Abstract
Background
The effects of different photoperiods on plant phytochemical synthesis can be improved by adjusting the daily light integral. Photoperiod is one of the most important environmental factors that control growth, plant’s internal rhythm and the synthesis of secondary metabolites. Information about the appropriate standard in terms of photoperiod for growing basil microgreens as one of the most important medicinal plants is limited. In this study, the effects of five different photoperiods, 6 (6 h × 3 cycles), 8 (8 h × 2 cycles), 16, 18, and 24 h day− 1 on the yield, photosynthesis and synthesis of secondary metabolites of three cultivars and one genotype of basil microgreens in floating system were evaluated. The purpose of this research was to determine the feasibility of using permanent light in growing basil microgreens and to create the best balance between beneficial secondary metabolites and performance.
Results
The results showed that the effects of photoperiod and cultivar on all investigated traits and their interaction on photosynthetic pigments, antioxidant capacity, total phenolic compounds, proline content and net photosynthesis rate were significantly different at the 1% level. The highest levels of vitamin C, flavonoids, anthocyanins, yield and antioxidant potential composite index (APCI) were obtained under the 24-h photoperiod. The highest antioxidant capacity was obtained for the Kapoor cultivar, and the highest total phenolic compound and proline contents were measured for the Ablagh genotype under a 24-h photoperiod. The highest yield (4.36 kg m− 2) and APCI (70.44) were obtained for the Ablagh genotype. The highest nitrate content was obtained with a photoperiod of 18 h for the Kapoor cultivar. The highest net photosynthesis rate was related to the Violeto cultivar under a 24-hour photoperiod (7.89 μmol CO2 m− 2 s− 1). Antioxidant capacity and flavonoids had a positive correlation with phenolic compounds and vitamin C. Yield had a positive correlation with antioxidant capacity, flavonoids, vitamin C, APCI, and proline.
Conclusions
Under continuous light conditions, basil microgreens resistance to light stress by increasing the synthesis of secondary metabolites and the increase of these biochemical compounds made basil microgreens increase their performance along with the increase of these health-promoting compounds. The best balance between antioxidant compounds and performance was achieved in continuous red + blue light. Based on these results, the use of continuous artificial LED lighting, due to the increase in plant biochemical with antioxidant properties and yield, can be a suitable strategy for growing basil microgreens in floating systems.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Microgreens have high nutritional value due to the presence of phytochemical compounds with antioxidant properties and have been introduced as a fresh superfood or functional food in the 21st century [1]. Due to the presence of plant antioxidants, including total phenolic compounds (TPC), vitamin C, total flavonoid compounds (TFC), anthocyanins (ACNs), carotenoids (CARs), and macro and micronutrients, basil (Ocimum basilicum L.) has emerged as one of the most advantageous fragrant plants for cultivating microgreens [2].
The photoperiod, as one of the most important factors of light conditions, is one of the foremost basic environmental factors involved in altering morphogenesis, physiology, photosynthesis, and the generation of plant phytochemicals, and yield through changes within the development cycle of microgreens [3]. The response of horticultural crops to different photoperiods is known to some extent, and the results of many studies have been reported so far. However, there is inadequate information about the response of different basil microgreen cultivars and genotypes to short and long photoperiods as well as continuous lighting (CL). The two photoperiods that are most frequently utilized in plant production are 12 and 16 h, according to a study by Appolloni et al. [4]. The response of plants to long photoperiods is highly genetically dependent; however, exceeding tolerable photoperiod limits can be associated with photooxidative damage to plant leaves, the production of harmful reactive oxygen species (ROS), and a mismatch between internalized circadian rhythms, which can cause a yield reduction in some species and cause economic loss for producers [5]. When a plant is exposed to constant light, the photoreceptor components in the reaction centers of photosystems I and II are negatively affected, which causes a decrease in transcription and a loss of light energy [6]. The response of different plants to long photoperiods is different, and more recent research has provided evidence that CL can lead to crop production without photodamage [7]. Using a 24-h photoperiod means having photon energy continuously available for plant CO2 fixation in the Calvin cycle and providing continuous growth and biomass enhancement [8]. Continuous lighting has increased the yield and plant biochemical of lettuce cultivars and some members of the Brassicaceae family [9, 10]. The effects of different photoperiods (8-12-16–24 h day− 1) on the growth of red and green crocus microgreens were studied, and the results showed that a 16 h light period increased yield, chlorophyll a (Chl a), chlorophyll b (Chl b), total CARs, ACNs, vitamin C (Vit C), and total antioxidant capacity (TAC) in both species [11]. In another experiment, the effects of different photoperiods (12, 14, 16, 18 and 20 h day− 1) on the antioxidant potential and nutritional value of two microgreens, cabbage (Brassica oleracea L.) and Chinese cabbage (Brassica oleracea var. Alboglabra Bailey), showed that the biomass of two microgreens reached a maximum with a photoperiod of 14 h, while the highest amount of Chl and CARs was obtained with a photoperiod of 16 h [12]. Research on the effect of LED light with 24 and 16 h day− 1 photoperiods on the performance and plant phytochemicals of radish, mizuna, cabbage, broccoli and arugula showed that the leaf weight and leaf area in all plants with a 16 h photoperiod were significantly greater than those with a 24 h photoperiod, but a 24 h photoperiod caused the accumulation of ACNs, TFC, and proline (Pro) in all species, and only in arugula did it cause a decrease in nitrate content [10].
Unlike in tomatoes and peppers [13, 14], in microgreens, CL stress usually lasts less than one month, and if the plant can balance the photo-oxidative stress with the synthesis of antioxidants without damaging yield [15], CL can be used as a viable strategy for growing basil microgreens. As a product rich in plant biochemicals with antioxidant properties, it is used in plant factories. In a previous study among 21 basil microgreeen genotypes and cultivars, the Violeto, Red Rubin, Kapoor, and Ablagh genotypes had the highest APCI index [16]. In another study, it was shown that changing control conditions and growth as well as nutrient solution changes can increase the TAC of these cultivars [17]. However, no information has been reported about their antioxidant response, photosynthesis and performance under different photoperiods and under CL. Accordingly, this research was conducted to investigate the effect of short and long photoperiods (including CL) on the yield, photosynthesis and antioxidant compounds of basil microgreens in a floating system. In addition to the feasibility of using a long photoperiod, the highest balance between yield and antioxidant compounds was determined using the different photoperiods.
Methods
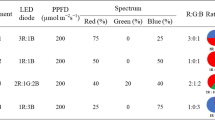
In this study, the effects of five photoperiods, 6 (6 h × 3 cycles), 8 (8 h × 2 cycles), 16, 18 and 24 h day− 1, on the yield and antioxidant response of three cultivars (Violeto, Kapoor, and Red Rubin) and one genotype (Ablagh genotype) of basil microgreens were investigated using a split-plot approach based on a randomized complete block design in a floating system with four replications. The seeds were sown in growth trays (105 cells) with 48.5 g m− 2 density in a mixture of cocopeat and perlite (v/v: 50%), and for faster germination, the trays were stored at a temperature of 25 °C and 60% relative humidity and 400 ppm of CO2 for 48 h in the dark. After seed germination, the different photoperiods were established using an LED artificial light with a combination of blue (450 nm) + red (650 nm) light (1:1) (Iran Grow Light Co. Ltd., Iran) and the light intensity was adjusted to 300 ± 15 μmol photons m− 2 s− 1 by using an AP4 porometer (Delta-T Devices, Cambridge, UK) in the plant growth chamber. The day and night temperatures were set at 24/22 ± 1 °C, and the relative humidity during the growth period was 65–75 ± 5%. Hoagland’s nutrient solution (Supplementary Table S1) with an EC of 2.40 mS cm− 1 and pH of 6 ± 0.2 was used to feed the basil microgreens in a floating system [17]. Due to the high volume of the nutrient solution and no change in EC level, the nutrient solution was not renewed during the growth period. The pH of the nutrient solution was adjusted using 1 M nitric acid and 1 M NaOH daily. All sampling to evaluate biochemical compounds on the day of harvesting was done. Five samples were prepared from each replication and their average was used to measure studied trains.
Determination of photosynthetic pigments
Arnon’s method was modified to quantify the Chl a, Chl b, total Chl, and CARs of leaves [18]. First, 30 mg of leaves was extracted using 300 μL of 80% acetone. Following centrifugation at 3000 rpm for 10 min on a Sigma 2–16 KL centrifuge (Sigma Lab. GmbH), 250 μL of the extract was added to each well of a microplate reader (INNO, LTEK, Gyeonggi-do, Korea), and the absorbance was measured at wavelengths of 663, 645, and 470 nm. The contents of Chl a, Chl b, total Chl, and CARs were determined using formulae 1–4.
W = sample weight (g), V = sample volume (mL).
Total antioxidant capacity, polyphenols, flavonoids, and anthocyanins
Firstly, 0.5 g of fresh leaves was extracted with 500 μL of 80% methanol and incubated in the dark for 24 h in a refrigerator. The extracted materials were centrifuged at 3000 rpm for 15 min to calculate the levels of antioxidant and biochemical compounds [17].
The TAC was determined using the method described by Sharma and Bhat [19]. The percentage of inhibition of 2-diphenyl-1-picrylhydrazyl (DPPH) was determined as follows: radical scavenging activity DPPH% = {(Abs of control − Abs of sample)/(Abs of control)}×100.
To determine the TPC, 160 μL of 1 M Na2CO3 solution and 20 μL of 10% (w/v) Folin-Ciocalteu reagent were mixed with 20 μL of the leaf extract [20]. After 20 min of incubation in the dark, the absorbance at 765 nm was measured using a microplate reader. The TPC was estimated in terms of mg gallic acid g− 1 fresh weight using the gallic acid calibration curve (y = 105.88x, R2 = 0.99).
The flavonoids were evaluated by combining 20 μL of the extracted sample with 85 μL of distilled water and 5 μL of 5% NaNO2. After a 6 min reaction, 10 μL of 10% AlCl3•6H2O was added to the mixture. After another 5 min of reaction, 35 μL of 1 M NaOH and 20 μL of distilled water were added, and the absorbance at 520 nm was measured [21].
Two buffers were used to measure the ACNs: the first solution was potassium chloride with a pH of 1 (0.025 M), which was adjusted using HCl. The second solution was sodium acetate with a pH of 4.5 (0.4 M). Next, 40 μl of basil extract was added to the microplate well along with 160 μl of buffer solution, and the absorbance was measured at wavelengths of 520 and 700 nm using a microplate reader after 20 to 50 min [22].
Proline content
Firstly, 0.1 g of fresh leaves was extracted with 10 mL of 80% methanol. Ninhydrin reagent was prepared by dissolving 2.25 g of ninhydrin in 54 ml of glacial acetic acid and 36 ml of 6 M phosphoric acid and then adding 450 ml of distilled water. The microtube was filled with 50 μl of extract and 250 μl of (ninehydride reagent, water) and incubated at 65 °C for 45 min. After incubation, 250 μl of the solution was added to the microplate, and the samples were analyzed at 515 nm. The proline content was determined using the L-proline standard curve (y = 4.9804x – 0.0309, R² = 0.99) and is given in mg g− 1 fresh weight [23].
Determination of vitamin C content
To calculate the Vit C content of leaves, 300 mg of sample was isolated and combined with 1000 μl of 1% metaphosphoric acid before centrifugation at 850 g for 15 min. The supernatant was then combined with an equivalent quantity of 2,6-dichloroindophenol (DCIP) sodium salt (30 ppm) and incubated at 25 °C for 5 min [24]. The microplate reader described above was used to detect the absorbance at a wavelength of 515 nm in the last stage. The concentration of Vit C was estimated using a standard curve for Vit C (mg Vit C g− 1 fresh weight) (y = 629.42x × 5.3205, R2 = 0.99).
Calculation of the antioxidant potential composite index
The antioxidant capacity of basil microgreens was quantified using the antioxidant potential composite index (APCI) [17, 25]. The APCI was calculated as the sum of six antioxidant activity indices, which included TAC, Vit C, CARs, TFC, TPC, and ACNs, using formula 5:
X1: Antioxidant capacity; X2: Polyphenols; X3: Vitamin C; X4: Flavonoids; X5: Anthocyanins; X6: Carotenoids; Max: The maximum amount of each antioxidant activity indices; N: Number of trains.
Nitrate content
The modified microplate spectrophotometer method of Hachiya and Okamoto was used to determine the nitrate concentration [26]. A tube containing 500 μl of deionized water and 50 mg of frozen microgreen powder was placed in a boiling water bath for 20 min. The sample was centrifuged for 10 min at 3000 rpm after cooling to ambient temperature. A mixture of 10 μl of the extract and 40 μl of salicylic acid in 0.05% (w/v) sulfuric acid was then incubated for 20 min at room temperature. Each sample was then given 1 mL of 8% NaOH (w/v) solution. Using a microplate reader to determine the absorbance at 410 nm, the quantity of nitrate was determined using the nitrate standard curve in mg kg− 1 fresh weight (y = 5149x-60.696, R² = 0.99).
Yield
At the two-leaf stage, basil microgreens were collected under photoperiods of 24 h day− 1 (18 days after sowing), 18 and 16 h day− 1 (25 days after sowing), and 8 and 6 h day− 1 (21 days after sowing), and the yield was determined based on kg m− 2.
Net photosynthesis rate
The net photosynthesis rate was measured using an LCi-SD portable photosynthesis system (ADC Bioscientific Ltd., Hoddesdon, UK) between 10 and 11 AM on the day before harvesting. For this purpose, 10 readings were taken from each replication, and the average of 10 readings was used for mean comparison and analysis of variance. The leaf temperature at the time of measuring photosynthesis was 24.35 °C and intercellular CO2 was 454 μmol m− 2 s− 1.
Statistical analysis
For the data analysis, IBM SPSS software version 22 was used. Duncan’s multiple range test (p ≤ 0.05) was used to compare means. To find any linear relationships between the trains under study, a bivariate Pearson correlation analysis was carried out.
Results
Photosynthetic pigments
The simple effects and the interaction effects of photoperiod and cultivar on the amount of Chl a, Chl b, Chl a + b, and CARs were significantly different at the 1% level (Table 1). The greatest amounts of Chl a, b, a + b, and CARs related to the basil genotype were detected under the 18, 24, 18 and 24-h photoperiods, respectively (Table 2). Total Chl had a positive and significant correlation with CARs, TPC, and Pro (Table 3).
Total antioxidant capacity
The simple effects and the interaction effects of photoperiod and cultivar on the amount of TAC were significantly different at the 1% level (Table 4). The greatest amount of TAC was observed for the Kapoor cultivar under the 24-h photoperiod, which was 178% greater than that of the Red Rubin cultivar under the 8-h photoperiod (Table 2). TAC had a positive and significant correlation with TFC, TPC, Vit C, and Pro (Table 3).
Total phenolic compounds
The simple effects and the interaction effect of photoperiod and cultivar on the TPC were significantly different at the 1% level (Table 4). The greatest TPC was observed for the Ablagh genotype under the 24 h photoperiod, which was 140% greater than that of the Red Rubin cultivar under the 6 h photoperiod (Table 2). TPC had a positive and significant correlation with Vit C, Pro, and nitrate (Table 3).
Proline content
The simple effects and interaction effects of photoperiod and cultivar on the amount of Pro were significantly different at the 1% level (Table 4). The greatest amount of Pro was detected in the Ablagh genotype in under the 24 h photoperiod, which was 475% greater than the Violeto cultivar under the 18 h photoperiod (Table 2).
Total flavonoid compounds
The simple effects of photoperiod and cultivar on the TFC of basil microgreens were significantly different at the 1% level, but their interaction did not significantly differ (Table 4). The highest TFC was calculated for the 24 h photoperiod, which increased by 52% compared to 8-h photoperiod (Table 5). The greatest TFC was calculated for the Red Rubin cultivar, which was 98% greater than the Kapoor cultivar (Table 6). TFC had a positive and significant correlation with TPC and Vit C (Table 3).
Vitamin C content
The simple effects of photoperiod and cultivar on the Vit C content of basil microgreens were significantly different at the 1% level, but their interaction did not significantly differ (Table 4). The greatest amount of Vit C was detected under the 24-h photoperiod, which increased by 60% compared to that under the 8-h photoperiod (Table 5). The highest amount of Vit C was detected in the Ablagh genotype, which was 98.51% greater than that in the Red Rubin cultivar (Table 6). The Vit C had a positive and significant correlation with ACNs and pro (Table 3).
Anthocyanin content
The simple effects of photoperiod and cultivar on the ACN content of basil microgreens were significantly different at the 1% level, but their interaction did not significantly differ (Table 4). The highest ACN content was calculated under the 24-hour photoperiod, which increased by 50% compared to that under the 8-hour photoperiod (Table 5). The greatest amount of ACNs was detected in the Red Rubin cultivar, which was 119.21% greater than that in the Kapoor cultivar (Table 6).
Antioxidant potential composite index
The simple effects of photoperiod and cultivar on the APCI of basil microgreens were significantly different at the 1% level, but their interaction did not significantly differ (Table 4). The highest APCI was calculated for the 24-hour photoperiod, which was 50% greater than that for the 8-hour photoperiod (Table 5). The highest APCI was calculated for the Ablagh genotype, which was 38.30% greater than that for the Red Rubin cultivar (Table 6).
Nitrate content
The simple effects of photoperiod and cultivar on the nitrate content of basil microgreens were significantly different at the 1% level, but their interaction did not significantly differ (Table 4). The highest amount of nitrate was calculated for the 18 h photoperiod, which increased by 78% compared to that for the 6 h photoperiod (Table 5). The highest amount of nitrate was calculated in the Kapoor cultivar, which was 115% greater than that in the Violeto cultivar (Table 6).
Yield
The simple effects of photoperiod and cultivar on basil microgreens yield were significantly different at the 1% level, but their interaction did not significantly differ (Table 4). The highest yield was calculated for the 24-h photoperiod, which increased by 34% compared to that of the 16-hour photoperiod (Table 5). The highest yield was calculated for the Violeto cultivar, which was 45% greater than that of the Red Rubin cultivar (Table 6). Yield had a positive and significant correlation with antioxidant capacity, TFC, Vit C, APCI, and Pro (Table 3).
Balancing between the APCI and yield of basil microgreens under different photoperiods
According to Fig. 1, the best balance between APCI and performance after 24 h of light treatment was established due to the greater performance and APCI of basil microgreens, and the worst balance between APCI and performance was observed after 16 h of light treatment.
Net photosynthesis rate
The simple effect of photoperiod and the interaction effect of photoperiod and cultivar on the net photosynthesis rate significantly differed at the 1% level, but the simple effect of cultivar did not significantly differ (Table 4). The highest net photosynthesis rate was related to the Violeto cultivar under a 24-hour photoperiod, which was 51% greater than that of the Kapoor cultivar under a 6 h photoperiod (Fig. 2). Photosynthesis had a positive and significant correlation with yield and APCI (Table 3).
Interaction effect of photoperiod and cultivar on the net photosynthesis rate of basil microgreens. Means followed by different lowercase letters in a column were significantly different according to Duncan’s multiple-range test (p ≤ 0.05). The photoperiods included 6 (6 h × 3 cycles), 8 (8 h × 2 cycles), 16, 18, and 24 h day− 1
Discussion
The photoperiod can be effective in the synthesis of photosynthetic pigments such as Chl and CARs, although these effects are completely dependent on light conditions, plant species and growth stage [8]. According to Solymosi et al. [27], various aspects of the antioxidant capacity of Chl have been identified. The results showed that the amount of Chl increased linearly with increasing photoperiod from short to long cycles, but after exposure to constant light, the content of Chl decreased. The increase in total Chl indicates that there is a combined effect between the photoperiod and the amount of Chl that allows plants to continue the process of photosynthesis in an ascending manner to light saturation point. The concentration of photosynthetic pigments changed in different photoperiods. In the CL, the amount of Chl decreased and the amount of CARs increased compared to the 18 h. This reaction has been due to excessive absorption of light by the photosynthetic system of the plant in CL so that the plant can increase resistance in these conditions [28]. The decrease in the amount of Chl under CL indicates exposure to light stress and the destruction of Chl pigments probably by increasing the synthesis of the Chl degrading enzyme, which is produced in young leaves under light stress conditions [29]. In photosynthetic systems, CARs (with beta-carotene being the most prominent) act as one component of light-harvesting complexes and protect Chl antennae against of photo-oxidative damage [30]. The importance of ROS in the oxidative damage caused by photo-oxidative stress is well known [31]. The photo-protection of CARs is due to the presence of conjugated double bonds, which can reduce the damaging effects of ROS and remove other ROS, such as hydrogen peroxide, hydroxyl radicals, and superoxide anions [32]. The balance and modification of photosynthetic pigment contents such as the ratio of Chl a/b and CARs/Chl are part of the photosynthesis process and help to achieve a proper balance between input and output energy [33]. Shibaeva et al. [10] stated in their review that the CARs/Chl ratio of Brassicaceae family microgreens increases in the CL. The increase in CARs in CL indicates an increase in the synthesis of these pigments to prevent and reduce the photodamage of light-harvesting complexes against excessive light, which somehow acts as a plant defense mechanism against light stress [34]. Research has shown that a photoperiod of 18 h can increase the production of Chl pigments, but basil microgreens produce more CARs under CL, and by using these pigments to address photo-oxidative stress, they can transform, absorb, and transfer energy. Increasing the ratio of CARs/Chl under CL, increases light absorbtion more effectively than other photoperiods and reduces possible membrane damage [32]. Therefore, CARs pigments, as part of a defense mechanism, control photo-oxidative stress, prevent possible damage to membranes, and increase performance under constant light.
The antioxidant potential increases under biotic and abiotic stresses such as fluctuations in environmental conditions during the production of microgreens. Basil microgreens respond to the photo-oxidative stress caused by continuous light by producing more bioactive antioxidant chemicals. In this study, the contents of TPC, CARs, TFC, ACNs, and Vit C, the most important non-enzymatic plant biochemical with antioxidant properties under CL conditions were increased. The ability of plant antioxidants to scavenge free radicals shows that they act as essential compounds to protect plant organisms against ROS [35]. Therefore, CL increases the food health of microgreens by increasing their antioxidant properties, and the synthesis of plant biochemical with antioxidant properties can protect this superfood from light damage.
TPCs are the most abundant and widespread group of secondary metabolites. TPC rings and hydroxyl groups create strong antioxidant activity and inhibit free radicals [36]. Different mechanisms, including the inhibition of ROS biosynthesis, the trapping of ROS, and the reduction of metal ion catalysts for ROS formation, can be used to explain the antioxidant activity of TPC [37]. Under the influence of long photoperiods, especially CL, the TPC of basil microgreens increased, and these changes were due to differences in the enzymatic activity of hydrolases and TPC oxidases, the synthesis of new TPCs, changes in polymerization and oxidation processes, and the destruction of free or bound TPCs [38]. The defensive mechanisms of plants in response to light stress, which activate various metabolic pathways to improve antioxidant responses in microgreens, might also be responsible for the increase in TPC observed following light exposure. Therefore, basil microgreens under long photoperiods (CL) are a good source of phenolic compounds and can be considered a natural alternative source.
Pro is regarded as one of the most potent osmotic protectors of cells, signaling molecules, and antioxidant defense chemicals, and due to its cyclic structure and secondary amino group (𝛼-amino group), which is different from proteinogenic amino acids, it is a good source of nitrogen for plants. The relationship between Pro synthesis and increasing plant resistance under stress conditions has been determined [36, 37, 39, 40]. Ablaq genotype showed the highest amount of Pro under CL, which indicates that this genotype is stressed under CL conditions. In addition to proline, Ablaq genotype under CL was able to show a high amount of TPC, TAC and CARs content, which by synthesizing these compounds along with proline was able to show resistance to light stress. In this experiment, Pro production in basil microgreens did not change much between different short and long photoperiods, except under constant light, during which the greatest amount of Pro was produced (Table 2). Basil is a summer plant, so it can be expected that this plant is not sensitive to long days (between 14 and 18 h), but basil microgreens are stressed under constant light conditions, and their Pro content is very high, which is among other antioxidant compounds, causing increased resistance to photo-oxidative conditions. Extended photoperiod itself is a reason of the excess of absorbed light that increases photooxidative pressure and makes plant to produce photoprotective antioxidants. Thus, CL provided by LEDs may add nutritional value to microgreens by increasing the antioxidant properties. These results are consistent with several studies that have reported the benefits of LEDs for nutritional value of horticultural crops [10, 41].
TFC, which are phenolic compounds with various structural variations and are categorized as flavones, flavonols, flavonones, flavonols, flavanols, isoflavones, ACNs, and chalcones [42], is another essential component of basil. The two benzene rings are separated by an oxygen-containing pyrene ring, serving as the basis of the flavon core structure. TFCs have pigment functions, signaling molecules, stress resistance, and reproductive regulatory roles in plants. These plants provide protection against photo-damage by exposure to harmful light inside the plant [43]. Several reports have shown that the accumulation of TFCs in plant tissues occurs in response to exposure to longer photoperiods [44]. The present study also showed that a longer photoperiod stimulates the biosynthesis and accumulation of TFC, which is probably related to the absorption of more light, which causes the expression of genes encoding the enzyme phenylalanine ammonia lyase, which is the most important factor in the synthesis of TPC and TFC [45].
The Vit C has several functions in plants, including antioxidant capacity, photosynthesis, electron transport through the membrane, and growth regulation [42]. As in previous research [43], the duration of illumination in this study was related to the increase in the Vit C content of the basil microgreens. In this study, the highest amount of Vit C content was measured in CL, which shows that this light treatment increases the synthesis of antioxidants and vitamin C during plant stress compared to other photoperiods. The enhanced expression of many genes involved in the Vit C biosynthesis pathway probably appears to be the primary cause of the light-dependent buildup of Vit C in plants [44,45,46]. The Ablaq genotype had a high potential in Vit C synthesis compared to other cultivars. This potential is probably due to the origin of the collection of that genotype. Considering that this genotype was collected from the temperate regions of Iran. The cold environment during the growth of basil has increased antioxidants, especially vitamin C, so that the plants can develop a strong defense system. The Vit C content had a positive and significant correlation with the CARs (Table 3). The Vit C and CARs are important antioxidants that can reduce or eliminate ROS caused by photo-oxidative stress in some plant species [47,48,49,50]. Based on these results, increasing the photoperiod and using CL as a suitable strategy for regulating light-dependent pathways increases the synthesis of Vit C and has made basil microgreens rich in Vit C.
The ACNs are considered defensive TFCs with the additional cost of cellular energy for plant growth, which can delay plant metabolism [51]. The presence of ACNs, which are identified by their red color in the leaves, can increase the antioxidant capacity of red basil microgreen cultivars and genotypes [52]. Some of the pigments analyzed, especially ACNs, whose content is high in red basil cultivars and genotypes, apparently contribute to the total TAC [52]. Past research has shown that the red color of leaves can indicate ACN and TPC contents [53]. Light is one of the most important factors involved in ACN biosynthesis, and light intensity, light quality, and photoperiod determine the concentration of ACNs. In addition, the activity of enzymes and genes related to ACN synthesis increases when crops are continuously exposed to radiation [54]. Therefore, the activity of the ACN synthetase enzyme increases under long photoperiods, which increases the ACN content. The results of this research, similar to previous results showed that increasing the photoperiod can increase the ACN content and be used as a factor to produce basil microgreens with high marketability by creating a visual effect along with increasing the amount of antioxidant compounds [55].
The nitrate content in leaves depends on many factors, such as plant genotype and prevailing environmental conditions during plant growth [56]. Nitrate is mainly processed during the day and accumulates in the vacuole at night [57]. Several plant treatment strategies involving a 24-hour photoperiod at the pre-harvest stage have been reported to be successful in reducing nitrate content in leafy vegetables [33]. Greater nitrate reductase (NR) activity as a result of greater NR gene expression may be the reason for the decrease in nitrate content under CL [58]. NR is the key enzyme that limits the rate of reduction of nitrate ions to nitrite ions. Light may control NR activity in two different ways by controlling the transcription, translation, and post-translational activity of NR genes, namely, by controlling the expression of NR genes by photosynthetic products and by controlling the NR status through NADPH [59]. The nitrate content in plants is negatively correlated with the concentration of soluble and non-structural forms of carbon, such as sugars and organic acids, because they complement each other in maintaining cell turgorescence. Therefore, a significant reduction in nitrate content under CL may be associated with an increase in carbohydrate synthesis and an increase in ferredoxin and NADPH content, which are used in nitrate reduction in leaves [6]. In this experiment, the nitrate content was the lowest in the CL treatment, and the nitrate content increased with increasing photoperiod from 8 to 18 h. Compared with a normal photoperiod (16 h), long and short photoperiods decreased the nitrate content. This result can be attributed to the higher activity of NR due to more received light, which leads to a reduction in nitrate accumulation [60]. Since the amount of light received at 18 and 16 h was equal to the total PPFD at 6 and 8 h, respectively, the results showed that the light treatment of 6 h compared to 18 h and the light treatment of 8 h compared to 16 h, respectively, increased the nitrate content to 178.08 and reduced it by 19.55%. Therefore, the use of CL along with short photoperiods can be more effective at reducing nitrate content.
Since microgreens are sold based on fresh weight, the main goal of microgreens production plants is to increase their fresh biomass and nutritional value. A traditional way to increase biomass is to increase DLI by increasing photoperiods [61]. In general, as the DLI increases, biomass can be expected to increase (up to the light saturation point) because a greater DLI means more photosynthetic assimilates [62]. The major mechanism by which plants build biomass is photosynthesis, and the total productivity of crops is closely correlated with whole-plant photosynthesis. Since photosynthesis and carbon absorption are always driven by light energy, CL usage has the potential to improve crop production [63]. Under the conditions of constant PPFD, the electron transfer rate and quantum efficiency of photosystem II (ΦPSII) are uniform and constant; therefore, maximizing ΦPSII under constant light intensity conditions is not feasible. However, there may be opportunities to breed basil microgreen cultivars with higher ΦPSII, which may increase growth and overall resource use efficiency in plant factories [64]. In this experiment, photosynthesis differed among photoperiods, which shows the different responses of basil microgreens to light conditions and day length. In general, the increase in the rate of photosynthesis of basil microgreens with constant ΦPSI, is due to the longer duration of photosynthesis, not the increase in the rate of photosynthesis, which leads to the production of more carbohydrates and increased yield [61].
Basil microgreens under DLI and CL respond to light stress by increasing the production of plant phytochemicals, which are compounds that promote human health, and under these conditions, plant performance increases. The results of earlier research on microgreen species of the Brassicaceae family are similar to the biomass increase shown in this study with increased DLI [10]. According to Pennisi et al. [65], the yield of basil did not increase when it was grown under a 24-hour photoperiod. This difference in observations could be the result of differences in the genetics of different cultivars because, under abiotic stressors such as light stress, gene biosynthesis is expressed differently in basil cultivars, and their expression patterns are different in each cultivar. In this study, under 24-hour CL, the plant was under CL and was continuously photosynthesizing and accumulating carbohydrates. As a result, respiration eliminates darkness during the night; therefore, no carbon loss occurs during the night [66]. In the tested basil microgreens, visible physical damage in CL due to carbohydrate accumulation did not occur in any of the four studied microgreens. Past research on the cultivation of microgreens of the Brassicaceae family has shown that CL increases the growth rate of plants [10]. Therefore, the use of CL can be introduced as a suitable strategy for growing basil microgreens in floating systems by shortening the growth period and increasing yield.
In a prior study, among 21 basil microgreen cultivars, the Ablagh genotype had the greatest APCI [16]. In general, due to its origin (Tabriz city), this genotype has high potential for the production of secondary metabolites and thus produces the greatest amounts of Vit C, TFC, and ACNs. High concentrations of TFC, Vit C, ACNs, CARs, antioxidant capacity, and TPC were detected in basil microgreens. Since a total of these compounds make up APCI, using CL increased their synthesis and produced biochemical compounds with greater antioxidant properties. The contribution of antioxidant compounds to photo-damage may vary significantly depending on environmental conditions and biological characteristics (plant species, plant age, and growth stage) [67]. The results of this study show that the application of CL boosts productivity by increasing yield and triggering the synthesis of secondary metabolites with greater antioxidant qualities, which enhances the nutritional value of microgreens, without causing any harm to basil microgreens. Therefore, the basil microgreens studied in this research can tolerate CL and can be added to other types of microgreens [10], and lettuce [9].
Conclusions
Photoperiod affected the synthesis of secondary metabolites, the performance of basil microgreens and the net photosynthesis rate. Compared with a shorter photoperiod, CL led to an increase in the content of phytochemicals with antioxidant properties such as CARs, ACNs, TFC, total phenolic compounds, and Vit C. According to the best balance between the APCI and yield obtained in CL and the high net photosynthesis rate, CL can be considered a good strategy for growing basil microgreens by increasing yield and nutritional value and drastically reducing nitrate.
Data availability
Data and materials will be made available on request.
References
Bhamra SK, Heinrich M, Johnson MR, Howard C, Slater A. The cultural and commercial value of tulsi (Ocimum tenuiflorum L.): multidisciplinary approaches focusing on species authentication. Plants. 2022;11(22):3160.
Sipos L, Balázs L, Székely G, Jung A, Sárosi S, Radácsi P, Csambalik, L. Optimization of basil (Ocimum basilicum L.) production in LED light environments–a review. Sci Horti. 2021;289:110486.
Hernández-Adasme C, Palma-Dias R, Escalona VH. The effect of light intensity and photoperiod on the yield and antioxidant activity of beet microgreens produced in an indoor system. Horticulturae. 2023;9:493.
Appolloni E, Pennisi G, Zauli I, Carotti L, Paucek I, Quaini S, Orsinia F, Gianquinto G. Beyond vegetables: effects of indoor LED light on specialized metabolite biosynthesis in medicinal and aromatic plants, edible flowers, and microgreens. J Sci Food Agric. 2021;102:472–87.
Marie TRJG, Leonardos ED, Lanoue J, Hao X, Micallef BJ, Grodzinski, B. A perspective emphasizing circadian rhythm entrainment to ensure sustainable crop production in controlled environment agriculture: dynamic use of LED cues. Front Sustain Food Syst. 2022;6:856162.
Velez-Ramirez AI, van Ieperen W, Vreugdenhil D, Millenaar FF. Plants under continuous light. Trends Plant Sci. 2011;16:310.
Lanoue J, St Louis S, Little C, Hao X. Continuous lighting can improve yield and reduce energy costs while increasing or maintaining nutritional contents of microgreens. Front Plant Sci. 2022;13:983222.
Shibaeva TG, Mamaev AV, Sherudilo EG, Titov AF. The role of photosynthetic daily light integral in plant response to extended photoperiods. Russ J Plant Physiol. 2022a;69:1–8.
Ohtake N, Ishikura M, Suzuki H, Yamori W, Goto E. Continuous irradiation with alternating red and blue light enhances plant growth while keeping nutritional quality in lettuce. HortScience. 2018;53:1804–9.
Shibaeva TG, Sherudilo EG, Rubaeva AA, Titov AF. Continuous LED lighting enhances yield and nutritional value of four genotypes of brassicaceae microgreens. Plants. 2022b;11:1–14.
Meas S, Luengwilai K, Thongket T. Enhancing growth and phytochemicals of two amaranth microgreens by LEDs light irradiation. Sci Hortic. 2020;265:109204.
Liu K, Gao M, Jiang H, Ou S, Li X, He R, Li Y, Liu H. Light intensity and photoperiod affect growth and nutritional quality of brassica microgreens. Molecules. 2022;27:883.
Shibaeva TG, Sherudilo EG, Ikkonen E, Rubaeva AA, Levkin IA, Titov AF. Effects of extended light/dark cycles on solanaceae plants. Plants. 2024;13(2):244.
Shibaeva TG, Mamaev AV, Sherudilo EG, Ikkonen EN, Titov AF. Age-related changes in sensitivity of tomato (Solanum lycopersicum L.) leaves to continuous light. Russ J Plant Physiol. 2021;68:948–57.
Zhang S, Zhang L, Zou H, Qiu L, Zheng Y, Yang D, Wang Y. Effects of light on secondary metabolite biosynthesis in medicinal plants. Front Plant Sci. 2021;12:781236.
Fayezizadeh MR, Ansari NA, Sourestani MM, Hasanuzzaman MB, Compounds. Antioxidant capacity, leaf color profile and yield of Basil (Ocimum sp.) microgreens in floating System. Plants. 2023a;12(14):2652.
Fayezizadeh MR, Ansari NA, Sourestani MM, Hasanuzzaman MB. Yield and antioxidant capacity in Basil microgreens: an exploration of nutrient solution concentrations in a floating system. Agriculture. 2023b;13(9):1691.
Arnon A. Method of extraction of chlorophyll in the plants. Agron J. 1967;23:112–21.
Sharma OP, Bhat TK. DPPH antioxidant assay revisited. Food Chem. 2009;113:1202–5.
Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 1996;239:70–6.
Dou H, Niu G, Gu M, Masabni JG. Responses of sweet Basil to different daily light integrals in photosynthesis, morphology, yield, and nutritional quality. Hortic Sci. 2018;53:496–503.
Lee J, Durst RW, Wrolstad RE, Eisele T, Giusti MM, Hach J, Hofsommer H, Koswig S, Krueger DA, Kupina S. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88:1269–78.
Paquin R, Lechasseur P. Observations sur une method dosage de l proline libre dans les extraits de plantes. Can J Bot. 1979;57:1851–4.
Ochoa-Velasco CE, Valadez-Blanco R, Salas-Coronado R, Sustaita-Rivera F, Hernández-Carlos B, García-Ortega S, Santos-Sánchez NF. Effect of nitrogen fertilization and Bacillus licheniformis biofertilizer addition on the antioxidants compounds and antioxidant activity of greenhouse cultivated tomato fruits (Solanum lycopersicum L. var. Sheva) Sci Hortic. 2016;201:338–45.
Ghoora MD, Babu DR, Srividya N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J Food Compos Anal. 2020;91:103495.
Hachiya T, Okamoto Y. Simple spectroscopic determination of nitrate, nitrite, and ammonium in Arabidopsis thaliana. Bio Protoc. 2017;7(10):e2280–2280.
Solymosi K, Mysliwa-Kurdziel B. Chlorophylls and their derivatives used in food industry and medicine. Mini Rev Med Chem. 2017;17:1194–222.
Avgoustaki DD. Optimization of photoperiod and quality assessment of basil plants grown in a small-scale indoor cultivation system for reduction of energy demand. Energies. 2019;12(20):3980.
Tian YN, Zhong RH, Wei JB, Luo HH, Eyal Y, Jin HL, Pang XQ. Arabidopsis CHLOROPHYLLASE 1 protects young leaves from long-term photodamage by facilitating FtsH-mediated D1 degradation in photosystem II repair. Mol. Plant. 2021;14(7):1149–67.
Zerres S, Stahl W. Carotenoids in human skin. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids. 2020;1865:158588.
Baswan SM, Klosner AE, Weir C, Salter-Venzon D, Gellenbeck KW, Leverett J, Krutmann J. Role of ingestible carotenoids in skin protection: a review of clinical evidence. Photodermatol Photoimmunol Photomed. 2021;37:490–504.
Torres-Contreras AM, Garcia-Baeza A, Vidal-Limon HR, Balderas-Renteria I, Ramírez-Cabrera MA, Ramirez-Estrada K. Plant secondary metabolites against skin photodamage: Mexican plants, a potential source of UV-radiation protectant molecules. Plants. 2020;11:220.
Samuolienė G, Viršilė A, Miliauskienė J, Haimi PJ, Laužikė K, Brazaitytė A, Duchovskis P. The physiological response of lettuce to red and blue light dynamics over different photoperiods. Front Plant Sci. 2021;11:610174.
Puah BP, Jalil J, Attiq A, Kamisah Y. New insights into molecular mechanism behind anti-cancer activities of lycopene. Molecules. 2021;26:3888.
Pinto E, Almeida AA, Aguiar AA, Ferreira IM. Comparison between the mineral profile and nitrate content of microgreens and mature lettuces. J Food Compos Anal. 2015;37:38–43.
Moazzen A, Öztinen N, Ak-Sakalli E, Koşar M. Structure-antiradical activity relationships of 25 natural antioxidant phenolic compounds from different classes. Heliyon. 2022;8(9):e10467.
Girish YR, Sharathkumar KS, Prashantha K, Rangappa S, Sudhanva MS. Significance of antioxidants and methods to evaluate their potency. Mater Chem Horizons. 2023;2(2):93–112.
Xu M, Jin Z, Ohm JB, Schwarz P, Rao J, Chen B. Improvement of the antioxidative activity of soluble phenolic compounds in chickpea by germination. J Agric Food Chem. 2018;66:6179–87.
Qirat M, Shahbaz M, Perveen S. Beneficial role of foliar-applied proline on carrot (Daucus carota L.) under saline conditions. Pak J Bot. 2018;50:1735–44.
Signorelli S, Tarkowski ŁP, O’Leary B, Tabares-da Rosa S, Borsani O, Monza J. GABA and proline metabolism in response to stress. In: Gupta DK, Corpas FJ, editors. Hormones and plant response. Plant in challenging environments. 2021; vol 2. Cham: Springer, pp. 291–314.
Loi M, Villani A, Paciolla F, Mulè G, Paciolla C. Challenges and opportunities of light-emitting diode (LED) as key to modulate antioxidant compounds in plants. Rev Antioxid. 2021;10:42.
Shomali A, Das S, Arif N, Sarraf M, Zahra N, Yadav V, Aliniaeifard S, Chauhan DK, Hasanuzzaman M. Diverse physiological roles of flavonoids in plant environmental stress responses and tolerance. Plants. 2022;11:3158.
Martín J, Navas MJ, Jiménez-Moreno AM, Asuero AG. Anthocyanin pigments: importance, sample preparation and extraction. In: Soto-Hernandez M, Palma-Tenango M, Garcia-Mateos R, editors. Phenolic compounds—Natural sources, importance and applications. London, UK: IntechOpen; 2017.
Singh B, Kumar A, Malik AK. Flavonoids biosynthesis in plants and its further analysis by capillary electrophoresis. Electrophoresis. 2017;38(6):820–32.
Smirnoff N. Ascorbic acid metabolism and functions: a comparison of plants and mammals. Free Radic Boil Med. 2018;122:116–29.
Ntagkas N, Woltering EJ, Marcelis LF. Light regulates ascorbate in plants: an integrated view on physiology and biochemistry. Environ Exp Bot. 2018;147:271–80.
Paciolla C, Fortunato S, Dipierro N, Paradiso A, De Leonardis S, Mastropasqua L, De Pinto MC. Vitamin C in plants: from functions to biofortification. Antioxidants. 2019;8(11):519.
Hang WY, Lorence A, Gruszewski HA, Chevone BI, Nessler CL. AMR1, an Arabidopsis gene that coordinately and negatively regulates the mannose/L-galactose ascorbic acid biosynthetic pathway. Plant Physiol. 2009;150:942–50.
Truffault V, Fry SC, Stevens RG, Gautier H. Ascorbate degradation in tomato leads to accumulation of oxalate, threonate and oxalyl threonate. Plant J. 2017;89:996–1008.
Zhou WL, Liu WK, Yang QC. Quality changes in hydroponic lettuce grown under pre-harvest short-duration continuous light of different intensities. J Hortic Sci Biotech. 2012;87:429–34.
Toscano S, Trivellini A, Cocetta G, Bulgari R, Francini A, Romano D, Ferrante A. Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front Plant Sci. 2019;10:1212.
Sytar O, Zivcak M, Bruckova K, Brestic M, Hemmerich I, Rauh C, Simko I. Shift in accumulation of flavonoids and phenolic acids in lettuce attributable to changes in ultraviolet radiation and temperature. Sci Hort. 2018;239:193–204.
Kim MJ, Moon Y, Kopsell DA, Park S, Tou JC, Waterland NL. Nutritional value of crisphead ‘Iceberg’ and Romaine lettuces (Lactuca sativa L.). J. Agric. Sci. 2016;8(1):37–45.
Guo X, Shakeel M, Wang D, Qu C, Yang S, Ahmad S, Song Z. Metabolome and transcriptome profiling unveil the mechanisms of light-induced anthocyanin synthesis in rabbiteye blueberry (vaccinium ashei: Reade). BMC Plant Biol. 2022;22(1):1–14.
He D, Kozai T, Niu G, Zhang X. In: Li J, Zhang G, editors. Light-emitting diodes for horticulture, in light-emitting diodes. Cham: Springer; 2019. pp. 513–48.
Colla G, Kim HJ, Kyriacou MC, Rouphael Y. Nitrates in fruits and vegetables. Sci Hortic. 2018;237:221–38.
Viršilė A, Brazaitytė A, Vaštakaitė-Kairienė V, Miliauskienė J, Jankauskienė J, Novičkovas A, Samuolienė G. Lighting intensity and photoperiod serves tailoring nitrate assimilation indices in red and green baby leaf lettuce. J Sci Food Agric. 2019;99(14):6608–19.
Proietti S, Moscatello S, Riccio F, Downey P, Battistelli A. Continuous lighting promotes plant growth, light conversion efficiency, and nutritional quality of Eruca vesicaria (L.) Cav. In controlled environment with minor effects due to light quality. Front. Plant Sci. 2021;12:730119.
Signore A, Bell L, Santamaria P, Wagstaff C, Van Labeke MC. Red light is effective in reducing nitrate concentration in rocket by increasing nitrate reductase activity, and contributes to increased total glucosinolates content. Front Plant Sci. 2020;11:604.
Fu W, Li P, Wu Y, Tang J. Effects of different light intensities on anti-oxidative enzyme activity, quality and biomass in lettuce. Hortic Sci. 2012;39:129–34.
Palmer S, van Iersel MW. Increasing growth of lettuce and mizuna under sole-source LED lighting using longer photoperiods with the same daily light integral. Agronomy. 2020;10(11):1659.
Poorter H, Niinemets Ü, Ntagkas N, Siebenkäs A, Mäenpää M, Matsubara S. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019;223:1073–105.
Lanoue J, Zheng J, Little C, Grodzinski B, Hao X. Continuous light does not compromise growth and yield in mini-cucumber greenhouse production with supplemental LED light. Plants. 2021;10(2):378.
Elkins C, van Iersel MW. Longer photoperiods with the same daily light integral increase daily electron transport through photosystem II in lettuce. Plants. 2020;9:1172.
Pennisi G, Orsini F, Landolfo M, Pistillo A, Crepaldi A, Nicola S. Optimal photoperiod for indoor cultivation of leafy vegetables and herbs. Eur J Hortic Sci. 2020;85:329–38.
Pham MD, Hwang H, Park SW, Cui M, Lee H, Chun C. Leaf chlorosis, epinasty, carbohydrate contents and growth of tomato show different responses to the red/blue wavelength ratio under continuous light. Plant Physiol Biochem. 2019;141:477–86.
Shibaeva TG, Mamaev AV, Titov AF. Possible physiological mechanisms of leaf photodamage in plants grown under continuous lighting. Russ J Plant Physiol. 2023;70(2):1–11.
Acknowledgements
We are thankful to the Research Council of Shahid Chamran University of Ahvaz for financial support.
Funding
This study was funded by “Shahid Chamran University of Ahvaz”.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.R.F, N.A.A and M.H; methodology, M.R.F, N.A.A and M.M.S; formal analysis, M.R.F and M.H; investigation, M.R.F, N.A.A and M.M.S; writing—original draft preparation, M.R.F, N.A.A and M.M.S and M.H; writing—review and editing, M.H; visualization, M.R.F, N.A.A and M.H; supervision, N.A.A; project administration, N.A.A. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fayezizadeh, M.R., Ansari, N.A., Sourestani, M.M. et al. Variations in photoperiods and their impact on yield, photosynthesis and secondary metabolite production in basil microgreens. BMC Plant Biol 24, 712 (2024). https://doi.org/10.1186/s12870-024-05448-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-05448-z