Abstract
Background
Acute kidney injury (AKI) is a common and severe complication of cardiac surgery with cardiopulmonary bypass (CPB). This study aimed to establish a model to predict the probability of postoperative AKI in patients undergoing cardiac surgery with CPB.
Methods
We conducted a retrospective, multicenter study to analyze 1082 patients undergoing cardiac surgery under CPB. The least absolute shrinkage and selection operator regression model was used to optimize feature selection for the AKI model. Multivariable logistic regression analysis was applied to build a prediction model incorporating the feature selected in the previously mentioned model. Finally, we used multiple methods to evaluate the accuracy and clinical applicability of the model.
Results
Age, gender, hypertension, CPB duration, intraoperative 5% bicarbonate solution and red blood cell transfusion, urine volume were identified as important factors. Then, these risk factors were created into nomogram to predict the incidence of AKI after cardiac surgery under CPB.
Conclusion
We developed a nomogram to predict the incidence of AKI after cardiac surgery. This model can be used as a reference tool for evaluating early medical intervention to prevent postoperative AKI.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is a common and severe complication of cardiac surgery with CPB [1, 2]. The apparent characteristics of AKI are rapid development, poor prognosis, high mortality, and complicated pathogenesis [3,4,5]. Studies have shown that as many as 42% of patients undergoing CPB heart surgery have cardiac surgery-related AKI after surgery, of which 2–6% of patients require continuous renal replacement therapy (CRRT), and the associated survival time is reduced, which usually bring a heavy burden to society. At present, there are few effective treatments for CPB-related AKI in the world, mainly including conservative medication and CRRT as supportive measures [6, 7]. And clinically, the diagnosis of AKI is mainly based on serum creatinine (Scr) and urea nitrogen levels, but these indicators are not sensitive to the early prediction of kidney damage [8, 9]. Furthermore, an emerging method in recent years is to detect biomarkers such as NGAL, KIM-1, miRNA-21 in blood or urine for early diagnosis of AKI. Whereas, these biomarkers are usually only a few hours earlier than the detection of Scr, and some biomarkers are also present in other diseases and cause false positives [10]. Thereby, it is necessary to identify high-risk patients early and give appropriate postoperative AKI support.
In order to rationally manage AKI after cardiac surgery, a model that can accurately predict high-risk patients to optimize postoperative AKI treatment strategies is very urgent. The Cleveland University Acute Renal Failure Score System (Cleveland Score) [11], Simplified Kidney Index Score (SRI Score) and other researchers have established predictive models of AKI after cardiac surgery, which are widely used [12]. However, these models are mainly based on old clinical data collected more than ten years ago, and mainly come from western populations. For clinicians, early prediction, early diagnosis and management of AKI after cardiac surgery are still challenging. Hence, the establishment of risk models for such patients still has important clinical significance.
The nomogram transforms the complex regression equation into a visual graph, making the results of the prediction model easier to read and more convenient to evaluate the patient’s condition. Due to its intuitive and easy-to-understand characteristics, nomogram has gradually received more and more attention and applications in medical research and clinical practice [13, 14].
However, nomograms are rarely used in AKI after cardiac surgery. Therefore, this study intends to use perioperative clinical information to include some preoperative and intraoperative risk factors that may affect the occurrence of postoperative AKI into the study. According to the AKI standard proposed by KDIGO in 2012, a nomogram prediction model was established through multivariate logistic regression analysis. Predict the prevalence of AKI in patients undergoing CPB cardiac surgery, and provide clinical guidance for early identification and intervention of high-risk patients [15].
Materials and methods
Study population
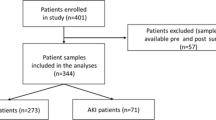
A retrospective analysis (2015–2020) of patients > = 18 years of age who underwent CPB cardiac surgery collected from two Grade A hospitals in China (Foshan First People’s Hospital; Zhongshan People’s Hospital). Excluding long-term dialysis patients with chronic renal failure and patients with incomplete clinical data, a total of 1082 patients were included in the study. 758 patients assigned to the modeling group and 324 assigned to the verification group. In addition, a total of 108 patients who met the inclusion criteria in Guangzhou First People’s Hospital from January 1, 2020 to October 1, 2020 were retrospectively collected. These 108 patients were used as an external validation group to verify the effectiveness of the model.
Data collection and definitions
The risk factors for preoperative AKI included the patient’s age, sex, and pre-surgical history of hypertension, diabetes, or anemia. We investigated CPB time, blood transfusion volume, urine volume, and the use of vasoactive and other drugs as intraoperative variables.
According to the clinical practice guidelines for AKI in Kidney Disease: Improving Global Outcomes (KDIGO), AKI is defined as any of the following: an increase in Scr of 0.3 mg/dl (26.5µmol/L) within 48 h or Scr increased to 1.5 times the baseline level within 7 days, or urine volume < 0.5 ml/kg/h for 6 h [16]. In this study, we used blood creatinine values to define acute kidney injury. Baseline Scr levels were defined as the values obtained closest to the date of surgery.
Statistical analysis
Statistical analyses were performed using the R software (Version 3.6.2; https://www.R-project.org) and SPSS21.0 software. First, the minimal absolute contraction and selection operator (LASSO) method for reducing high-dimensional data was used to select the best predictive characteristics of risk factors for postoperative AKI in patients who had undergone cardiac surgery with CPB [17, 18]. Select variables with non-zero coefficients in the LASSO regression model. Subsequently, through multivariate logistic regression analysis, the variables with p-value < 0.05 were used to construct a prediction model. Harrell’s C index was measured to quantify the discriminative performance of the nomogram [19]. Following this, calibration curves were plotted to assess the calibration of the nomogram. We analyzed the decision curve to determine the clinical effectiveness of the nomogram by quantifying the net benefits under different threshold probabilities in the cohort. Moreover, internal verification is performed by calculating the C index of the verification group. Finally, we included the variables included in the logistic regression model, reconstructed the model using a neural network, and evaluated the variable importance and model performance.
Results
Patient characteristics
We divided the 1082 patients included in this study into AKI group (288) and non-AKI group (794) according to KDIGO standards. Table 1 shows the preoperative and intraoperative factors of the two groups of patients. 70% (758) patients were randomly selected to establish the prediction model, and 30% (324) patients were used to verify the accuracy of the model.
Feature selection
In terms of preoperative and intraoperative influencing factors, according to the validation group of 758 patients (~ 2:1 ratio; Fig. 1 A and Fig. 1B), 17 variables were reduced to 10 potential predictors, and they were returned in LASSO. There are non-zero coefficients in the model. These variables include age, sex, hypertension, anemia, CPB duration, intraoperative succinylated gelatin and 5% bicarbonate solution infusion (SBS), as well as red blood cell (RBC) infusion, intraoperative blood loss and urine output (Table 2).
The LASSO binary logistic regression model was used to select the risk factors of AKI after CPB. A The choice of the best parameter (lambda) in the LASSO model passed the lowest standard for five times of cross-validation. The partial likelihood deviation (binomial deviation) curve is plotted against log (λ). Draw a dashed vertical line with the best value by using the minimum standard and the minimum standard 1 SE (1-SE standard). B LASSO coefficient curve of 17 features. A coefficient distribution map for the logλ sequence is generated. Vertical lines are drawn at the values selected using five-fold cross-validation, where the optimal λ produces sixteen features with non-zero coefficients
Predictive nomogram for the probability of AKI
Based on the final regression analysis, a nomogram containing seven essential risk factors for predicting AKI in patients who had undergone cardiac surgery with CPB was constructed. The total score was calculated based on gender, age, hypertension, CPB duration, intraoperative 5% SBS and RBC transfusion, and urine output. The value of each variable was assigned a score on the point scale axis. The total score could be calculated easily based on addition of individual scores. Following this, the overall score was projected into a total score (Fig. 2). In this manner, we could estimate the probability of AKI in patients who had undergone cardiac surgery with CPB.
The nomogram of AKI after CPB surgery. The Nomogram of AKI after CPB surgery estimates the probability of AKI after the operation. Mark the patient value on each axis, draw a straight line perpendicular to the point axis, and add the points of each variable. Next, mark the sum on the total point axis and draw a straight line perpendicular to the probability axis. RBC- intraoperative transfusion of red blood cells; SBS-intraoperative 5% sodium bicarbonate solution infusion; Urine-intraoperative urine output
Apparent performance of the AKI risk nomogram in the cohort
The calibration curve of the nomograms used to predict the risk of AKI in patients after surgery showed good agreement (Fig. 3). The C index of the predicted nomogram of this group was 0.725 (95% confidence interval: 0.685–0.765). Furthermore, the value was confirmed as 0.709 by the verification group, which indicated good degree of recognition of the model. In order to further verify the effectiveness of the model, we also retrospectively collected 108 patients who met the inclusion criteria in Guangzhou First People’s Hospital from January 1, 2020 to August 1, 2020 as an external validation. The median age of the external validation group was 58.5 years (50, 66), the proportion of male patients was 35.2%, the preoperative hypertension patients were 27.8%, the median CPB time was 94.5 min (60, 135), and the median red blood cell transfusion volume was 2u (0, 4.5), the median intraoperative sodium bicarbonate injection infusion was 0 ml (0, 0), and the median urine output was 800 ml (500, 1000). According to the establishment of the above model, the C-index of the external validation group is 0.973.
Calibration curve for nomogram prediction of AKI after CPB in the cohort. The x-axis represents the predicted risk of AKI after CPB. The y-axis represents the actual occurrence of AKI after CPB. The dotted line on the diagonal represents the perfect prediction of the ideal model. The solid line represents the performance of the nomogram. The closer the fit to the diagonal dashed line is, the better the prediction effect
Clinical applications
Analysis of the decision curve of the AKI nomogram is shown in Fig. 4. The decision curve shows that if the threshold probability is greater than 4%, the use of the nomogram of AKI after CPB in the current study to predict the risk of AKI will increase the benefit more than the full-patient intervention program or the non-intervention program.
Decision curve analysis for the AKI nomogram. The y-axis is net income. The blue line represents the distribution of AKI after CPB. The thin solid line indicates that it is assumed that all patients undergoing CPB surgery do not develop AKI after surgery. The thick solid line represents the hypothesis that all patients have AKI. The decision curve shows that if the threshold probability is greater than 4%, the use of the nomogram of AKI after CPB in the current study to predict the risk of AKI will increase the benefit more than the full-patient intervention program or the non-intervention program
Neural network validation
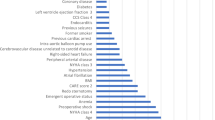
Artificial Neural Network (ANN) is a statistical method with similar characteristics generated by people simulating the structure and function of the human brain [20]. Logistic regression and ANN have essential differences. Although both are popular algorithms in the field of machine learning, they have different advantages in different aspects: Compared with the readability of the model, Logistic regression has a great advantage over neural network. In most cases, because of the complex internal operation mechanism of neural network, the output results are directly obtained from the input, and people can use the knowledge induced by the model without understanding its operation [21]. We reconstruct the neural network model with the independent variables included in the above logistic regression model. First, we used a multilayer perceptron to perform neural network model with postoperative AKI as the dependent variable and gender, age, hypertension, CPB time, intraoperative red blood cell transfusion, intraoperative SBS transfusion, and urine volume as covariates. The 1082 patient data were randomly split into 761 training sets (70.3%) and 321 validation sets (29.7%). In addition, the function of model building activation is a hyperbolic tangent function, and the weights are updated using the scaled conjugate gradient method. The model confusion matrix is shown in Table 3. Next, the importance of independent variables is evaluated according to the results of feature importance analysis of the neural network model, and the ranking is shown in Fig. 5; Table 4. The results showed that the order of importance of independent variables was age, CPB time, RBC infusion, SBS infusion, urine output, gender, and hypertension. We unexpectedly found that the results were consistent with the order of axis lengths represented by the independent variables of the above nomogram. Finally, we evaluate the performance of the neural network model. The ROC curve of the model is shown in Fig. 6, and the area under the curve is 0.749, which indicates that the model has good generalization ability.
The importance of normalizing. The independent variable importance map can be used to analyze which factors have a greater impact on the predicted value, and the more obvious the importance, the greater the impact on the predicted value. This figure suggests that Age has the greatest impact on postoperative AKI.
ROC curve of neural network model. The ROC curve is a curve reflecting the relationship between sensitivity and specificity. The abscissa (X-axis) is 1 – specificity, also known as the false positive rate (false positive rate), the closer the X-axis is to zero, the higher the accuracy; the ordinate (Y-axis) is called sensitivity, also known as true positives rate (sensitivity), the larger the Y-axis, the better the accuracy. The area under the ROC curve (AUC) can evaluate the quality of the model. If the area under the ROC curve is greater than 0.5, it proves that the model has certain value. The closer the AUC is to 1, the better the authenticity of the model is proved. The area under the ROC curve of this neural network model was 0.749, which confirmed the good value of this model
Discussion
In 1953, the successful implementation of the first open heart surgery under CPB officially opened the prelude to cardiac surgery. This is considered to be one of the most important clinical developments in the medical field [22]. The CPB technology temporarily replaces the human heart and lungs with an external circuit composed of a pump and an oxygenation membrane. Its purpose is to replace the heart and lung function to maintain the blood perfusion of the whole body tissues and organs when performing heart surgery, and at the same time create a good surgical field of vision for the surgeon [23]. Although CPB has now become an indispensable technique for many heart operations, the potential adverse effects of this technique on sensitive organs such as the brain or kidney cannot be ignored. During CPB, patients are exposed to a complex set of non-physiological conditions. Blood coagulation, pro-inflammatory, activation of survival cascade and changes in redox state can all trigger systemic inflammatory response syndrome, leading to multiple organ dysfunction [24]. Compared to other specialized surgeries, the operative morbidity and mortality of patients undergoing cardiac surgery with CPB are higher. The incidence of postoperative complications has been of great concern [25]. As a common clinical complication of cardiac surgery, AKI significantly reduces the cure rate and quality of life of the patients [26, 27]. We observed that AKI occurred in approximately 27% of the patients in our study. In the analysis of risk factors, postoperative AKI was related to gender, age, hypertension, CPB duration, intraoperative 5% SBS and RBC transfusion, and urine output. The nomogram shows that males, older age, preoperative hypertension, longer CPB duration, intraoperative RBC and 5% SBS infusion, and less urine output will increase the risk of AKI after surgery. Hence, it is necessary and meaningful to take preventive measures. The current clinical diagnosis of AKI chiefly relies on assessment of Scr and urea nitrogen levels [28]. However, these traditional biomarkers do not enable early referral to clinicians, thus preventing early intervention to avoid the occurrence of AKI. Therefore, an accurate prediction method of postoperative AKI in patients undergoing cardiac surgery with CPB is clinically crucial.
In the present study, we built a model to predict the risk factors of AKI in patients who underwent cardiac surgery with CPB. The nomogram, which transforms the statistical prediction model into a simple and intuitive graph, so as to calculate the numerical probability of the occurrence of clinical events. The nomogram first builds a multi-factor regression model, assigns points to each value level of each influencing factor, and adds up the points of each segment to obtain the total score. Finally, the total score and the probability of an outcome event are converted by a function to obtain the predicted probability of an individual outcome event. We specifically studied the relationship between preoperative and intraoperative influencing factors and postoperative AKI. Internal validation of the cohort showed good discrimination and calibration capability. In particular, the high C index value in the interval validation indicated that the collinear map could be used widely and accurately for large samples.
Each factor of the nomogram represents the length of the line segment, which reflects the contribution of the factor to the end event. Sexual dimorphism has been correlated with AKI. Large-scale epidemiological studies have shown that female have a lower incidence of AKI after non-cardiac surgery. Interestingly, based on various retrospective and prospective studies, national and international registration data, most researches on AKI after cardiac surgery consider female to be an independent risk factor for its development [29,30,31]. Nevertheless, our findings contradict the widely accepted view that female are an independent risk factor for the development of AKI after cardiac surgery. In 2016, Neugarten et al. also reached a contradictory conclusion through meta-analysis, they think this may simply reflect a different definition of AKI after heart surgery [32]. Whereas, this simple explanation may obscure the more complex interaction between gender and cardiovascular surgery. Furthermore, previous studies on animal models have reported that female sex had an inherent protective role against ischemia reperfusion induced AKI, and testosterone increases the susceptibility of the kidney to ischemic injury [9, 33,34,35]. The relationship between gender and AKI after cardiac surgery still needs further research.
Recent studies have shown that with age, the structure and function of the kidney undergo characteristic changes. These changes impair the ability of the kidney to withstand and recover from injury, resulting in a high sensitivity of the elderly to AKI [36]. Previous studies have shown that nephrons are stably lost, and the glomerular filtration rate decreases with age (in individuals aged ≥ 30 years) [37]. Moreover, a large number of clinical studies have also confirmed that the elderly are more likely to develop AKI after cardiac surgery and have a worse prognosis than young patients [38]. Similarly, our study confirmed that age is an independent risk factor for AKI after cardiac surgery with CPB.
The relationship between kidneys and hypertension has been extensively studied. Persistent high blood pressure will increase the hydrostatic pressure of the renal blood vessels and cause thickening of the arterial wall. This is due to the increase in the smooth muscle and hyaluronic acid content of the medium and the narrowing of the lumen, which in turn leads to a decrease in renal blood flow. In CPB heart surgery, the kidney is in a state of hypoperfusion, these changes also make the kidney more prone to prerenal AKI [39]. Studies have confirmed that preoperative hypertension is an independent risk factor for AKI after CPB. They believe that long-term persistent hypertension can increase the pressure of the glomerulus, leading to pathological changes such as glomerular fibrosis and vascular sclerosis. Even if the renal function is normal before surgery, there may be renal parenchymal ischemia and nephron reduction. This will increase the risk of AKI after CPB. In 2018, Barkhordari et al. conducted a cross-sectional study of 3473 patients, and the results showed that hypertension was significantly associated with the incidence of AKI after coronary artery bypass graft surgery [40]. The results of this study indicate that patients diagnosed with hypertension before surgery have an increased risk of AKI after CPB, which is consistent with most studies.
Acute blood loss or decreased hematocrit is common in heart surgery, so blood transfusion is often performed during surgery to improve oxygen supply to the kidneys and other important organs [41]. Throughout the ages, restrictive blood transfusion and liberal blood transfusion programs have been controversial and have been widely studied. However, studies have confirmed that blood transfusion is not a benign intervention and has been associated with multiple organ failure, including AKI in patients undergoing CPB. Recently, data from a randomized controlled trial showed that in patients undergoing cardiac surgery, restrictive blood transfusion regimens are not inferior to free blood transfusion regimens [42]. Rasmussen et al. confirmed that the transfusion of all blood products increased the risk of AKI in a dose-dependent manner. However, in a multivariate analysis combining all blood products, only red blood cell transfusion is still significantly associated with the occurrence of acute kidney injury [43]. This is consistent with our conclusion. At present, several pathophysiological mechanisms of kidney injury after red blood cell infusion have been proposed. The consumption of 2,3-diphosphoglycerate in stored red blood cells may change the oxygen dissociation curve and may impair oxygen delivery [44]. In addition, mechanical damage and turbulent channels in CPB may enhance hemolysis. Hemolysis of red blood cells increases the concentration of free hemoglobin and iron, as well as pro-inflammatory molecules, which are toxic to the kidneys. Our findings confirmed that with the increase of intraoperative blood transfusion, the incidence of postoperative AKI also increases.
Furthermore, our study confirmed that the risk of AKI after surgery increased with an increase in the CPB time, which was similar to that reported in previous studies [45]. AKI may be related to renal hypoperfusion and ischemia reperfusion injury caused by CPB [46, 47]. Moreover, CPB stimulates the systemic inflammatory response, leading to release of a series of pro-inflammatory mediators and factors, which further causes AKI [48, 49]. Therefore, based on current clinical evidence, reducing the CPB time to the maximum possible extent remains the focus to decrease the risk of postoperative AKI.
SBS is widely used considering its theoretically protective effects on renal tubules. A double-blind, randomized, controlled trial that recruited many patients who had undergone cardiac surgery proposed that perioperative urinary alkalization reduced the incidence of postoperative AKI from 52 to 32%, with no apparent side effects [50]. The mechanism related to this effect is thought to be related to the ability of bicarbonate to alkalize urine and slow-down the Haber-Weiss reaction that produces active oxygen through iron-dependent pathways [51]. Moreover, another mechanism is that bicarbonate can directly remove other reactive substances present in blood, including hydroxyl radicals and peroxynitrite [52]. However, the protective role of alkalization against AKI has received widespread attention in recent years and has also raised queries. Recent studies have suggested that infusion of SBS does not reduce the incidence of AKI after open-heart surgery [53]. In 2007, a study by Haase et al. showed that sodium bicarbonate is an effective, simple, practical, and inexpensive treatment that can prevent the toxic effects of hemolysis on the kidneys during CPB [50]. Whereas, they reached conflicting conclusions through a multi-center double-blind randomized controlled experiment in 2013. They proposed that in high-risk patients with AKI after open-heart surgery, bicarbonate infusion can alkalinize blood and urine, but cannot reduce the incidence of AKI or reduce acute tubular injury. Importantly, prophylactic use of sodium bicarbonate injection may increase mortality [54]. Our study supports the aforementioned view. We found that increased intraoperative infusion of 5% SBS was associated with higher incidence of AKI after surgery.
Urine volume is closely related to renal function and has been used as a diagnostic criterion for AKI. It has been clinically recognized that insufficient renal perfusion is the main factor that promotes AKI after cardiac surgery [55]. Song et al. divided the patients into two subgroups according to the urine output of less than or more than 4 mL/kg/h during CPB. The urine output was determined to be an independent predictor of AKI, with odds ratios of 0.43 and 1.11, respectively [56]. They believe that urine output can be used as a simple and feasible method to early predict the development of AKI after cardiac surgery. Yilmaz M et al. also believe that urine output during CPB is an important criterion for predicting AKI after coronary artery bypass graft surgery [57]. However, some scholars have proposed that the damage of renal tubular reabsorption and the heterogeneity of nephron function may contradictorily increase the urine output, and maintaining the urine flow may not guarantee the normal function of the kidney. In the same context, excessive urination during CPB should not be interpreted as a favorable signal, as renal tubular damage caused by inflammation and thrombosis during CPB may increase urine flow. Our findings believe that intraoperative urine output is a friendly factor for postoperative AKI. More targeted research should be conducted to understand the relationship between intraoperative urine volume and postoperative AKI in patients undergoing CPB cardiac surgery.
Conclusion
The prediction of postoperative AKI in patients undergoing cardiac surgery with CPB is critical as the complication can affect the overall survival of patients. A novel nomogram was developed and its application was reported in this study. The nomogram demonstrated relatively good accuracy to help clinicians understand the risk of postoperative AKI in patients undergoing cardiac surgery with CPB, thereby enabling appropriate measures to be taken for early medical intervention.
Availability of data and materials
The release of data in this study did not involve the identity information of patients. The full text does not contain any direct or indirect identifiers that expose the basic information of patients, and the clinical information of all participants is completely anonymous. All data generated during this study are included in this published article [and its supplementary information files].
References
Krawczeski CD. Cardiopulmonary bypass and AKI: AKI is bad, so let’s get beyond the diagnosis. Front Pediatr. 2019;7:492.
Gaffney AM, Sladen RN. Acute kidney injury in cardiac surgery. Curr Opin Anaesthesiol. 2015;28(1):50–9.
Kališnik JM, Hrovat E, Hrastovec A, Žibert J, Jerin A, Skitek M, Santarpino G, Klokocovnik T. Creatinine, neutrophil gelatinase-associated lipocalin, and cystatin C in determining acute kidney injury after heart operations using cardiopulmonary bypass. Artif Organs. 2017;41(5):481–9.
Kallel S, Triki Z, Abdenadher M, Frikha I, Jemel A, Karoui A. Acute renal failure after cardiac surgery: evaluation of the RIFLE criteria. Nephrol Ther. 2013;9(2):108–14.
Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, Bihorac A. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119(18):2444–53.
Peng K, McIlroy D, Bollen B, Billings F, Zarbock A, Popescu W, Fox A, Shore-Lesserson L, Zhou S, Geube M, et al. Society of cardiovascular anesthesiologists clinical practice update for management of acute kidney injury associated with cardiac surgery. Anesth Analg. 2022;135(4):744–56.
Long Y, Feng X, Shan X, Chen Q, Xia Z, Ji F, Liu H, Peng K. Remote ischemic preconditioning reduces acute kidney Injury after Cardiac surgery: a systematic review and Meta-analysis of Randomized controlled trials. Anesth Analg. 2022;134(3):592–605.
Saydam O, Turkmen E, Portakal O, Arici M, Dogan R, Demircin M, Pasaoglu I, Yilmaz M. Emerging biomarker for predicting acute kidney injury after cardiac surgery: cystatin C. Turk J Med Sci. 2018;48(6):1096–103.
Hutchens MP, Dunlap J, Hurn PD, Jarnberg PO. Renal ischemia: does sex matter? Anesth Analg. 2008;107(1):239–49.
Xiao Z, Huang Q, Yang Y, Liu M, Chen Q, Huang J, Xiang Y, Long X, Zhao T, Wang X, et al. Emerging early diagnostic methods for acute kidney injury. Theranostics. 2022;12(6):2963–86.
Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16(1):162–8.
Wijeysundera DN, Karkouti K, Dupuis JY, Rao V, Chan CT, Granton JT, Beattie WS. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA. 2007;297(16):1801–9.
Chen S, Ma C, Zhang C, Shi R. Nomogram to predict risk for early ischemic stroke by non-invasive method. Med (Baltim). 2020;99(39):e22413.
Lin H, Hou J, Tang H, Chen K, Sun H, Zheng Z, Hu S. A novel nomogram to predict perioperative acute kidney injury following isolated coronary artery bypass grafting surgery with impaired left ventricular ejection fraction. BMC Cardiovasc Disord. 2020;20(1):517.
Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–e180.
Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, Tolwani AJ, Waikar SS, Weisbord SD. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J kidney diseases: official J Natl Kidney Foundation. 2013;61(5):649–72.
Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26(30):5512–28.
Kidd AC, McGettrick M, Tsim S, Halligan DL, Bylesjo M, Blyth KG. Survival prediction in mesothelioma using a scalable Lasso regression model: instructions for use and initial performance using clinical predictors. BMJ Open Respir Res. 2018;5(1):e000240.
Jin C, Cao J, Cai Y, Wang L, Liu K, Shen W, Hu J. A nomogram for predicting the risk of invasive pulmonary adenocarcinoma for patients with solitary peripheral subsolid nodules. J Thorac Cardiovasc Surg. 2017;153(2):462–9.e461.
Kuan Y, Hong C, Chen P, Liu W, Chung C. Logistic regression and artificial neural network-based simple predicting models for obstructive sleep apnea by age, sex, and body mass index. Math Biosci engineering: MBE. 2022;19(11):11409–21.
Cheng X, Han W, Liang Y, Lin X, Luo J, Zhong W, Chen D. Risk prediction of coronary artery stenosis in patients with coronary heart disease based on logistic regression and artificial neural network. Comput Math Methods Med. 2022;2022:3684700.
Gibbon J. The development of the heart-lung apparatus. Am J Surg. 1978;135(5):608–19.
Hessel E. What’s New in Cardiopulmonary Bypass. J Cardiothorac Vasc Anesth. 2019;33(8):2296–326.
Evora P, Bottura C, Arcêncio L, Albuquerque A, Évora P, Rodrigues A. Key points for curbing cardiopulmonary bypass inflammation. Acta Cirurgica Brasileira. 2016;31(Suppl 1):45–52.
Mazzone AL, Baker RA, Gleadle JM. Mending a broken heart but breaking the kidney. Nephrol (Carlton Vic). 2016;21(10):812–20.
Alge JL, Arthur JM. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol. 2015;10(1):147–55.
O’Neal JB, Shaw AD, Billings FT. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care (London England). 2016;20(1):187.
Teo SH, Endre ZH. Biomarkers in acute kidney injury (AKI). Best Pract Res Clin Anaesthesiol. 2017;31(3):331–44.
Neugarten J, Golestaneh L. Gender and the prevalence and progression of renal disease. Adv Chronic Kidney Dis. 2013;20(5):390–5.
Parida S, Badhe AS. Cardiac surgery-associated acute kidney injury. J Anesth. 2013;27(3):433–46.
Romagnoli S, Ricci Z. Postoperative acute kidney injury. Minerva Anestesiol. 2015;81(6):684–96.
Neugarten J, Sandilya S, Singh B, Golestaneh L. Sex and the risk of AKI following cardio-thoracic surgery: a Meta-analysis. Clin J Am Soc Nephrol. 2016;11(12):2113–22.
Takayama J, Takaoka M, Sugino Y, Yamamoto Y, Ohkita M, Matsumura Y. Sex difference in ischemic acute renal failure in rats: approach by proteomic analysis. Biol Pharm Bull. 2007;30(10):1905–12.
Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem. 2004;279(50):52282–92.
Müller V, Losonczy G, Heemann U, Vannay A, Fekete A, Reusz G, Tulassay T, Szabó AJ. Sexual dimorphism in renal ischemia-reperfusion injury in rats: possible role of endothelin. Kidney Int. 2002;62(4):1364–71.
O’Sullivan E, Hughes J, Ferenbach D. Renal aging: causes and consequences. J Am Soc Nephrology: JASN. 2017;28(2):407–20.
Glassock R, Denic A, Rule AD. When kidneys get old: an essay on nephro-geriatrics. J Bras Nefrol. 2017;39(1):59–64.
Conlon PJ, Stafford-Smith M, White WD, Newman MF, King S, Winn MP, Landolfo K. Acute renal failure following cardiac surgery. Nephrol Dial Transplant. 1999;14(5):1158–62.
Infante B, Franzin R, Madio D, Calvaruso M, Maiorano A, Sangregorio F, Netti G, Ranieri E, Gesualdo L, Castellano G, et al. Molecular Mechanisms of AKI in the Elderly: from animal models to therapeutic intervention. J Clin Med. 2020;9(8):2574.
Barkhordari K, Fakhre Yasseri A, Yousefshahi F, Shafiee A. Risk factors for acute kidney Injury in Coronary artery bypass graft surgery patients based on the Acute kidney Injury Network Criteria. J Tehran Heart Cent. 2018;13(2):52–7.
Habib RH, Zacharias A, Schwann TA, Riordan CJ, Engoren M, Durham SJ, Shah A. Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: implications on operative outcome. Crit Care Med. 2005;33(8):1749–56.
Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, Fukushima J, Kalil Filho R, Sierra DB, Lopes NH, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304(14):1559–67.
Rasmussen S, Kandler K, Nielsen R, Jakobsen P, Ranucci M, Ravn H. Association between transfusion of blood products and acute kidney injury following cardiac surgery. Acta Anaesthesiol Scand. 2020;64(10):1397–404.
Karkouti K. Transfusion and risk of acute kidney injury in cardiac surgery. Br J Anaesth. 2012;109:i29–38.
Ghincea CV, Reece TB, Eldeiry M, Roda GF, Bronsert MR, Jarrett MJ, Pal JD, Cleveland JC Jr, Fullerton DA, Aftab M. Predictors of acute kidney injury following aortic arch surgery. J Surg Res. 2019;242:40–6.
Valenzuela-Flores AG, Valenzuela-Flores AA, Ortega-Ramírez JA, Penagos-Paniagua M, Pérez-Campos JP. Physiopathological alterations secondary to extracorporeal circulation in cardiac surgery. Cir Cir. 2005;73(2):143–9.
Evans RG, Lankadeva YR, Cochrane AD, Marino B, Iguchi N, Zhu MZ, Hood SG, Smith JA, Bellomo R, Gardiner BS, et al. Renal haemodynamics and oxygenation during and after cardiac surgery and cardiopulmonary bypass. Acta Physiol (Oxford, England). 2018;222(3).
Jahnukainen T, Keski‐Nisula J, Tainio J, Valkonen H, Pätilä T, Jalanko H, Suominen P. Efficacy of corticosteroids in prevention of acute kidney injury in neonates undergoing cardiac surgery—A randomized controlled trial. Acta Anaesthesiol Scand. 2018;00:1–8.
Morgan CJ, Gill PJ, Lam S, Joffe AR. Peri-operative interventions, but not inflammatory mediators, increase risk of acute kidney injury after cardiac surgery: a prospective cohort study. Intensive Care Med. 2013;39(5):934–41.
Haase M, Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Reade MC, Bagshaw SM, Seevanayagam N, Seevanayagam S, et al. Sodium bicarbonate to prevent increases in serum creatinine after cardiac surgery: a pilot double-blind, randomized controlled trial. Crit Care Med. 2009;37(1):39–47.
Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Meth Enzymol. 1990;186:1–85.
Caulfield JL, Singh SP, Wishnok JS, Deen WM, Tannenbaum SR. Bicarbonate inhibits N-nitrosation in oxygenated nitric oxide solutions. J Biol Chem. 1996;271(42):25859–63.
Kristeller JL, Zavorsky GS, Prior JE, Keating DA, Brady MA, Romaldini TA, Hickman TL, Stahl RF. Lack of effectiveness of sodium bicarbonate in preventing kidney injury in patients undergoing cardiac surgery: a randomized controlled trial. Pharmacotherapy. 2013;33(7):710–7.
Haase M, Haase-Fielitz A, Plass M, Kuppe H, Hetzer R, Hannon C, Murray PT, Bailey MJ, Bellomo R, Bagshaw SM. Prophylactic perioperative sodium bicarbonate to prevent acute kidney injury following open heart surgery: a multicenter double-blinded randomized controlled trial. PLoS Med. 2013;10(4):e1001426.
Abu-Omar Y, Ratnatunga C. Cardiopulmonary bypass and renal injury. Perfusion. 2006;21(4):209–13.
Song Y, Kim D, Kwak Y, Kim B, Joo H, Ju J, Yoo Y. Urine output during cardiopulmonary bypass predicts acute kidney Injury after Cardiac surgery: a Single-Center Retrospective Analysis. Medicine. 2016;95(22):e3757.
Yilmaz M, Aksoy R, Kilic Yilmaz V, Balci C, Duzyol C, Tekeli Kunt A. Urine output during cardiopulmonary bypass predicts acute kidney injury after coronary artery bypass grafting. Heart Surg Forum. 2016;19(6):E289-93.
Acknowledgements
Many thanks to those who helped me with the writing of the article.
Funding
This work was supported by the Natural Science Foundation of Guangdong Province: 2019A1515011087 to ZHOU J.
Author information
Authors and Affiliations
Contributions
Huan Jing collected and analyzed data and wrote manuscripts. Simin Tang, Meijuan Liao, Sen Lin and Li Ye collected data. Jiying Zhong, Hanbin Wang helped analyze data. Jun Zhou oversaw the research and revised the manuscript. The author(s) read approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki. This study has received an exemption from the need for informed consent and ethical approval from the Medical Ethics Committee of the First People's Hospital of Foshan.
Consent for publication
Not applicable
Competing interests
There is no conflict of interest involved in this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jing, H., Liao, M., Tang, S. et al. Predicting the risk of acute kidney injury after cardiopulmonary bypass: development and assessment of a new predictive nomogram. BMC Anesthesiol 22, 379 (2022). https://doi.org/10.1186/s12871-022-01925-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-022-01925-w