Abstract
Study objective
The purpose of the present study was to evaluate the efficacy of levosimendan in patients with acute myocardial infarction related ventricular septal rupture (AMI-VSR) underwent cardiac surgery.
Design
Prospective observational cohort study with propensity score analysis.
Patients
There were 261 patients with AMI-VSR in our study. After 1:1 propensity matching, 106 patients (53 levosimendan and 53 control) were selected in the matched cohort.
Interventions
None.
Measurements
Patients who received levosimendan were assigned to the levosimendan group (n = 164). The patients who were not received were levosimendan assigned to the control group (n = 97). The levosimendan was initiated immediately after cardiopulmonary bypass. Then, it has been maintained during the postoperative 3 days. The poor outcomes were identified as follows: death and postoperative complications (postoperative stroke, low cardiac output syndromeneeded mechanical circulatory support after surgery, acute kidney injury (≥ stage III), postoperative infection or septic shock, new developed atrial fibrillation or ventricular arrhythmias).
Main results
Before matching, the control group had more length of ICU stay (6.69 ± 3.90 d vs. 5.20 ± 2.24 d, p < 0.001) and longer mechanical ventilation time (23 h, IQR: 16–53 h vs. 16 h, IQR: 11–23 h, p < 0.001). Other postoperative outcomes have not shown significant differences between two groups. After matching, no significant difference was found between both groups for all postoperative outcomes. The Kaplan–Meier survivul estimate and log-rank test showed that the 90-day survival had no significant differences between two groups before and after matching.
Conclusion
Our study found that a low-dose infusion of levosimendan in AMI-VSR patients underwent surgical repair did not associated with positively affect to postoperative outcomes.
Similar content being viewed by others
Introduction
Ventricular septal rupture (VSR) is a fatal complication of acute myocardial infarction (AMI) [1]. The mortality among VSR patients is nearly 41–80% [2, 3]. Surgical repair may be the best choice for VSR compared with other treatments [4]. However, it had been reported that the mortality of surgical repair was from 38.2 to 65% [4,5,6], it is the highest mortality among all kinds of cardiac surgery. Meanwhile, the surgical repair may have some severe postoperative complications which related to poor outcome, such as acute kidney injury (AKI), low cardiac output syndrome (LOCS) and hepatic failure [6, 7]. Thus, preserving hemodynamic stabilization is necessary and crucial. Inotropic agents and mechanical circulatory support (MCS) devices (intra-aortic balloon pump and extracorporeal membrane oxygenation) were usually administrated for hemodynamic stabilization in these patients. However, the traditional inotropic agents have adverse effects in patients with severe left ventricular dysfunction and coronary vascular disease [8]. And the MCS could cause some life-threatening complications [9]. In addition, recent studies reported that the MCS might not reduce short- or long-term mortality [10, 11]. Thus, some novel inotropic agents might be need to develop for patients with VSR.
Levosimendan is a calcium sensitizer that exerts its inotropic effect by interacting with troponin C (the binding protein for calcium) to enhance the calcium sensitivity of cardiac myocytes [12]. Therefore, levosimendan can improve cardiac performance while not increasing myocardial oxygen consumption or changing myocardial substrate utilization [12]. Some previous studies had shown that levosimendan improved cardiac function in high-risk patients underwent cardiac surgery [13, 14]. It could significantly decreased mortality and postoperative complications [15,16,17]. However, some trials found that levosimendan might have no benefit in patients undergoing cardiac surgery [18,19,20]. In a word, the efficacy of levosimendan in patients undergoing cardiac surgery is still controversial. Moreover, there is no study that has been developed to evaluated levosimendan in patients with VSR. Thus, we designed this prospective observational cohort study to evaluate the efficacy of levosimendan in patients with VSR underwent cardiac surgery.
Materials and methods
Study design and patients
The VSR is a rare complication of AMI. It was not easy to get an accepted sample size in a single-center random control trial (RCT). Therefore, we designed a prospective observational cohort in two medical centers. This study included all those patients in two tertiary hospitals with VSR and undergoing cardiac surgery from January 1, 2015 to October 1, 2021. According to the ethical guidelines of the Helsinki declaration, ethical committees of Nanjing Drum Tower Hospital and Chinese Academy of Medical Sciences Fuwai Hospital had approved the study. The written informed consents were obtained from the patients or a member of their authorized delegator. Whether to use levosimendan was based on the treating physician’s treatment strategy.
All patients who developed VSR diagnosed by echocardiography with low ejection fraction (≤35%) and scheduled for concomitant coronary artery bypass grafting (CABG) with cardiopulmonary bypass (CPB) were included. The exclusion criteria were as follows: 1) The patients who died before surgery; 2) post-AMI VSR with free wall rupture or papillary muscle dysfunction [21]; 3) urgent operation; 4) re-operation for postoperative residual shunt and tearing attributed to sutures poorly; 5) preoperative MCS used; 6) Other medical diseases or conditions (i.e. cancers, pregnant, lactation period, autoimmune disease,multi-organ failure, ongoing infection).
Study treatment protocol
Before operation, Swan-Ganz catheter was placed routinely. Durgs and dosage used during anesthesia were as described below. For inducing general anesthesia, Vecuronium: 0.07–0.15 mg/kg, Etomidate: 0.1–0.4 mg/kg, midazolam: 0.1–0.4 mg/kg, and sufentanil: 0.5–1.0 μg/kg were used. In continuous anesthesia, sevoflurane mixed with oxygen (< 4.0%), 25–75 mg/kg/min of propofol,: 0.1–0.2 mg/kg/min of remifentanil and 1.0–2.0 mg/kg/min of vecuronium were used. Intermittent positive pressure breathing (IPPB) for provide intraoperative intermittent mechanical ventilation. Tidal volume was 6–10 mL/kg, fraction of inspired oxygen (FiO2) was 0.6–1.0 and 4–7 cm H2O of positive end expiratory pressure (PEEP). β-blocker and amiodarone were used for control arrhythmia and/or tachycardia after excluded contraindications.
All operations were performed with standard CPB. Arterial cannulation used an appropriate size cannula inserted into the ascending aorta, single-stage cannulae with superior and inferior vena cavae or dual-stage cannulae with right atrium were chosen for venous cannulations. The CPB circuit priming with 1500–2000 mL Sodium Lactate Ringer’s Injection contained 25–50 g albumin and 20 mL 10% Magnesium Sulfate Injection. Intravenous infusion 200–400 U/kg of heparin for anticoagulation, and CPB was started when whole-blood active clotting time (ACT) was over 480 s. Antegrade cardioplegia used hyperkalemic cold blood cardioplegia (cardioplegia solution to blood ratio was 4:1), which was delivered every 20 to 30 min through the aortic root during the aortic cross-clamp (ACC). At the end of CPB, 1:1 ratio of protamine for reverse heparin. The CABG was done before VSR repair. Two surgical techniques were used for VSR repair (Daggett procedure and David procedures), and determined by the cardiac surgeons. The Dagget procedure used single or multiple patches to cover the defect and sewed to the LV and RV to close the VSR [22]. The David procedure placed all sutures in the LV, which is also named “infarct exclusion technique” [23].
For patients in the levosimendan group, intravenous infusion of levosimendan was initiated immediately after CPB. The levosimendan was administrated as follows: loading dose was 6 μg/kg in the first hour, followed by a maintenance dose of 0.1 μg /kg/min. The infusion of levosimendan was maintained during the postoperative 3 days if clinically appropriate. Other inotropes and vasopressors were routinely used. For patients in control groups, the treatment strategy was the same excepted levosimendan.
Definition
Postoperative complications were the following in-hospital postoperative complications included postoperative stroke, low cardiac output syndrome (LCOS) needed mechanical circulatory support (MCS) after surgery, postoperative acute kidney injury (≥ stage III), postoperative infection or septic shock, new devoleped atrial fibrillation or ventricular arrhythmias. Diagnosis and classification of AKI was based on KDIGO clinical practice guideline [24].
Vasoactive inotropic score (VIS) was used for quantifies the amount of cardiovascular support required by patients postoperatively and includes dopamine, dobutamine, epinephrine, milrinone, vasopressin, and norepinephrine. VIS was calculated (VIS = dopamine dose [μg kg − 1 min − 1] + dobutamine [μg kg − 1 min − 1] + 100 × epinephrine dose [μg kg − 1 min − 1] + 10 × milrinone dose [μg kg − 1 min − 1] + 10,000 × vasopressin [units kg − 1 min − 1] + 100 × norepinephrine dose [μg kg − 1 min − 1]). VISmax defined as using the maximum dosing rates of vasoactive and inotropic medications (μg kg − 1 min − 1) during the first 24, 48, 72 h after postoperative ICU admission.
Statistical analysis
IBM SPSS Statistics (version 26; IBM Corporation, Armonk, NY) was used for analysis. Continuous variables were described as mean ± SD or median with interquartile ranges (IQR). Discrete variables were described as frequencies (n, %). continuous normally distributed variables were compared by independent samples Student’s t test, Mann-Whitney U test were used to compare variables not normally distributed. The categorical variables were compared using Chi-square or Fisher exact test when appropriate. A P value < 0.05 was considered as significant.
Further assessment used propensity score analysis for adjustment indicated bias and keep the homogeneity comparable between two groups.
We selected age, EuroSCORE, NYHA class, hypertension, LVEF, ACC and CPB time for including in matching. They were proven to be related with poor outcomes after cardiac surgery [25,26,27,28]. For each patient, the probability of administrating levosimendan and compared with patients who had not in a 1:1 ratio, matched by the closest propensity score with ±0. 01 difference. Standardized Mean Difference (SMD) was used for the assessment of balance after match. Then, we used Kaplan–Meier survival estimate to contrast the postoperative 90- day survival of two groups befor and after matching.
Results
Clinical characteristics
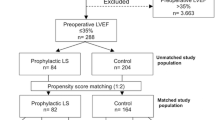
From January 1, 2015 to September 1, 2021, the study enrolled 280 patients totally, and 261 patients were included in the statistic analysis and were followed for 90 days after surgery (Fig. 1).
In 261 patients, 164 patients used levosimendan, 97 patients were not. The demographic data and preoperative clinical characteristics have no difference between two groups (Table 1). Chinese Academy of Medical Sciences Fuwai Hospital enrolled 241 patients and mortality was 32.37%, Nanjing Drum Tower Hospital enrolled 20 patients and mortality was 35.00%, There was no significant difference in mortality between two centers (P = 0.739).
Outcomes in the pre-matched patients
After surgical repair, 90-day mortality was 31.96 and 32.93% in the control and levosimendan groups, 30-day mortality were 27.84 and 31.71%, respectively. There was no significant difference in mortality and other outcomes between two groups (Table 2). However, control group had more length of ICU stay (6.69 ± 3.90 d vs. 5.20 ± 2.24 d, p < 0.001) and longer MV time (median: 23 h, IQR: 16–53 h vs. median: 16 h, IQR: 11–23 h, p < 0.001). The Kaplan–Meier curves and log-rank test of the 90-day survival did not shown significant differences in this pre-matched groups (Fig. 2, P = 0.77).
Outcomes in the propensity-matched patients
We used propensity score analysis for further assessment. Levosimendan administration was associated with multiple clinical variables, including age, EuroSCORE, NYHA class, hypertension Left Ventricular Ejection Fraction (LVEF), Aortic Cross Clamp (ACC) time and cardiopulmonary bypass time. Finally, there were 106 patients (53 levosimendan and 53 control) in the propensity-matched cohort. Standardized Mean Difference (SMD) was used for the assessment of balance after match, and all the SMD of clinical variables was < 0.1 (Fig. 3). The baseline characteristics among propensity-matched groups was no significant difference (Table 1). No significant difference was found between both groups for all outcomes and complications. The Kaplan–Meier curves and log-rank test of the 90-day survival did not shown significant differences in matched groups (Fig. 4, P = 0.48).
Discussion
In our study, we focused on patients undergoing VSR repair with CABG. Our results were shown as follows: ① The mortality, AKI, MCS use and stroke had no differences significantly between the levosimendan and control group. Before matching, the levosimendan group might decrease length of ICU stay and mechanical ventilation time compared with control group; ② In order to eliminated potential bias, we used propensity score analysis for further assessment. After matching, all postoperative outcomes, including length of ICU stay and mechanical ventilation time, were not different among two groups; ③The Kaplan–Meier curves showed 90- days survival had no differences among two groups before and after matching.
Levosimendan had been identified as a helpful agent to decrease mortality in patients undergoing cardiac surgery [15, 16, 29]. However, there were some contrary results that had been reported in recent studies [18,19,20]. It is still controversial whether levosimendan had positive effects or not on patients undergoing CABG. And to our best knowledge, there was no study that had been focused on AMI-VSR. We, therefore, designed this study to evaluate the efficacy of levosimendan in patients with VSR underwent cardiac surgery. In our study, levosimendan did not reduce the 90-day mortality. Moreover, no benefit of levosimendan was found on postoperative complications.
Series of experimental studies reported that levosimendan was an activator of potassium ATPase channel which improved potassium flux to the mitochondrial matrix [30], and modulated mitochondrial ATP production and implicated a pharmacological mechanism for cardioprotection [31]. Additionally, levosimendan also as a Ca2+ sensitizer to increase Ca2 + −saturated cardiac troponin C in cardiomyocytes [32], which improved cardiac performance without oxygen wasting [33, 34]. Theoretically, the levosimendan may be beneficial in patients with ischemic cardiomyopathy and heart failure. Then, some clinical studies were managed to investigate the efficacy of levosimendan. Erikkson et al. [35] reported that levosimendan significantly enhanced primary weaning from CPB and decreased IABP use in on-pump coronary artery bypass grafting (op-CABG). De Hert et al. [36] reported that levosimendan could significantly improve ventricular function in patients with low preoperative LVEF. Then, three large multicenter randomized trials were following developed to supply high-quality evidence. However, they found that levosimendan had no benefit for patients with pre-operative left ventricular (LV) dysfunction. In the LEVO-CTS trial, it demonstrated that levosimendan did not show the superiority in the poor outcomes in patients with LVEF < 35% compared with placebo [19]. In the CHEETAH trial, 30-day mortality had no differences between levosimendan and placebo in patients with severely perioperative LV dysfunction [37]. The LICORN trial reported that there were no any clinical advantages in patients with LVEF≤40% underwent cardiac surgery [20]. However, some recent researches reposted that levosimendan was associated with lower 90-day mortality or LCOS in patients underwent isolated CABG, but not in those underwent other procedures [38,39,40]. It may be necessary to evaluate the levosimendan by a single disease. However, the previous studies did not report what effects of levosimendan on AMI-VSR patients.
Our study failed to demonstrate any advantage in levosimendan supported patients with VSR. However, there are still many issues that deserve further consideration. First, for patients who has developed VSR after AMI and undergoing surgical repair, the operative mortality has not been improved significantly over the past half century [4]. This trend may suggested that advances in surgical technology may not be beneficial for such patients. The theoretical basis of levosimendan for the treatment of ischemic cardiomyopathy was still solid, at the same time we are in absence of more and new options with inotropic agents, in fact, levosimendan represents a rare case of an inotrope for short-term hemodynamic treatments for acute cardiac care which approved by regulatory authorities in the past 20 years. Some studies showed that levosimendan was a valid pharmacological strategy for perioperative management of VSR [41, 42]. This result prompts optimal use timing of levosimendan need more exploration. Then, we can not deny the possibility that higher doses levosimendan might have been effective in reducing mortality and complications, although higher doses might also have increased the risk of hypotension and arrhythmias. Third, it is hard to known about actual viable myocardium in patients between two groups and we did not systematically collect cardiac output data, which can lead to the real efficacy of levosimmendan being masked.
In summary, our study found that a low-dose infusion of levosimendan in VSR patients underwent surgical repair and CABG did not associated with lower mortality and not associated with positively affect to postoperative outcomes.
Study limitation
Our study was two centers prospective observational cohort study. Some potential biases could have been influenced this study and we used propensity score matching to avoid them. In another side, some hard to be observed factors but actually affect assignment to treatment and outcomes could have been lost in the matching procedure. Hidden bias attribute to latent variables might still remain after matching, which may cause statistical errors. Furthermore, propensity score matching removed a large number of patients may lead to an increase in statistical error. In addition, the condition of AMI-VSR was very complicated, the recruited patients in our study might not reflect well to the clinical reality. This study could not cover every part of hemodynamic situations. It may cause some potential errors.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to patients did not signed consents about upload the data but are available from the corresponding author on reasonable request.
References
Birnbaum Y, Fishbein MC, Blanche C, Siegel RJ. Ventricular septal rupture after acute myocardial infarction. N Engl J Med. 2002;347(18):1426–32.
French JK, Hellkamp AS, Armstrong PW, Cohen E, Kleiman NS, O'Connor CM, et al. Mechanical complications after percutaneous coronary intervention in ST-elevation myocardial infarction (from APEX-AMI); 2010. p. 59–63.
Moreyra AE, Huang MS, Wilson AC, Deng Y, Cosgrove NM, Kostis JB. Trends in incidence and mortality rates of ventricular septal rupture during acute myocardial infarction; 2010. p. 1095–100.
Matteucci M, Ronco D, Corazzari C, Fina D, Jiritano F, Meani P, et al. Surgical repair of Postinfarction ventricular Septal rupture: systematic review and Meta-analysis. Ann Thorac Surg. 2021;112(1):326–37.
Jones BM, Kapadia SR, Smedira NG, Robich M, Tuzcu EM, Menon V, et al. Ventricular septal rupture complicating acute myocardial infarction: a contemporary review. Eur Heart J. 2014;35(31):2060–8.
Cinq-Mars A, Voisine P, Dagenais F, Charbonneau É, Jacques F, Kalavrouziotis D, et al. Risk factors of mortality after surgical correction of ventricular septal defect following myocardial infarction: retrospective analysis and review of the literature. Int J Cardiol. 2016;206:27–36.
Lomivorotov VV, Efremov SM, Kirov MY, Fominskiy EV, Karaskov AM. Low-cardiac-output syndrome after cardiac surgery. J Cardiothor Vasc An. 2017;31(1):291–308.
Gillies M, Bellomo R, Doolan L, Buxton B. Bench-to-bedside review: Inotropic drug therapy after adult cardiac surgery -- a systematic literature review. Crit Care (London, England). 2005;9(3):266–79.
Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97(2):610–6.
Thiele H, Zeymer U, Neumann F, Ferenc M, Olbrich H, Hausleiter J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–96.
Romeo F, Acconcia MC, Sergi D, Romeo A, Muscoli S, Valente S, et al. The outcome of intra-aortic balloon pump support in acute myocardial infarction complicated by cardiogenic shock according to the type of revascularization: a comprehensive meta-analysis. Am Heart J. 2013;165(5):679–92.
Pathak A, Lebrin M, Vaccaro A, Senard JM, Despas F. Pharmacology of levosimendan: inotropic, vasodilatory and cardioprotective effects. J Clin Pharm Ther. 2013;38(5):341–9.
Ersoy O, Boysan E, Unal EU, Yay K, Yener U, Cicekcioglu F, et al. Effectiveness of prophylactic levosimendan in high-risk valve surgery patients. Cardiovasc J Afr. 2013;24(7):260–4.
Leppikangas H, Järvelä K, Sisto T, Maaranen P, Virtanen M, Lehto P, et al. Preoperative levosimendan infusion in combined aortic valve and coronary bypass surgery. Brit J Anaesth. 2011;106(3):298–304.
Sanfilippo F, Knight JB, Scolletta S, Santonocito C, Pastore F, Lorini FL, et al. Levosimendan for patients with severely reduced left ventricular systolic function and/or low cardiac output syndrome undergoing cardiac surgery: a systematic review and meta-analysis. Crit Care (London, England). 2017;21(1):252.
Treskatsch S, Balzer F, Geyer T, Spies CD, Kastrup M, Grubitzsch H, et al. Early levosimendan administration is associated with decreased mortality after cardiac surgery; 2015. p. 851–9.
Elahi MM, Lam J, Asopa S, Matata BM. Levosimendan versus an intra-aortic balloon pump in adult cardiac surgery patients with low cardiac output; 2011. p. 1154–62.
Guarracino F, Heringlake M, Cholley B, Bettex D, Bouchez S, Lomivorotov VV, et al. Use of Levosimendan in cardiac surgery: an update after the LEVO-CTS, CHEETAH, and LICORN trials in the light of clinical practice. J Cardiovasc Pharmacol. 2018;71(1):1–9.
Mehta RH, Leimberger JD, van Diepen S, Meza J, Wang A, Jankowich R, et al. Levosimendan in patients with left ventricular dysfunction undergoing cardiac Surgery; 2017. p. 2032–42.
Cholley B, Caruba T, Grosjean S, Amour J, Ouattara A, Villacorta J, et al. Effect of Levosimendan on low cardiac output syndrome in patients with low ejection fraction undergoing coronary artery bypass grafting with cardiopulmonary bypass: the LICORN randomized clinical trial. JAMA. 2017;318(6):548–56.
Elbadawi A, Elgendy IY, Mahmoud K, Barakat AF, Mentias A, Mohamed AH, et al. Temporal trends and outcomes of mechanical complications in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2019;12(18):1825–36.
Daggett WM, Guyton RA, Mundth ED, Buckley MJ, McEnany MT, Gold HK, et al. Surgery for post-myocardial infarct ventricular septal defect. Ann Surg. 1977;186(3):260–71.
David TE, Dale L, Sun Z. Postinfarction ventricular septal rupture: repair by endocardial patch with infarct exclusion. J Thorac Cardiovasc Surg. 1995;110(5):1315–22.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84.
Aronson S, Dyke CM, Levy JH, Cheung AT, Lumb PD, Avery EG, et al. Does perioperative systolic blood pressure variability predict mortality after cardiac surgery? An exploratory analysis of the ECLIPSE trials; 2011. p. 19–30.
Wang J, Xiao F, Ren J, Li Y, Zhang M. Risk factors for mortality after coronary artery bypass grafting in patients with low left ventricular ejection fraction. Chinese Med J Peking. 2007;120(4):317–22.
Roques F, Nashef SA, Michel P, Gauducheau E, de Vincentiis C, Baudet E, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15(6):816–22 822–3.
Wang W, Bagshaw SM, Norris CM, Zibdawi R, Zibdawi M, MacArthur R. Association between older age and outcome after cardiac surgery: a population-based cohort study; 2014. p. 177.
Jiménez-Rivera JJ, Álvarez-Castillo A, Ferrer-Rodríguez J, Iribarren-Sarrías JL, García-González MJ, Jorge-Pérez P, et al. Preconditioning with levosimendan reduces postoperative low cardiac output in moderate-severe systolic dysfunction patients who will undergo elective coronary artery bypass graft surgery: a cost-effective strategy; 2020. p. 108.
Kopustinskiene DM, Pollesello P, Saris NL. Potassium-specific effects of levosimendan on heart mitochondria. Biochem Pharmacol. 2004;68(5):807–12.
Papp Z, Édes I, Fruhwald S, De Hert SG, Salmenperä M, Leppikangas H, et al. Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol. 2012;159(2):82–7.
Sorsa T, Pollesello P, Rosevear PR, Drakenberg T, Kilpeläinen I. Stereoselective binding of levosimendan to cardiac troponin C causes Ca2+−sensitization; 2004. p. 1–8.
Lilleberg J, Nieminen MS, Akkila J, Heikkilä L, Kuitunen A, Lehtonen L, et al. Effects of a new calcium sensitizer, levosimendan, on haemodynamics, coronary blood flow and myocardial substrate utilization early after coronary artery bypass grafting; 1998. p. 660–8.
Ukkonen H, Saraste M, Akkila J, Knuuti J, Karanko M, Iida H, et al. Myocardial efficiency during levosimendan infusion in congestive heart failure; 2000. p. 522–31.
Eriksson HI, Jalonen JR, Heikkinen LO, Kivikko M, Laine M, Leino KA, et al. Levosimendan facilitates weaning from cardiopulmonary bypass in patients undergoing coronary artery bypass grafting with impaired left ventricular function. Ann Thorac Surg. 2009;87(2):448–54.
De Hert SG, Lorsomradee S, Cromheecke S, Van der Linden PJ. The effects of levosimendan in cardiac surgery patients with poor left ventricular function. Anesth Analg. 2007;104(4):766–73.
Landoni G, Lomivorotov VV, Alvaro G, Lobreglio R, Pisano A, Guarracino F, et al. Levosimendan for hemodynamic support after cardiac surgery. N Engl J Med. 2017;376(21):2021–31.
Wang W, Zhou X, Liao X, Liu B, Yu H. The efficacy and safety of prophylactic use of levosimendan on patients undergoing coronary artery bypass graft: a systematic review and meta-analysis. J Anesth. 2019;33(4):543–50.
van Diepen S, Mehta RH, Leimberger JD, Goodman SG, Fremes S, Jankowich R, et al. Levosimendan in patients with reduced left ventricular function undergoing isolated coronary or valve surgery; 2020. p. 2302–9.
Desai PM, Sarkar MS, Umbarkar SR. Prophylactic preoperative levosimendan for off-pump coronary artery bypass grafting in patients with left ventricular dysfunction: single-centered randomized prospective study. Ann Card Anaesth. 2018;21(2):123–8.
Camilli M, Ciampi P, Pedicino D, D’Aiello A, Mazza A, Montone RA, et al. Use of Levosimendan as bridge therapy to surgical correction of post-infarction ventricular septal defect: a case report; 2021. p. 3296–9.
Anastasiadis K, Antonitsis P, Vranis K, Kleontas A, Asteriou C, Grosomanidis V, et al. Effectiveness of prophylactic levosimendan in patients with impaired left ventricular function undergoing coronary artery bypass grafting: a randomized pilot study. Interact Cardiov Th. 2016;23(5):740–7.
Acknowledgements
Not Applicable.
Funding
This research has been supported by National Natural Science Foundation of China (to Dong-Jin Wang, No. 81970401).
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. Ze-Shi Li and Kuo Wang drafted and edited the final manuscript; reviewed the literature, and prepared the manuscript; Tuo Pan designed the manuscript, helped supervise the data collection, analyze the data, provided surgical content expertise; Yan-Hua Sun provided anesthesiologic content expertise; Chang Liu helped provided cardiopulmonary bypass content expertise; Yong-Qing Cheng provided intensive care content expertise, and edited part of the manuscript; He Zhang and Hai-Tao Zhang helped drafted the manuscript; Dong-Jin Wang provided surgical content expertise, designed the study, guided and reviewed the manuscript; Zu-Jun Chen provided intensive care content expertise,analyze the data, designed the study, guided and reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
According to the ethical guidelines of the Helsinki declaration, ethical committees of Nanjing Drum Tower Hospital and Chinese Academy of Medical Sciences Fuwai Hospital had approved the study. The written informed consents were obtained from the patients or a member of their authorized delegator.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Li, ZS., Wang, K., Pan, T. et al. The evaluation of levosimendan in patients with acute myocardial infarction related ventricular septal rupture undergoing cardiac surgery: a prospective observational cohort study with propensity score analysis. BMC Anesthesiol 22, 135 (2022). https://doi.org/10.1186/s12871-022-01663-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-022-01663-z