Abstract
Background
Remifentanil induced hyperalgesia (RIH) is characterized by stimulation evoked pain including allodynia and thermal hyperalgesia after remifentanil infusion. N-methyl-D-aspartate (NMDA) receptor was reported to be involved in the progress of RIH. We hypothesized that intrathecal MgSO4 could relieve hyperalgesia after remifentanil infusion via regulating phosphorylation of NMDA receptor NR2B subunit activity in this study.
Methods
Thirty two rats were randomly allocated into control group, model of RIH group, RIH plus 100ug MgSO4 group, RIH plus 300ug MgSO4 group. Mechanical and thermal hyperalgesia were tested at -24th h, 2nd h, 6th h, 24th h, 48th h after remifentanil infusion. Following sacrifice of rats after the last behavioral test, we performed the western blot to detect the expression of spinal phosphorylated NMDA receptor NR2B subunit (pNR2B) in the L4-L5 segments.
Results
Intrathecal MgSO4 (100, 300 μg) dose-dependently reduced thermal and mechanical hyperalgesia from 2 h to 48 h after remifentanil infusion. Remifentanil infusion remarkably stimulated the expression of pNR2B. Nevertheless, the increased amount of pNR2B by RIH was dose-dependently suppressed by intrathecal infusion of MgSO4 in rats.
Conclusions
Remifentanil induced hyperalgesia/allodynia could be ameliorated by Mg-mediated blockade targeting the NR2B subunit in NMDA receptors.
Similar content being viewed by others
Background
Due to the property of rapid onset and recovery, remifentanil is quite widely used in clinic. However, remifentanil was reported to be associated with the development of hyperalgesia. it was noted that general anesthesia based on remifentanil infusion resulted in severe postoperative pain after surgery [1]. Additionally, analgesic consumption after operation can be increased following the intraoperative use of remifentanil [2].
N-methyl-D aspartate (NMDA) receptors was known to play a critical role in excitatory synaptic transmission. Remifentanil could enhance the activation of spinal NMDA receptor, which was attributed to the progress of remifentanil induced hyperalgesia (RIH) [3].
NR2B subunit is an essential part in the NMDA receptors. Tyrosine-1472 phosphorylation in NR2B is known to be associated with neuropathological conditions [4]. Phosphorylation of Tyr-1472 in NR2B (pNR2B) has previously been demonstrated in the progress of RIH [5].
NMDA receptor antagonist was implicated to decrease the extended area of RIH in human [6]. Prior administration of magnesium could inhibit delayed fentanyl induced hyperalgesia [7, 8] in rats. Our previous study suggested that magnesium sulphate could dose-dependently inhibit RIH in rats [9]. The purpose of this study was to explore whether intrathecal magnesium administration could prevent RIH via decreasing the amount of pNR2B expression in superficial dorsal horn of spinal cord.
Methods
Animals
After approval of experimental animal center of Wenzhou Medical university, Adult male Sprague–Dawley rats (230 ± 30 g) were obtained and maintained under a 12 h light −12 h dark cycle with food and water freely available.
Intrathecal catheter placement
Intrathecally catheterization was performed after an acclimation period of at least 5 days for the rats. Under sevoflurane anesthesia, plastic PE-10 tube (OD: 0.5 mm, ID: 0.25 mm, AniLab Co. Ltd, China) was implanted into intrathecal space. The rostral part of the catheter was then sutured to the muscle to immobilize it.
Surgical procedure
Under sevoflurane anesthesia, a longitudinal incision (0.8 cm) was made starting from edge of the heel and extending toward the toes of the right hind paw. The underlying plantaris muscle was exposed and incised longitudinally, leaving the muscle origin and insertion intact. After hemostasis, skin was closed covered with antiseptic gauze.
Remifentanil infusion
Remifentanil (1.2 μg.kg−1.min−1) was infused via tail vein over a period of 60 min using a pump.
Behavioral test
Mechanical nociception was determined by measuring paw withdrawal mechanical thresholds (PWMT). Through a mesh bottom (1 × 1 cm), the electronic von Frey filament (0.8 mm diameter, LS instrument, USA) was applied vertically to the plantar surface of the right hind paw. Positive nociceptive-like response was defined as clear paw withdrawal or licking.
Thermal nociception was determined by measuring paw withdrawal thermal latency (PWTL) using thermal stimulation system (Model 336, Series 8, IITC INC, USA). A radiant thermal source below a glass floor (5 mm thick) was positioned to deliver a thermal stimulus to the midplantar region adjacent to the wound of right hind paw. When the rat had response of clear paw withdrawal or flinching, the thermal source was switched off, and the timer stopped, measuring the PWTL. Thermal stimulation was automatically cut off after 25 s if the rat fails to withdraw.
Animals were allowed to acclimatize for 30 min before testing. Mean PWTL and PWMT were established by averaging the values of three tests with a five minute interval between each test.
Drugs
Remifentanil hydrochloride (Ultiva) (batch number: 6587129, Ren Fu Co, China), Magnesium sulfate (M2643, Sigma. St. Louis, USA), sevoflurane (batch number: 4Z132, maruishi-pharm.co. Japan).
Western blotting
After the last behavioral test, the animals were sacrificed with sevoflurane and lumbar spinal cord L4-L5 segments were removed in 2 min. Tissue samples were homogenized in lysis buffer containing protease inhibitors (Sigma-Aldrich Co.). The homogenate was centrifuged at 12,000 rpm for 5 min at 4 °C and supernatant was removed as the total protein. Proteins (70 μg) were separated on a 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membrane (Bio-Rad, CA) with a Trans-Blot Transfer Cell system (Bio-Rad, CA). The filters were blocked with 5% nonfat milk in TBS buffer at room temperature for 1 h. Then the blot was incubated with the primary antibody against phosphorylated Tyr 1472 NR2B (1:1000; Cell signaling Technology, USA) overnight at 4 °C. The membrane was washed with TBS buffer and incubated for 1 h with the secondary anti- rabbit IgG horseradish peroxidase (1:2000; Jackson ImmunoResearch, USA) at room temperature and visualized in enhanced chemiluminescence solution (Amersham Biosciences) followed by film exposure. β-Actin was used as endogenous control (1:10,000, Biotechnology, USA). Those who did the western blot were blinded to the group allocation. Densitometric quantification of each specific band was performed using Gene Tools Match software (Syngene, Cambridge, UK). The results were expressed as the percentage of β-actin immunoreactivity.
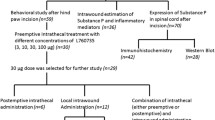
Experiment protocol
Experiments were performed 7 days later after intrathecal catheterization. To evaluate thermal and mechanical hyperalgesia induced by remifentanil, 32 rats were assigned into the following four experimental groups (n = 8): 1: Group C (a control group with the administration of sevoflurane inhalation without incision); 2: Group RI (model of RIH with surgical procedure and remifentanil infusion, 10 μl normal saline was intrathecally administration); 3: Group RIM3 (300 μg MgSO4 was intrathecally given to the group RI); 4: Group RIM1 (100 μg MgSO4 was intrathecally given to the group RI). 30 min before remifentanil infusion and plantar incision, MgSO4 or normal saline were intrathecally administration in a volume of 10 μl, followed by additional normal saline (20 μl) to flush the catheter. Remifentanil infusion and plantar incision were performed at the same time. PWMT and PWTL tests were performed at −24 h, 2 h, 6 h, 24 h, and 48 h after remifentanil infusion. The L4-L5 segments for western blot analysis were collected just after behavioral testing at 48 h to investigate whether magnesium could prevent RIH via modulating spinal pNR2B activity.
All behavioral tests were carried out in a quiet test room by the same investigator (Doc Lin) who was blinded to the group allocation.
Statistical analysis
Quantitative parametric data obtained from the groups were expressed as mean ± SD. Data from thermal and mechanical hyperalgesia were analyzed using repeated measures analysis of variance. The expression of pNR2B across all experimental groups were statistically tested by one-way ANOVA with Bonferroni correction. The level of statistical significance was set at p = 0.05. Data were analyzed with the SPSS 15.0 software (SPSS Inc., Chicago, IL, USA).
Result
There were no statistical differences by the basal PWTL and PWMT values among the experimental groups (p > 0.05).
Compared with normal saline, both of 300 μg and 100 μg MgSO4 significantly inhibited thermal (Fig. 1a) and mechanical (Fig. 1b) hyperalgesia. Compared with 100 μg MgSO4, 300 μg MgSO4 had less thermal hyperalgesia at 2 h, 6 h, 24 h, 48 h (p = 0.000) and less mechanical hyperalgesia at 2 h (p = 0.009),6 h (p = 0.005), 24 h (p = 0.004). There was no statistical difference in mechanical hyperalgesia at 48 h (p = 0.286) (Fig. 1, Additional file 1).
a-b Effect of MgSO4 on remifentanil-induced hyperalgesia. MgSO4 or normal saline were administered intrathecally 30 min before remifentanil infusion and surgical incision. PWTL (a) and PWMT (b) were evaluated at 24 h before incision and 2, 6, 24, 48 h. Data are expressed as means ± SD. * p < 0.001 compared with Group C, # p < 0.001 compared with Group RI, + p < 0.01 compared with GroupRIM1
The levels of tyrosine phosphorylated NR2B of NMDA receptors were significantly lower in GroupRIM3 compared with the levels in GroupRIM1 and Group RI (p < 0.001). No significant change was detected between GroupRIM3 and Group C (p = 0.078). (Fig. 2, Additional file 2).
a-b The expression level of tyrosine phosphorylation of NR2B in superficial spinal cord in each group. L4-L5 segments of spinal cord were collected at 48 h after the remifentanil infusion. a Bands of Western blot for pNR2B. β-Actin is a loading control. b Quantification of pNR2B in each group. Data are expressed as means ± SD. * p < 0.001 compared with Group RI, # p < 0.001 compared with Group RIM1
Discussion and conclusions
In the current study, remifentanil was found to aggravate both mechanical and thermal hyperalgesia after remifentanil infusion. Intrathecal Mg2+ provided a dose-dependent anti-nociceptive effect [10]. The authors have previously demonstrated that the MgSO4 doses of 100ug and 300 μg were safe for intrathecal injection in rats [9]. MgSO4 was reported to alleviate the hyperalgesia via inhibiting the tyrosine phosphorylation of NMDA receptor in dorsal horn of lumber spinal cord [11].
Remifentanil was report to induce dose-dependent hyperalgesia in human [2]. The hyperalgesic effect of opioid was complicated and was once seemed to be mediated by opioid receptors. However, hyperalgesia could still be provoked by opioid in mice in which μ, κ and δ opioid receptors were completely knocked out [12, 13]. On the contrary, infusing the opioid could still cause hyperalgesia instead of analgesia in these knockout mice [13]. Based on the results of these studies, it is supposed that opioid-induced hyperalgesia may be explained by mechanism other than opioid receptors.
MgSO4 in the trial was found to decrease the remifentanil induced hyperalgesia. RIH may be associated with NMDA activation via an intracellular pathway to up-regulate the function of NMDA activity [3]. MgSO4 could occlude the Ca2+ ion current, which resulted in a further release of glutamate and initiating a series of central sensitization to induce opioid-induced hyperalgesia [14, 15]. As illustrated by Angst’s study, even short-term administration of remifentanil could enhance hyperalgesia during withdrawal, while ketamine, the NMDA receptor antagonist, could abolish such hyperalgesic response [6]. NMDA antagonists could reverse opioid hyperalgesia by antagonizing NMDA receptors [16]. Taken together, NMDA palyed a key role in pathogenesis of RIH.
Distribution of NMDA receptor NR2B was demonstrated to almost limit in the superficial dorsal horn [17]. The spinal sensitization and increased nociceptive input may present due to the condition that noxious stimulus occurred within the superficial dorsal horn in the central primary afferent terminals. Subsequently, nociceptive substances such as glutamic acids activate the specific nociceptive neurons and create the pain impulses. Following such injury, the expression of NR2B subunit in superficial dorsal horn was detected to increase significantly [18].
Protein phosphorylation is a major mechanism for the regulation of receptor activity. Tyr-1472 phosphorylation of NR2B subunit is important for synaptic plasticity and the development of central sensitization [19]. Furthermore, tyr-1472 phosphorylation in the superficial dorsal horn of the spinal cord contributed to the progress of hyperalgesia in neuropathic pain [20, 21]. Abrupt withdrawal from opioid use could activate NMDA receptors and increase postsynaptic calcium concentrations [22]. NMDA antagonist was found to block increased Tyr1472 phosphorylation and inhibit remifentanil induced hyperalgesia [23].
Mg deficiency could facilitate NMDA receptor activation and long-term sensitization of the nociceptive pathways [11]. Previous study has showed that intrathecal administration of MgSO4 was beneficial in rats with NMDA receptor-dependent hyperalgesia [15].
Our findings support our hypothesis that NR2B tyrosine phosphorylation could be alleviated by NMDA antagonists. However, direct evidence of magnesium to the NR2B tyrosine phosphorylation and hyperalgesia remains to be further explored. It is the first study to demonstrate that magnesium could prevent RIH via decreasing Tyr-1472 phosphorylation in NMDA receptor.
Sevoflurane was previously proved to have no influence on nociceptive thresholds, so it was used for anesthesia and animal sacrifice in this study [24]. The doses of MgSO4 employed in the trial was based on the previous study [9]. Limitations:
-
1.
NMDA receptors are positioned in either cellular membrane or cytoplasm. In the previous study, it was demonstrated that membrane trafficking of NR2B subunits in the spinal cord were significantly promoted after remifentanil infusion [25]. Due to inadequate funding, we only extracted the total protein for western blot analysis. In the further study, we should extract the membrane protein of dorsal horn to detect the amount of pNR2B subunits after magnesium intervention.
-
2.
Sole surgical incision can also induce hyperalgesia after operation. Why the hyperalgesia in the paper was attributed to the remifentanil infusion? Previous report from our research have demonstrated that remifentanil infusion alone could trigger more severe hyperalgesia than surgical incision alone [9]. In the figure, the experiment indicated that MgSO4 could inhibit RIH with statistical difference. However, in the Group RIM3 and Group RIM1, the thresholds of the behavior tests in the Fig. 1 were found to have the same level compared with the thresholds in the Group surgical incision alone in the figure (shown as Additional file 1). In the incision-absent group, it is virtually impossible to interpret our results. In order to present the result, we uploaded a new Figure as (shown as Additional files 3 and 4) which combined the Group magnesium in the study and the Group single incision from the model experiment in our previous study [9]. Both of the two study were performed by doc Sun at the same condition. Maybe we have made over-interpretation of the data. It was afraid that such differences did not have clinically meaningful. In conclusion, the increased expression of NR2B phosphorylation in the spinal dorsal horn played an important role in the progress of RIH. Intrathecal administration of MgSO4 could dose-dependently diminish RIH via decreasing the amount of pNR2B in the spinal dorsal horn. This study offered the potential therapeutic opportunities of MgSO4 for the management of opioid induced pain.
Abbreviations
- NMDA:
-
N-methyl-D-aspartate
- PWMT:
-
Paw withdrawal mechanical thresholds
- PWTL:
-
Paw withdrawal thermal latency
- RIH:
-
Remifentanil-induced hyperalgesia
References
Shin SW, Cho AR, Lee HJ, Kim HJ, Byeon G, Yoon JW, Kim KH, Kwon JY. Maintenance anaesthetics during remifentanil-based anaesthesia might affect postoperative pain control after breast Cancer. Br J Anaesth. 2010;105:661–7.
Guignard B, Bossard AE, Coste C, Sessler DI, Lebrault C, Alfonsi P, Fletcher D, Chauvin M. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93:409–17.
Zhao M, Joo DT. Enhancement of spinal N-methyl-D-aspartate receptor function by remifentanil action at delta-opioid receptors as a mechanism for acute opioid-induced hyperalgesia or tolerance. Anesthesiology. 2008;109:308–17.
Dunah AW, Wang Y, Yasuda RP, Kameyama K, Huganir RL, Wolfe BB, Standaert DG. Alterations in subunit expression, composition, and phosphorylation of striatal N-methyl-D-aspartate glutamate receptors in a rat 6-hydroxydopamine model of Parkinson’s disease. Mol Pharmacol. 2000;57:342–52.
Jiang M, Zhang W, Ma Z, Gu X. Antinociception and prevention of hyperalgesia by intrathecal administration of Ro 25–6981, a highly selective antagonist of the 2B subunit of N-methyl-D-aspartate receptor. Pharmacol Biochem Behav. 2013;112:56–63.
Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57.
Van Elstraete AC, Sitbon P, Mazoit J-X, Conti M, Benhamou D. Protective effect of prior administration of magnesium on delayed hyperalgesia induced by fentanyl in rats. Can J Anesth. 2006;53:1180–5.
Tufan M, Yasemin G, Dilek O, Ismail G. Magnesium modifies fentanyl-induced local antinociception and hyperalgesia. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:415–20.
Sun J, Lin H, Feng X, Dong J, Ansong E, Xu X. A comparison of intrathecal magnesium and ketamine in attenuating remifentanil-induced hyperalgesia in rats. BMC Anesthesiol. 2016;16:74.
Yoshito T, Eiru S, Toshihiko K, Isao S. Antihyperalgesic effects of intrathecally administered magnesium sulfate in rats. Pain. 2000;84:175–9.
Rondon LJ, Privat AM, Daulhac L, Davin N, Mazur A, Fialip J, Eschalier A, Courteix C. Magnesium attenuates chronic hypersensitivity and spinal cord NMDA receptor phosphorylation in a rat model of diabetic neuropathic pain. J Physiol. 2010;588:4205–15.
Waxman AR, Arout C, Caldwell M, Dahan A, Kest B. Acute and chronic fentanyl administration causes hyperalgesia independently of opioid receptor activity in mice. Neurosci Lett. 2009;46:68–72.
Juni A, Klein G, Pintar JE, Kest B. Nociception increases during opioid infusion in opioid receptor triple knock-out mice. Neuroscience. 2007;147:439–44.
Guntz E, Dumont H, Roussel C, Gall D, Dufrasne F, Cuvelier L, Blum D, Schiffmann SN, Sosnowski M. Effects of remifentanil on N-methyl-D-aspartate receptor: an electrophysiologic study in rat spinal cord. Anesthesiology. 2005;102:1235–41.
Parisa H, Mohsen P, Mansoor K, Kazem J, Marzieh M, Nepton S. Oral magnesium administration prevents thermal hyperalgesia induced by diabetes in rats. Diabetes Res Clin Pract. 2006;73:17–22.
Ossipov MH, Lai J, King T, Vanderah TW, Malan TP, Hruby VJ, Porreca F. Antinociceptive and nociceptive actions of opioids. J Neurobiol. 2004;61:126–48.
Nagy GG, Watanabe M, Fukaya M, Todd AJ. Synaptic distribution of the NR1, NR2A and NR2B subunits of the N-methyl-d-aspartate receptor in the rat lumbar spinal cord revealed with an antigen-unmasking technique. Eur J Neurosci. 2004;20:3301–12.
Wilson JA, Garry EM, Anderson HA, Rosie R, Colvin LA, Mitchell R, Fleetwood-Walker SM. NMDA receptor antagonist treatment at the time of nerve injury prevents injury-induced changes in spinal NR1 and NR2B subunit expression and increases the sensitivity of residual pain behaviours to subsequently administered NMDA receptor antagonists. Pain. 2005;117:421–32.
Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fynmediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2001;276:693–9.
Abe T, Matsumura S, Katano T, Mabuchi T, Takagi K, Xu L, Yamamoto A, Hattori K, Yagi T, Watanabe M, Nakazawa T, Mishina M, Nakai Y, Ito S. Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci. 2005;22:1445–54.
Guo W, Zou S, Guan Y, Ikeda T, Tal M, Dubner R, Ren K. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord during the development and maintenance of inflammatory hyperalgesia. J Neurosci. 2002;22:6208–17.
Drdla R, Gassner M, Gingl E, Sandkühler J. Induction of synaptic long-term potentiation after opioid withdrawal. Science. 2009;325:207–10.
Gu XP, Wu XL, Liu Y, Cui SQ, Ma ZL. Tyrosine phosphorylation of the N-Methyl-D-Aspartate receptor 2B subunit in spinal cord contributes to remifentanil-induced postoperative hyperalgesia: the preventive effect of ketamine. Mol Pain. 2009;5:76–84.
Célérier E, González JR, Maldonado R, Cabalvro D, Puig MM. Opioid-induced hyperalgesia in a murine model of postoperative pain: role of nitric oxide generated from the inducible nitric oxide synthase. Anesthesiology. 2006;104:546–55.
Wang CY, Li YZ, Wang HY, Xie KL, Shu RC, Zhang LL, Hu N, Yu YH, Wang GL. Inhibition of DOR prevents remifentanil induced postoperative hyperalgesia through regulating the trafficking and function of spinal NMDA receptors in vivo and in vitro. Brain Res Bull. 2015;110:30–9.
Acknowledgements
We would like to thank Ruichen Shu from Tianjin Medical University for technical assistance and statistical preparation. We should also thank the staff in the writing center of Ohio State University of USA for assistance in manuscript preparation.
Funding
The authors received the funding from the program of Wenzhou science and technology bureau [Y20140698].
Availability of data and materials
All data generated or analyzed during this study are included in this published article (Additional files 1, 2, 3 and 4).
Authors’ contributions
All authors read and approved the final manuscript. JS. Contribution: Design the trial, acquisition of data, data analysis and give interpretation of data. HL. Contribution: Design the trial, data analysis and give interpretation of data. GH. Contribution: Design the trial, acquisition of data. WL. Contribution: Design the trial, acquisition of data.. JY. Contribution: Design the trial, analysis and give interpretation of data. He also gave final approval of the version to be published.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The experimental protocol was approved by the Institutional Animal Care Committee, Wenzhou Medical University.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1:
Data of PWTL and PWMT values in the study. (XLS 19 kb)
Additional file 2:
Data of ratio of grey values by β-actin in the western blot. (XLS 19 kb)
Additional file 3:
The PWTL and PWMT tests in the previous model tests from the referred literature (A and B) and the ones after the NMDA antagonists therapy in this study (C and D). (XLS 18 kb)
Additional file 4:
Data of PWTL and PWMT values in the previous model tests from the referred literature. (TIF 812 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sun, J., Lin, H., He, G. et al. Magnesium sulphate attenuate remifentanil-induced postoperative hyperalgesia via regulating tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord. BMC Anesthesiol 17, 30 (2017). https://doi.org/10.1186/s12871-017-0325-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-017-0325-3