of patients having an inflammatory reaction or an ischemia–reperfusion event; M. Urner, L.K. Limbach, I.K. Herrmann, W.J. Stark, B. Beck Schimmer, applied as Patent Cooperation Treaty (PCT) (internationally), July 2009, as well as a patent application on bioconjugates of antibodies and functionalized magnetic nanoparticles. BBS and Martin Schläpfer have received Sedana Medical AB, Danderyd, Sweden, grant money as collaborators of a large multicenter study ‘Inhaled Sedation in COVID-19-related acute respiratory distress syndrome (ISCA): an international research data study in the recent context of widespread disease resulting from the 2019 (SARS-CoV2) coronavirus pandemics (COVID-19), not related to this topic.
Martin Schläpfer has received research money for an investigator-initiated trial from Roche AG, Switzerland, not related to this study, and travel support from Baxter AG, Switzerland, as well as research money from Sedana Medical AB, Danderyd, Sweden, for another investigator-initiated trial, not related to this study. Martin Schläpfer, Beatrice Beck-Schimmer, and Marc Studer have submitted a patent for the application of CO2/O2 mixtures to mitigate negative effects of surgery and anesthesia.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplemental information S1. shows the time difference to reach the lowest tissueoxygenation index (TOI = regional oxygen saturation, SrO2) value inboth treatment arms. Time of the O2/Air treatment has beensubtracted from the corresponding apnea time after O2/CO2treatment. Positive values indicate a longer apnea time to reach the lowest TOIvalue after O2/CO2treatment, negative values indicate ashorter apnea time apnea (A). In the subsequent figures, the course ofTOI during apnea after O2/Air and after application of O2/CO2is depicted for all individuals (B). The red lines indicate O2/Airtreatment, turquoise lines indicate O2/CO2 treatment, thefirst intervention is shown in solid, the second intervention in dashed lines.The blue line indicates the lowest TOI value, that was measured in bothtreatment groups.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Schmidt, M.T., Studer, M., Kunz, A. et al. There is no evidence that carbon dioxide-enriched oxygen before apnea affects the time to arterial desaturation, but it might improve cerebral oxygenation in anesthetized obese patients: a single-blinded randomized crossover trial. BMC Anesthesiol 23, 41 (2023). https://doi.org/10.1186/s12871-023-01982-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-023-01982-9