Abstract
Among plant-derived secondary metabolites are benzylisoquinoline alkaloids (BIAs) that play a vital role in medicine. The most conspicuous BIAs frequently found in opium poppy are morphine, codeine, thebaine, papaverine, sanguinarine, and noscapine. BIAs have provided abundant clinically useful drugs used in the treatment of various diseases and ailments With an increasing demand for these herbal remedies, genetic improvement of poppy plants appears to be essential to live up to the expectations of the pharmaceutical industry. With the advent of clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated9 (Cas9), the field of metabolic engineering has undergone a paradigm shift in its approach due to its appealing attributes, such as the transgene-free editing capability, precision, selectivity, robustness, and versatility. The potentiality of the CRISPR system for manipulating metabolic pathways in opium poppy was demonstrated, but further investigations regarding the use of CRISPR in BIA pathway engineering should be undertaken to develop opium poppy into a bioreactor synthesizing BIAs at the industrial-scale levels. In this regard, the recruitment of RNA-guided genome editing for knocking out miRNAs, flower responsible genes, genes involved in competitive pathways, and base editing are described. The approaches presented here have never been suggested or applied in opium poppy so far.
Similar content being viewed by others
Introduction
Benzylisoquinoline alkaloids (BIAs) are among the most structurally diverse group of plant secondary metabolites containing nitrogen in their scaffold, with nearing the 2,500 known structures [1]. As secondary metabolites, BIAs make a significant contribution to the plant's chemical defense against abiotic and biotic stresses, including water scarcity, salinity, herbivores, and pathogens [2]. BIAs are also believed to have repellent properties against pests [3, 4]. Likewise, the propagation of microorganisms and viruses is hindered by BIAs [5]. Interestingly, BIAs function as the major players in plant acclimatization to the ever-changing environment [6]. Among characterized BIAs, morphine, codeine, thebaine, papaverine, sanguinarine, berberine, and noscapine have attracted much more attention since they bring considerable medical benefits to humans (Table 1).
Ironically, little is known about their ecophysiological functions. Some studies document that the aforementioned BIAs play a crucial role in scavenging excessive free radicals generated as a result of various biotic and abiotic stresses [16, 17]. Sanguinarine and berberine put inhibitory effects on herbivores, bacteria, and fungi [5]. Berberine is recognized as a robust antifeedant compound preventing larval growth and feeding and subsequently reducing the population of pests such as fruit flies (Drosophila melongaster) [3, 4]. As a result of this, berberine has been applied to infected farmlands as a commercial insecticide [4]. While opium poppy plants are experiencing mechanical damage, morphine is being metabolized to bismorphine, which is integrated to the cell wall and confers resistance to hydrolysis mediated by pectinase [18].
BIAs are predominantly occurred in the Papaveraceae, Ranunculaceae, Berberidaceae, and Menispermaceae families [19]. Opium poppy (Papaver somniferum L.) belonging to the Papaveraceae family is assigned the commercial production of codeine, morphine, and the precursor thebaine. In addition, opium poppy is considered as the key supply of sanguinarine, noscapine, and papaverine. Thus, the pharmaceutical industries exclusively rely on opium poppy to satisfy their medical necessity. The chemical synthesis of most BIAs is far from being a possible option as they possess complex structures.
Opium poppy has been subjected to biotechnology-based approaches to boost the levels of pharmaceutically valued BIAs. This encompasses exposure to various biotic and abiotic elicitors and overexpression of BIA biosynthetic genes [20,21,22,23,24,25]. Likewise, the engineering of a rate-limiting enzyme, CYP82Y1, the construction of an artificial metabolon, and the overexpression of a WRKY transcription factor, PsWRKY, are proposed as promising methods for BIA enhancement [26, 27].
Metabolic engineering had suffered from the scarcity of a technique enabling efficient and precise modulation of metabolite biosynthetic pathways until the advent of clustered regularly interspaced short palindromic repeats (CRISPR)/ CRISPRassociated9 (Cas9) for genome editing in 2012 [28]. Genomic mutations made by CRISPR are permanent and transferable to the posterities, providing a great opportunity for plant breeding [29]. More importantly, CRISPR-edited plants with optimized secondary metabolite profiles can be generated without becoming transgenic [30]. The application of CRISPR exceeds beyond gene knockout. It has also been utilized for single base-pair modifications, gene substitution, insertion and/or deletion, and gene expression enhancement [31]. CRISPR/Cas9 heads its site‐directed mutagenesis with the help of two components, an endonuclease and an RNA guide (gRNA) [28]. The most widely adopted endonuclease enzyme for the CRISPR-mediated editing is type II Cas9 derived from Streptococcus pyogenes (SpCas9). gRNA is responsible for identifying a short stretch of nucleotide bases, 3 bp, known as protospacer adjacent motif (PAM) and then binding to target DNA sequences which is adjacent to the PAM [32]. The resulting RNA–DNA complex becomes prone to the cleavage by Cas9 that introduces a double-strand break (DSB) within the genome. Subsequently, nonhomologous end-joining (NHEJ) machinery assumes the responsibility of repair [33], resulting in either completely accurate repair or insertion/deletion (InDel) mutations at the cleavage site [34].
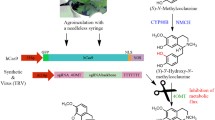
Unfortunately, despite the importance of opium poppy as the sole source of industrial production of BIAs and its high status in today′s society as a model system for studying the biosynthesis of secondary metabolites, especially alkaloids, the applicability of the CRISPR/Cas9 system to promote BIA biosynthesis in this species has not yet been widely explored. The only effort in this regard is the knockout of 3′-hydroxy-N-methylcoclaurine 4′-O-methyltransferase (4′OMT) producing a central branch point intermediate in BIA pathway, (S)-reticuline. In this study, sgRNA was transcribed from both viral-based (TRV) and synthetic binary plasmids and subsequently delivered into plant cells by Agrobacterium harboring a Cas9 encoding-synthetic vector. A dramatic decline in the levels of (S)-reticuline and BIAs was observed, confirming the regulatory role of 4′OMT in BIA pathway [35].
The narcotic analgesics morphine and codeine are introduced as World Health Organization (WHO)-approved medicines owning to their worldwide utility as robust pain-relief drugs [36]. Despite this, more than 50 percent of individuals living around the world are deprived of effective remedies to treat moderate or severe pain [37]. In addition to producing important pharmaceuticals, opium poppy is a source of illicit drugs like heroin synthesized from morphine. Because of this, opium poppy is regarded as a double-edged sword, offering both beneficial and harmful compounds to human health [38]. Safer alternative platforms to producing opium poppy-derived medicinal agents are preferred but the occurrence of one or more chiral centers in the chemical structures of BIAs of medicinal significance hinders their large-scale chemical synthesis [39]. Until recently, extensive attempts have been made to synthesize morphine and its derivatives enzymatically, but none is currently applicable for the commercial production of them [40]. Regarding the microbial synthesis of opioids, both bacteria and fungi such as Escherichia coli and Saccharomyces cerevisiae have been engineered to assemble complex biosynthetic pathways of noscapine, sanguinarine, morphine, codeine, thebaine, and several derivatives. Nevertheless, although achieving significant progresses, synthetic biology is not yet a viable option for BIA industrial synthesis [41]. Taken together, opium poppy crops remain the sole commercial source of BIAs.
In this review, we discuss CRISPR/Cas9-associated strategies that target different genes involved in competitive pathways, adjusting flowering time, and miRNA biosynthesis. In addition, CRISPR-mediated modulation of SNPs named base editing is summarized. Noteworthy, the concurrent overproduction of all BIAs can be accomplished using the adoption of these strategies, avoiding labor-intensive and time-consuming step of increasing each BIA separately. Presumably, these proposed CRISPR-based approaches enable the development of methods for precision breeding of opium poppy tailored to the heightened BIA synthesis.
Biosynthesis of major BIAs in opium poppy
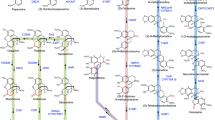
The biosynthesis of BIAs can be considered as an indication of exploiting resources efficiently. Despite considerable structural diversity, BIAs are biosynthetically originated from a single amino acid: L-tyrosine (Fig. 1). L-tyrosine undergoes the decarboxylation, meta-hydroxylation, and transamination, resulting in the formation of two intermediates dopamine and 4-hydroxyphenylacetaldehyde (4-HPAA) condensed by the first committed enzyme (S)-norcoclaurine synthase (NCS) (Fig. 1) [42]. The resulting (S)-norcoclaurine is converted to (S)-coclaurine by the enzyme norcoclaurine 6-O-methyltransferase (6OMT). The formation of (S)-coclaurine is the gateway reaction to papaverine synthesis (Fig. 1). Alternatively, (S)-coclaurine is transformed to (S)-reticuline through 3′-hydroxylation and consecutive O- and N-methylation reactions [39]. Different pathways, including morphinan (morphine, codeine, and thebaine), benzo[c]phenanthridine (sanguinarine), and phthalideisoquinoline (noscapine) are arisen from (S)-reticuline (Fig. 1) [39].
The biosynthetic pathways of dominant BIAs in opium poppy, leading to the formation of papaverine, noscapine, sanguinarine, thebaine, codeine, and morphine. All enzymes have been purified from opium poppy and functionally characterized. AT1, 1,13-dihydroxy-N-methylcanadine 13-O-acetyltransferase; BBE, berberine bridge enzyme; CAS, canadine synthase; CEX1, 3-O-acetylpapaveroxine carboxylesterase; CFS, cheilanthifoline synthase; CNMT, coclaurine N-methyltransferase; CODM, codeine O-demethylase; COR, codeinone reductase; CYP82X1, 1-hydroxy-13-O-acetyl-N-methylcanadine 8-hydroxylase; CYP82X2, 1-hydroxy-N-methylcanadine 13-O-hydroxylase; CYP82Y1, N-methylcanadine 1-hydroxylase; DBOX, dihydrobenzophenanthridine oxidase; 3HOase, L-tyrosine/tyramine 3-hydroxylase; 4-HPAA, 4-hydroxyphenylacetaldehyde; 4-HPP, 4-hydroxyphenylpyruvate; 4HPPDC, 4-hydroxyphenylpuruvate decarboxylase; MSH, N-methylstylopine 14-hydroxylase; NCS, norcoclaurine synthase; NISO, neopinone isomerase; NMCH, N-methylcoclaurine 3′-hydroxylase; NOS, noscapine synthase; OMT2/3, 4′-O-desmethyl-3-O-acetylpapaveroxine 4′-O-methyltransferase; 4′OMT, 3′-hydroxy-N-methylcoclaurine 4′-O-methyltransferase; 6OMT, norcoclaurine 6-O-methyltransferase; 7OMT, norreticuline 7-O-methyltransferase; PAAS, phenylacetaldehyde synthase; P6H, protopine 6-hydroxylase; PPO, polyphenol oxidase; REPI, reticuline epimerase; RNMT, reticuline N-methyltransferase; SalAT, salutaridinol 7-O-acetyltransferase; SalR, salutaridine reductase; SalSyn, salutaridine synthase; SanR, sanguinarine reductase; SOMT, scoulerine 9-O-methyltransferase; SPS, stylopine synthase; STOX, tetrahydroprotoberberine oxidase; T6ODM, thebaine 6-O-demethylase; THS, thebaine synthase; TNMT, tetrahydroprotoberberine N-methyltransferase; TYDC, L-tyrosine/DOPA decarboxylase; TAT, L-tyrosine aminotransferase
CRISPR/Cas9-based strategies to enhance BIAs
Repressing competitive pathways
L-tyrosine, in addition to tryptophan and phenylalanine, results from chorismate which is in turn derived from the glycolysis and pentose phosphate pathways through the seven enzymatic reactions of shikimate pathway (Fig. 2) [43]. Chorismate has been recognized as the central intermediate in which amino acid biosynthetic pathways are diverged from it (Fig. 2). Chorismate serves as the origin of numerous primary metabolites, including vitamins K and B9 and the major defense-responsive hormone, salicylic acid, as well (Fig. 2) [44]. It has been documented that in the majority of circumstances, the central carbon flux acts in favor of phenylalanine biosynthesis. It means that the highest amount of chorismate is directed to its biosynthetic pathway to synthesize myriad phenylalanine–derived compounds, such as phenolics, lignin, flavonoids, and anthocyanin [43]. As a result, a much smaller flux flows towards L-tyrosine biosynthesis compared to that of phenylalanine.
The shikimate pathway supports the synthesis of numerous primary metabolites. The final product of this pathway, chorismate, is channeled into the pathways generating K and B9 vitamins (Vits), L-tyrosine (Tyr), phenylalanine (Phe), salicylic acid (SA), and tryptophan (Trp). L-tyrosine is further converted to benzylisoquinoline alkaloids (BIAs), cyanogenic glycosides (CNgles), and tocopherol. The cleavage of CYP79A1 involved in the biosynthesis pathway of CNgles by the means of the molecular scissor, Cas9, results in InDels in the given gene, generating loss-of-function allele. As a consequence, more L-tyrosine is directed toward BIA pathway
In addition to BIAs, L-tyrosine has also been found to be consumed for the synthesis of tocopherol and cyanogenic glycosides (dhurrin) [43]. Tocopherol in plants is similar to vitamin E in animals in terms of biological activity. Both represent the antioxidant activity. The advantages of tocopherol offered to plants have been enormously studied. Plants containing sufficient levels of tocopherol strongly resist several abiotic stresses, such as water deficiency, salinity, UV radiation, extreme temperature, and so on [45]. Moreover, a pivotal role is played by tocopherol in physiological processes, including growth and development, signal transduction, phytohormonal regulation, and senescence [46].
Cyanogenic glycosides, also known as dhurrin, are biologically active compounds initially identified in Sorghum bicolor, but their occurrence has then been evidenced in excess of 3,000 plant species belonging to 130 families alike [47]. Cyanogenic glycosides actively participate in several biological processes, including seed germination, bud burst, and carbon and nitrogen transport across the cells [48]. Also, they participate in the mitigation of oxidative stress [49]. To generate dhurrin, the activity of CYP79A1 allows the entry of L-tyrosine to its biosynthetic pathway [50]. This rate limiting enzyme catalyzes the conversion of L-tyrosine to p-hydroxyphenylacetaldoxime [51]. Next, the resulting compound is transformed to dhurrin by CYP71E1 and UDP-glucosyltransferase (UGT85B1) [52].
It is ironic that despite the principle functions, cyanogenic glycosides are not counted as essential components in most environmental conditions [47]. Therefore, plant species can bear the elimination of cyanogenic glycosides and stay alive. However, the opposite has been reported for tocopherol. In other words, plants are highly susceptible to the reduction of tocopherol level and a reduction in its content poses adverse and irreversible effects on plant survivability and resilience [45]. In an experiment conducted to develop transgenic sorghum plants harboring reduced levels of cyanogenic glycosides, the expression of CYP79A1 was down-regulated by antisense mediated. The dhurrin content in the transgenics reduced remarkably to 5.1 and 149 μg/g on dry weight basis in comparison with 192.08 μg/g in the non-transformed control. Moreover, progenies in T3 generation produced considerably reduced levels of dhurrin, 62.9 and 76.2 μg/g compared to 221.4 μg/g in the control plants [53].
Hence, to channel more L-tyrosine into BIA pathway, cyanogenic glycoside pathway, not tocopherol, should be repressed by CRISPR. Through knocking out CYP79A1, first-committed enzyme in cyanogenic glycoside biosynthetic pathway, no competition for L-tyrosine consumption occurs between cyanogenic glycoside pathway and the other two pathways, tocopherol and BIA, so that L-tyrosine is exclusively redirected toward BIA and tocopherol pathways. As a result, the pathway of BIA synthesis receives more L-tyrosine compared to before. The BIA content is expected to elevate as a consequence (Fig. 2).
Delaying flowering time
Among floral attributes, flowering time stands out as one of the major determinants of the commercial-scale production of cut flowers, bioactive compounds, and even fodder. Indeed, flowering time ensures commercial success and economic gains. The transition from vegetative to productive stage occurs as a result of endogenous and exogenous cues. Several developmental and environmental signals impact the floral meristem [54]. In poppy plants, they mostly include, but are not limited to, temperature and photoperiod [55]. The flowering induction has been proven to be regulated by eight pathways, photoperiodic, autonomous, vernalization, hormonal, sugar, aging, and temperature. Although acting primarily dependently, these pathways interact with each other at times [54]. The most paramount regulator among genes involved in the floral transition pathways is FLOWERING LOCUS T (FT) [56]. Once translated in the leaf phloem, FT makes a journey to primary meristems where stimulates the expression of the other flowering induction genes, such as SUPPRESSOR OF OVEREXPRESSION OF CONSTANCE 1 (SOC1) and APETALA 1 (AP1) and the eight pathways mentioned previously, collectively inducing flowering [56].
Morphine, codeine, thebaine, sanguinarine, papaverine, and noscapine are abundantly occurring in vegetative, not productive, tissues, including mature capsule, root, unripe capsule, and stem. Flowers and seeds of poppy are deprived of BIAs [57]. Thus, breeding for the prolonged vegetative growth will contribute to the robust vegetative organs, extended harvest time, improved quality, higher biomass yield, and more importantly, higher BIA biosynthesis and accumulation. Functional information about gene networks associated with flowering enables manipulation of key genes in this process. Owning to the leading function of FT in flowering, its genetic alteration should be on the priority over other genes in this process. The high percentage of similarity with other proteins necessitates the sequence-specific and targeted editing of FT gene [58]. With the advent of CRISPR/Cas9 methodology, this goal is achievable via designing sgRNA unique to the FT sequence. FT mutagenesis could result in the hindrance of the expression of downstream genes acting in pathways that directly or indirectly affect flowering, preventing the transfer of the floral signal to primary meristems. Consequently, an extension of the vegetative growth and an enhancement in BIA quantity may be witnessed.
Suppressing microRNAs
MicroRNAs (miRNAs) are denoted as indigenous small, double-strand, non-protein-coding RNAs that are transcribed from MIR genes, with the maximum length of 23 nucleotides. Almost all processes that happen in a living organism bear a trace of miRNAs. miRNAs post-transcriptionally regulate the expression of genes involved in multitude developmental and biological activities, such as seed emergence, blooming, cell and tissue differentiation, apoptosis, signal transductions, phytohormone pathways, primary and secondary metabolisms, and responses to biotic and abiotic stresses in opium poppy [59, 60]. They bind with their complement binding sites situated within target mRNAs, resulting in the degradation or translation inhibition of the transcript via the RNA interference mechanism [61]. Interestingly, it has been recently found that miRNAs can assume the function of signaling molecules. In this situation, the communication between plants and interacting organisms can be established by exchanging miRNAs, allowing plant-to-plant and host-to-microbe interactions [62]. miRNAs and their binding sites act in a species-specific manner. In other words, they are conserved in a given species [63]. In spite of identification of numerous miRNAs in plants, there is insufficient evidence regarding miRNAs wrecking the control of secondary metabolite biosynthesis [64, 65]. Among up to 327 tissue-specific miRNAs in poppy plants, those that influence BIA biosynthesis have recently been functionally characterized (Table 2) [66]. The pso-miR2161 and pso-miR13 prevent the transcripts of 4′OMT and 7-O-methyltransferase (7OMT) from being translated, respectively (Table 2) [66]. The former enzyme catalyzes the methylation of 3′-hydroxy-N-methylcoclaurine, but the latter performs the same reaction on (S)-reticuline [67, 68]. mRNAs encoding codeinone reductase (COR) and salutaridinol 7-O-acetyltransferase (SalAT) are evaluated to be on the tight control of pso-miR t0047847 and pso-miR t0013376, respectively, whereas the L-tyrosine/L-DOPA decarboxylase (TYDC) transcript is inhibited by pso-miR t0000199 (Table 2) [66]. COR and SalAT undertake the responsibility of converting (S)-reticuline to morphinan alkaloids [69, 70], and the decarboxylation of both L-tyrosine and L-DOPA is mediated by TYDC [71]. Correspondingly, pso-miR408 is predicted to target the mRNAs of FAD-binding and berberine bridge enzyme (BBE) domain-containing protein (Table 2) [66], which generate (S)-scoulerine from (S)-reticuline [72].
Till date, CRISPR has been extensively adopted for the knockout of biosynthetic gene. However, a novel avenue has been opened to researchers to explore whether MIR genes can be subjected by this technique. The employment of CRISPR/Cas in miRNA coding genes to regulate metabolite biosynthetic pathway is promising and feasible; however, the modification of target MIRs is in its infancy and still challenging. miRNAs are resistant to frameshift mutations induced by one sgRNA [73]. For this reason, the application of dual sgRNAs to simultaneously edit both ends of a miRNA gene would be effective [74]. This results in the elimination of whole MIR gene or the impairment of the processing of miRNA [74]. The reliance on the 5′-NGG PAM sequence required for the classic Cas9 is restricted the usage of the CRISPR/Cas9 system to fewer MIR genes since due to the small size of miRNA genes, the 5′-NGG PAM site may not be found in all miRNA genes [75]. However, various Cas protein variants have been developed to address this issue. For instance, Cas9 nucleases from Staphylococcus aureus (SaCas9), Neisseria meningitides (NmCas9), and Campylobacter jejuni (CjCas9) can recognize NNGRRT, NNNNGATT, and NNNNACAC PAMs, respectively [75, 76]. Recently, a near-PAMless Cas9 variant termed SpRY is engineered to target NRN and NYN sequences [77]. Therefore, MIR genes can be modified as effective as protein-coding genes through employing these optimized Cas variants.
Single bp InDels introduced by CRISPR/Cas9 often reduce disruption to miRNA activity [78]. The solutions to this issue are to generate medium or large fragment deletions through inducing two DSBs and to increase the frequency of deletions at target miRNA gene [78, 79].
Until recently, the CRISPR/Cas-based miRNA editing has only been utilized in plant species Arabidopsis thaliana [80], Marchantia polymorpha [81], rice [80], wheat [82], soybean [83], and tomato [84]. The efforts to knockout MIR genes has been restricted to agronomically important trains, including higher yield, nutrition acquisition efficiency, and enhanced resistance against biotic and abiotic stresses [85]. Therefore, further studies on the identification and functional analysis of miRNAs involved in biosynthetic pathways of secondary metabolites as well as on the application of CRISPR-based technologies are necessary to routinely employ this technology in metabolic engineering in medicinal plants, especially opium poppy.
Moreover, given that miRNAs act cooperatively to modulate different pathways and in some cases, a single miRNA affects the expression of another miRNA [86], it is essential to determine whether the identified miRNAs in opium poppy work independently to precisely manipulate these miRNAs through genome editing. Another issue is that MIR genes are frequently located in fragile regions like exons or regulatory regions of protein-coding genes so their editing needs special care [86]. Hence, prior to editing of MIR genes in opium poppy, the distribution pattern of them in the genome is required to avoid destructing genes encoding proteins.
A schematic overview of the mechanism in which miRNA editing promotes the production of end products is illustrated in Fig. 3. When MIR genes are exposed to genetic mutations introduced by CRISPR/Cas, target gene′s transcripts are released from the inhibition of this miRNA. As a result, the gene is expressed constitutively and the abundance of its transcripts are continuously increasing. The translation of these transcripts provides higher precursor for downstream enzymes, resulting in a substantial increase in the level of end product, e.g. BIAs (Fig. 3).
a genetic information archived in DNA is transferred to mRNA transcript and to protein that finally gives instruction to secondary metabolism responsible for BIA biosynthesis. b mature mRNA processed by 5′ capping and 3′ polyadenylation is directed to the RNA-silencing pathway mediated by miRNAs. The miRNA complementary to mRNA inhibits its translation via cleavage the transcript. c CRISPR-based miRNA editing causes the modifications in the mRNA sequence. The resulting miRNA is incapable to bind with the target mRNA, culminating in the continuous protein synthesis and an improvement in the BIA accumulation.
 Adenine;
Adenine;
 Uracil;
Uracil;
 Cytosine;
Cytosine;
 Guanine
Guanine
Base editing
The assessment of plant metabolic diversity occupies an important position in plant breeding procedures. With the advent of metabolic profiling technologies, the interspecific and intraspecific variations of metabolites have been analyzed quantitatively [87]. Genome-wide association study (GWAS) accompanied by metabolomics broaden our knowledge regarding the genetic basis of plant metabolism via the determination of DNA variants having enormous importance on metabolic fluctuations [88]. GWAS explores a plethora of genetic loci associated with complex traits, such as metabolite of interest, facilitating the identification of the gene–metabolite association [88]. In poppy plants, just two molecular markers, including amplified fragment length polymorphism (AFLP) and simple sequence repeat (SSR), have been employed for the detection of the genomic regions linked to BIA content [89, 90]. Nevertheless, with the arrival of high-throughput sequencing technologies, differentiation of poppy chemotypes according to single nucleotide polymorphisms (SNPs) is on the rise. As the most pervasive markers in plant genomes, SNPs supply deeper and more comprehensive understanding of molecular mechanisms of plant metabolism. As well as that, SNPs enjoy high sensitivity, robustness, quality assurance, and commercial effectiveness [91]. The recognition of SNP variants responsible for differences in alkaloid content in poppy has been allowed by GWAS [92].
Given the fact that SNPs are underlying contributors to the BIA variation, they can be considered as one of the best options for editing by CRISPR. The CRISPR/Cas9 system relies on homology-directed repair (HDR) to introduce a desired alteration to a single base pair [93]. Since a competition between HDR and NHEJ almost always takes place at the DSB site and usually terminates in favor of NHEJ [94], a number of nucleotides are excluded from the genome. Hence, owning to the high frequency of InDel mutations, the endonuclease-based genome editing is not applicable for editing single base pair. A revised version of CRISPR, therefore, which avoids generation of DSB turns out to be essential. Base editing stands out as one of the most viable approaches that can specifically modify the sequence of a single-nucleotide [95]. To do so, two base editors have been optimized recently: Cytosine base editor (CBE) and adenine base editor (ABE) are assigned to the conversion of C to T and A to G and vice versa, respectively [95]. CBE and ABE share a catalytically impaired Cas9 nuclease (dCas9) fused to a base modification enzyme (cytidine deaminase or adenine deaminase). CBE is additionally composed of a uracil glycosylase inhibitor [95]. The modified Cas9 nicks the DNA strand opposite the PAM-containing one, providing the opportunity for the deaminases that are naturally act on single-strand DNA to catalyze the deamination reaction [34]. The underlying mechanisms whereby these base editors amend a single-point mutation are detailed in Fig. 4. To the best of our knowledge, base editing has not yet been applied for enhancing the contents of secondary metabolites of medical values. However, in several crops, CBE and ABE are currently used to improve agronomic traits, such as herbicide resistance, nutrition acquisition, flowering time, and grain yield [96]. With precise converting C to T at position P197 of Brassica napus acetolactate synthase (BnALS1) gene using CBE, a novel herbicide resistance oilseed rape was generated [97]. In Arabidopsis thaliana and Brassica napus, A-to-G replacement in the FT protein using ABE resulted in plants with late flowering [98].
The schematic overview of base editing. a cytosine base editor (CBE) enables the conversion of C to uracil (U) directed by cytidine deaminase (1). The resulting unmatched U*G is transformed to U*A pair after DNA repair on the non-edited strand (2). Ultimately, T*A pair is resulted from DNA replication (3). b adenine base editor (ABE) makes use of adenosine deaminase instead. The enzyme converts A to inosine (I) (1). The I*T base mismatch is corrected to I*C pair by repair mechanism on the non-edited strand (2). The final G*C pair is produced during DNA replication (3)
In BIA biosynthetic pathway, NCS has been found to catalyze the rate-limiting step [99]. Thus, the efficiency of (S)-norcoclaurine biosynthesis and the control of metabolic flux strongly rely on the catalytic efficiency of NCS [99]. In a study, through homology-based modeling and molecular dynamic, the structure of Papaver somniferum NCS (PsNCS), the impact of the residues residing around the active site on the activation energy, and the rate-enhancing mutations were predicted [100]. The charged residues that negatively affect the efficiency of enzyme were identified as Asp64, Asp71, Arg105, Lys110, Glu117, and Lys163, suggesting the favorable candidates for mutation. Since the residues were suggested to be localized in the enzyme’s surface, their charged groups face the surrounding solvent and do not interact with other residues via hydrogen bridges. They, therefore, guarantee the solubility of NCS. This provides a unique opportunity for mutation. If they are substituted with polar or oppositely charged residues, the structure of NCS will not be disturbed [100].
The findings of this study laid the groundwork for base editing of NCS in further experiments. The enzyme may bear the substitutions of Glu to Gln, Asp to Asn, Arg to Lys, and Lys to Gln because they differ only in a single base pair, For example, Lys is encoded by AAA/AAG but GLn is resulted from CAA/CAG. Moreover, in these SNP mutations, the former residue and the replaced one are both polar so the polarity of the environment surrounding residues is retained [100]. Additionally, although the side chains charge is removed, other characteristics of the residues remain unchanged and the structure of NCS may not be disturbed [100]. Consequently, the catalytic activity of NCS will be enhanced without any structure rearrangement, resulting in higher BIA production.
Single amino acid substitution using base editing is favored to computational design methods due to its independency from computer simulations, bioinformatics methods, and prior knowledge regarding protein structure, the structure–function relationship, accurate protein modelling, and intermolecular interactions.
Challenges in engineering BIA pathway by applying CRISPR/Cas technology
The availability of opium poppy reference genome sequence accounting for up to 95% of the whole genome size facilitates the applicability of CRISPR/Cas9-based genome editing in this plant.
However, enhancing BIA content with the help of CRISPR faces some hurdles that should be overcome. With regard to off-target effects, similarity in gene sequences limits its application [101]. Phylogenic analysis identified that morphinan pathway genes have a high degree of sequence homology, reflecting their evolutionary history. Interestingly, codeine 3-O-demethylase (CODM) and thebaine 6-O-demethylase (T6ODM) share more that 97% amino acid identity [102]. Thus, the target specificity of sgRNAs designed to edit genes involved in morphinan pathway must be strictly validated.
Based upon a logistic regression model, off-target occurrence is believed to be mainly impacted by five factors: mismatch frequency, mismatch position, GC-content, nuclease variants, and delivery methods [101]. The number mismatches is reversely correlated with off-target effects. Off-target genome editing is reduced to up to 59% when a single mismatch is present [103]. However, location of mismatches is more important than their quantity. Mismatches at the 5′ end of sgRNAs, even three to five base pair mismatches, can still contribute to off-target cleavage activity [104]. Due to close homology between 4′OMT and 4′OMT1, 4′OMT-targeting sgRNA showed 17 bp matches out of 20 bp with 4′OMT1 at the 5′ end. The presence of mismatches at the 3′ end was not tolerated by the CRISPR/Cas9 system and therefore, off-target cleavage was inhibited [35].
GC content is another principal factor influencing off-target gene editing. Increasing GC content positively affects on-target activity since higher GC content stabilizes the DNA: RNA hybridization [103]. Although GC contents of BIA biosynthetic genes are determined and are available in the web-based NCBI database, the impact of GC content on the gene editing efficacy in opium poppy needs to be unveiled. The current understanding of the endonuclease variants and delivery techniques on off-target effects is finite [105].
Establishing an efficient procedure for the transformation and regeneration is a significant obstacle to the application of CRISPR/Cas9 in opium poppy. Opium poppy has been recognized as a difficult to transform with Agrobacterium and very recalcitrant plant species because of the massive amount of alkaloids that hinder transformation and differentiation of plant cells [106]. Moreover, all regeneration protocols vary depending on varieties and genotypes [107]. To overcome these limitations, nanoparticle-based plasmid delivery and polyethylene glycol-mediated protoplast transformation have been proposed [108].
Other underlying contributors, including promoters, terminators, selectable markers, plasmid vectors, and driving machinery should also be taken into account to witness the effective utilization of the CRISPR tool in opium poppy.
Ethical issues surrounding gene editing
In recent years, agriculture and the environment have experienced advances in CRISPR/Cas-mediated gene editing. However, this technology has raised some legal and ethical issues on these sectors. Organic farming communities argue that gene editing disturbs the natural evolutionary processes via the conversion of wild-type alleles into drive alleles in a wild-type population, changing heterozygous to homozygous [109]. In RNA-targeted gene editing, the occurrence of off-target mutations is undeniable. This may result in a set of concomitant disadvantages. This unintentional failure will persist in each generation [110]. The mutations may be transferred to future generations. The frequency of mutations may increase as time passes [111]. The alleles with non-target modifications may be disappeared absolutely due to gene drift. More importantly, horizontal gene transfer may occur between the plant carrying off-target mutations and other living beings in the environment, increasing the possibility of the transmission of negative attributes to the associated organisms [112].
Gene editing applications should be in the realm of biosafety law to ensure the safety of people and the environment. When not observing legal and ethical regulations, the agricultural gene editing practices are not allowed to leave the laboratory. Nevertheless, from the perspective of the proponents of gene editing, gene-edited products are not genetically transformed with foreign genes, transgenes, thus, biosafety regulations should not be implemented on them [113]. On the other hand, the critics questioned the biosafety of gene editing because of very limited understanding about off-target effects [114]. Fortunately, newly developed CRISPR-Cas is capable of minimizing or even preventing non-target gene editing.
Due to the controversies about CRISPR/Cas, regulatory policies are required to be developed for future processing, research and development, and commercial uses [115]. Notably, rules and regulations should be clear, science-based, effective, defendable, credible, and proportional to the context which is planned to use to be accepted by the scientific community and bioethical and legal parties [109].
Concluding remarks and future perspectives
CRISPR-oriented metabolic engineering has emerged as a promising tool to tackle the insufficiency of opium poppy-derived medicines through introducing preferred and genome-wide genetic changes in the genome. However, future efforts must be focused on uncovering the untapped potentialities of CRISPR/Cas9. CRISPR/Cas9-based genome editing could counteract the negative characteristics of conventional methods for metabolic engineering suffering from accidental gene insertion, unpredicted results, position effect, and low efficiency. It is worth mentioning that genome editing by CRISPR/Cas9 is not limited to developing poppy plants with advanced pharmaceutical outputs; it can expand its applications to a range of traits beneficial to farmers and the food industry by creating biotic and abiotic stress-resilient genotypes, plants with delayed senescence, and essential oil-enriched seeds, respectively.
The global trend toward synthetic drugs has been reversed. The popularity and acceptability of herbal-based medicines such as opium poppy-extracted BIAs are continuously increasing since they offer a safe, inexpensive, easy to access, and no side-effects therapy [15]. Persons of all ages and genders have received herbal therapy at least once in their lives. A surprising number of people are treated with codeine or strong opioid like morphine, 69% and 99%, respectively [116]. The number of patients whose principal drugs of concern are BIAs is growing. Hence, it is apparent that the pharmaceutical companies will require a substantial amount of BIAs to meet the increasing demand of practitioners and patients as well. Additionally, with the continuous advent of novel strains of pathogenic microorganisms, the development of new drugs is urgent. Because the efficacy of BIAs against emerging diseases, such as SARS-COVID 9 and HIV has been proven [117,118,119], it can be projected that BIAs efficiently will combat unprecedented health-threatening problems in the upcoming years. Therefore, the exploitation of strategies enhancing their production is rational and necessary.
Furthermore, BIAs can supply raw materials for fabrication of semisynthetic derivatives. Thebaine, as an example, is used as the starting material for the semi-synthesis of oxycodone and hydrocodone mitigating acute pains; naltrexone reducing opioid cravings; and naloxone reversing opioid overdose [39].
The advancements in functional genomics tools, including VIGS-mediated analysis, transcriptomics, proteomics, and next-generation sequencing (NGS) projects have broaden our knowledge of the organization of BIA biosynthetic genes and enzymes, BIA metabolic networks, and regulatory mechanisms at different levels. These achievements are likely to substantially enhance the effectiveness of innovative metabolic engineering strategies like CRISPR/Cas and to provide an opportunity for BIA industrial biosynthesis in opium poppy.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Liscombe DK, Macleod BP, Loukanina N, Nandi OI, Facchini PJ. Evidence for the monophyletic evolution of benzylisoquinoline alkaloid biosynthesis in angiosperms. Phytochem. 2005;66:1374–93.
Hagel JM, Facchini PJ. Benzylisoquinoline alkaloid metabolism: a century of discovery and a brave new world. Plant Cell Physiol. 2013;54:647–72.
Sellier MJ, Reeb P, Marion-Poll F. Consumption of bitter alkaloids in Drosophila melanogaster in multiple choice test conditions. Chem Senses. 2011;36:323–34.
Shields VDC, Smith KP, Arnold NS, Gordon IM, Shaw TE, Waranch D. The effect of varying alkaloid concentrations on the feeding behavior of gypsy moth larvae, Lymantria dispar (L.) (Lepidoptera: Lymantriidae). Arthropod Plant Interact. 2008;2:101–7.
Schmeller T, Latz-Bruning B, Wink M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochem. 1997;44:257–66.
Jamwal K, Bhattacharya S, Puri S. Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. J Appl Res Med Aromat Plants. 2018;9:26–38.
Hamilton GR, Baskett TF. In the arms of morpheus the development of morphine for postoperative pain relief. Can J Anaesth. 2000;47:367–74.
Facchini PJ, Park SU. Developmental and inducible accumulation of gene transcripts involved in alkaloid biosynthesis in opium poppy. Phytochem. 2003;64:177–86.
Pathan H, Williams J. Basic opioid pharmacology: an update. Br J Pain. 2012;6:11–6.
Desgagne-Penix I, Facchini PJ. Systematic silencing of benzylisoquinoline alkaloid biosynthetic genes reveals the major route to papaverine in opium poppy. Plant J. 2012;72:331–44.
Dvorák Z, Vrzal R, Maurel P, Ulrichová J. Differential effects of selected natural compounds with anti-inflammatory activity on the glucocorticoid receptor and NF-kappaB in HeLa cells. Chem Biol Interact. 2006;159:117–28.
Eun JP, Koh GY. Suppression of angiogenesis by the plant alkaloid, sanguinarine. Biochem Biophys Res Comm. 2004;317:618–24.
Galadari S, Rahman A, Pallichankandy S, Thayyullathil F. Molecular targets and anticancer potential of sanguinarine–a benzophenanthridine alkaloid. Phytomed. 2017;4:143–53.
Jeng JH, Wu HL, Lin BR, Lan WH, Change HH, Ho YS, et al. Antiplatelet effect of sanguinarine is correlated to calcium mobilization, thromboxane and cAMP production. Atherosclerosis. 2007;191:250–8.
Rahmanian-Devin P, Baradaran Rahimi V, Jaafari MR, Golmohammadzadeh S, Sanei-Far Z, Askari VR. Noscapine, an emerging medication for different diseases: A mechanistic review. Evid Based Complement Alternat Med. 2021;2021:8402517.
Elyasi L, Eftekhar-Vaghefi SH, Esmaeili-Mahani S. Morphine protects SH-SY5Y human neuroblastoma cells against 6-hydroxydopamine-induced cell damage: involvement of anti-oxidant, calcium blocking, and anti-apoptotic properties. Rejuvenation Res. 2014;17:255–63.
Jayaraj RL, Beiram R, Azimullah S, Meeran M F N, Ojha SK, Adem A, et al. Noscapine prevents rotenone-induced neurotoxicity: Involvement of oxidative stress, neuroinflammation and autophagy pathways. Molecules. 2021;26:4627.
Morimoto S, Suemori K, Moriwaki J, Taura F, Tanaka H, Aso M, et al. Morphine metabolism in the opium poppy and its possible physiological function. J Biol Chem. 2001;276:38179–84.
Ziegler J, Facchini PJ. Alkaloid biosynthesis: metabolism and trafficking. Annu Rev Plant Biol. 2008;59:735–69.
Gurkok T, Ozhuner E, Parmaksiz I, Özcan S, Turktas M, Ipek A, et al. Functional characterization of 4′OMT and 7OMT genes in BIA biosynthesis. Front Plant Sci. 2016;7:98.
Larkin PJ, Miller JA, Allen RS, Chitty JA, Gerlach WL, Frick S, et al. Increasing morphinan alkaloid production by over-expressing codeinone reductase in transgenic Papaver somniferum. Plant Biotechnol J. 2007;5:26–37.
Pandey SS, Singh S, Babu CSV, Shanker K, Shrivastava NK, Kalra A. Endophytes of opium poppy differentially modulate host plant productivity and genes for the biosynthetic pathway of benzylisoquinoline alkaloids. Planta. 2016;243:1097–114.
Siahmansour Sh, Ismaili A, Nazarian Firouzabadi F. Effect of different elicitor treatments on hairy root of medicinal plant poppies (Papaver somniferum L.). J Plant Prod. 2016;41:29–42.
Sohrabi SM, Ismaili A, Nazarian-Firouzabadi F. Simultaneous over-expression and silencing of some benzylisoquinoline alkaloid biosynthetic genes in opium poppy. Ind Crops Prod. 2018;123:581–90.
Szabó B, Tyihák E, Szabó G, Botz L. Mycotoxin and drought stress induced change of alkaloid content of Papaver somniferum plantlets. Acta Bot Hung. 2003;45:409–17.
Aghaali Z, Naghavi MR. Engineering of CYP82Y1, a cytochrome P450 monooxygenase: a key enzyme in noscapine biosynthesis in opium poppy. Biochm J. 2023;480:2009–22.
Aghaali Z, Naghavi MR, Zargar M. Promising approaches for simultaneous enhancement of medicinally significant benzylisoquinoline alkaloids in opium poppy. Front Plant Sci. 2024;15:1377318.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21.
Liu D, Hu R, Palla KJ, Tuskan GA, Yang X. Advances and perspectives on the use of CRISPR/Cas9 systems in plant genomics research. Curr Opin Plant Biol. 2016;30:70–7.
Mackelprang R, Lemaux PG. Genetic engineering and editing of plants: an analysis of new and persisting questions. Annu Rev Plant Biol. 2020;71:659–87.
Soyars CL, Peterson BA, Burr CA, Nimchuk ZL. Cutting edge genetics: CRISPR/Cas9 editing of plant genomes. Plant Cell Physiol. 2018;59:1608–20.
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–6.
Shalem O, Sanjana NE, Zhang F. High-throughput functional genomics using CRISPR–Cas9. Nat Rev Genet. 2015;16:299–311.
Jiang F, Doudna JA. CRISPR-Cas9 structures and mechanisms. Annu Rev Biophys. 2017;46:505–29.
Alagoz Y, Gurkok T, Zhang B, Unver T. Manipulating the biosynthesis of bioactive compound alkaloids for next-generation metabolic engineering in opium poppy using CRISPR-Cas 9 genome editing technology. Sci Rep. 2016;6:30910.
World Health Organization (WHO) model list of essential medicines-22nd list. Geneva, Switzerland. 2021. https://who.int. Accessed 30 Sept 2021.
Lohman D, Schleifer R, Amon JJ. Access to pain treatment as a human right. BMC Med. 2010;8:8.
Leshner AI. Integrating tactics on opioids. Science. 2019;363:1367.
Singh A, Menéndez-Perdomo IM, Facchini PJ. Benzylisoquinoline alkaloid biosynthesis in opium poppy: an update. Phytochem Rev. 2019;18:1457–82.
Reed JW, Hudlicky T. The quest for a practical synthesis of morphine alkaloids and their derivatives by chemoenzymatic methods. Acc Chem Res. 2015;48:674–87.
Ehrenworth AM, Peralta-Yahya P. Accelerating the semisynthesis of alkaloid-based drugs through metabolic engineering. Nat Chem Biol. 2017;13:249–58.
Samanani N, Liscombe DK, Facchini PJ. Molecular cloning and characterization of norcoclaurine synthase, an enzyme catalyzing the first committed step in benzylisoquinoline alkaloid biosynthesis. Plant J. 2004;40:302–13.
Maeda H, Dudareva N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu Rev Plant Biol. 2012;63:73–105.
Herrmann KM, Weaver LM. The shikimate pathway. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:473–503.
Hasanuzzaman M, Nahar K, Fujita M. Role of tocopherol (vitamin E) in plants: abiotic stress tolerance and beyond. In: Ahmad P, editor. Emerging technologies and management of crop stress tolerance. Amsterdam: Elsevier, The Netherlands; 2014. p. 267–89.
Arrom L, Munne-Bosch S. Tocopherol composition in flower organs of Lilium and its variations during natural and artificial senescence. Plant Sci. 2010;179:289–95.
Gleadow RM, Møller BL. Cyanogenic glycosides: synthesis, physiology, and phenotypic plasticity. Annu Rev Plant Biol. 2014;65:155–85.
Selmar D, Lieberei R, Biehl B. Mobilization and utilization of cyanogenic glycosides: the linustatin pathway. Plant Physiol. 1988;86:711–6.
Møller BL. Functional diversifications of cyanogenic glucosides. Curr Opin Plant Biol. 2010;13:337–46.
Kahn RA, Fahrendorf T, Halkier BA, Møller BL. Substrate specificity of the cytochrome P450 enzymes CYP79A1 and CYP71E1 involved in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. Arch Biochem Biophys. 1999;363:9–18.
Sibbesen O, Koch B, Halkier BA, Mّller BL. Cytochrome P-450TYR is a multifunctional hemethiolate enzyme catalyzing the conversion of L-tyrosine to p-hydroxyphenylacealdehyde oxime in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. J Biol Chem. 1995;270:3506–3511.
Jones PR, Møller BL, Hoj PB. The UDP-glucose:p-hydroxymandelonitrile-O-glucosyltransferase that catalyzes the last step in synthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor: isolation, cloning, heterologous expression, and substrate specificity. J Biol Chem. 1999;274:35483–91.
Pandey AK, Madhu P, Bhat BV. Down-regulation of CYP79A1 Gene through antisense approach reduced the cyanogenic glycoside dhurrin in [Sorghum bicolor L.) Moench] to improve fodder quality. Front Nutr. 2019;6:122.
van Dijk ADJ, Molenaar J. Floral pathway integrator gene expression mediates gradual transmission of environmental and endogenous cues to flowering time. Peer J. 2017;2017: e3197.
Lisson SN. Temperature and photoperiod effects on the growth and development of opium poppy (Papaver somniferum). Aust J Exp Agric. 2007;47:742–8.
Fornara F, de Montaigu A, Coupland G. SnapShot: control of flowering in Arabidopsis. Cell. 2010;141:550.
Knutsen HK, Alexander J, Barregard L, Bignami M, Bruschweiler B, Ceccatelli S. Update of the scientific opinion on opium alkaloids in poppy seeds. EFSA J. 2018;16:5243.
Hodaei A, Werbrouck SPO. Unlocking nature’s clock: CRISPR technology in flowering time engineering. Plants. 2023;12:4020.
Karimi AA, Naghavi MR, Nasiri J. Identification of miRNAs and their related target genes in Papaver somniferum. Iran J Field Crop Sci. 2017;48:1161–70.
Unver T, Parmaksiz I, Dündar E. Identification of conserved micro-RNAs and their target transcripts in opium poppy (Papaver somniferum L.). Plant Cell Rep. 2010;29:757–69.
Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, et al. Widespread translational inhibition by plant miRNAs and siRNAs. Science. 2008;320:1185–90.
Middleton H, Yergeau É, Monard C, Combier JP, El Amrani A. Rhizospheric plant-microbe interactions: miRNAs as a key mediator. Trends Plant Sci. 2021;26:132–41.
Ferreira SS, Reis RS. Using CRISPR/Cas to enhance gene expression for crop trait improvement by editing miRNA targets. J Exp Bot. 2023;74:2208–12.
Najafabadi AS, Naghavi MR. Mining Ferula gummosa transcriptome to identify miRNAs involved in the regulation and biosynthesis of terpenes. Gene. 2018;645:41–7.
Sabzehzari M, Naghavi MR. Phyto-miRNAs-based regulation of metabolites biosynthesis in medicinal plants. Gene. 2019;682:13–24.
Boke H, Ozhuner E, Turktas M, Parmaksiz I, Ozcan S, Unver T. Regulation of the alkaloid biosynthesis by miRNA in opium poppy. Plant Biotechnol J. 2015;13:409–20.
Morishige T, Tsujita T, Yamada Y, Sato F. Molecular characterization of the S-adenosyl-l-methionine: 3′-hydroxy-N-methylcoclaurine 4′-O-methyltransferase involved in isoquinoline alkaloid biosynthesis in C. japonica. J Biol Chem. 2000;275:3398–23405.
Ounaroon A, Decker G, Schmidt J, Lottspeich F, Kutchan TM. (R,S)-Reticuline 7-O-methyltransferase and (R,S)-norcoclaurine 6-O-methyltransferase of Papaver somniferum––cDNA cloning and characterization of methyl transfer enzymes of alkaloid biosynthesis in opium poppy. Plant J. 2003;36:808–19.
Grothe T, Lenz R, Kutchan TM. Molecular characterization of the salutaridinol 7-O-acetyltransferase involved in morphine biosynthesis in opium poppy P. somniferum. J Biol Chem. 2001;276:30717–23.
Unterlinner B, Lenz R, Kutchan TM. Molecular cloning and functional expression of codeinone reductase: the penultimate enzyme in morphine biosynthesis in the opium poppy P. somniferum. Plant J. 1999;18:465–745.
Facchini PJ, De Luca V. Differential and tissue-specific expression of a gene family for tyrosine/dopa decarboxylase in opium poppy. J Biol Chem. 1994;269:26684–90.
Facchini PJ, Penzes C, Johnson A, Bull D. Molecular characterization of berberine bridge enzyme genes from opium poppy. Plant Physiol. 1996;112:1669–77.
Zhang D, Zhang Z, Unver T, Zhang B. CRISPR/Cas: a powerful tool for gene function study and crop improvement. J Adv Res. 2021;29:207–21.
Lukan T, Veillet F, Križnik M, Coll A, Mahkovec Povalej T, Pogačar K, et al. CRISPR/Cas9-mediated fine-tuning of miRNA expression in tetraploid potato. Hortic Res. 2022;9:uhac147.
Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun. 2018;9:1911.
Manghwar H, Lindsey K, Zhang X, Jin S. CRISPR/Cas system: recent advances and future prospects for genome editing. Trends Plant Sci. 2019;24:1102–25.
Walton RT, Christie KA, Whittaker MN, Kleinstiver BP. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science. 2020;368:290–6.
Zhou J, Deng K, Cheng Y, Zhong Z, Tian L, Tang X, et al. CRISPR-Cas9 based genome editing reveals new insights into microRNA function and regulation in rice. Front Plant Sci. 2017;8:1598.
Zhang Q, Yin K, Liu G, Li S, Li M, Qiu JL. Fusing T5 exonuclease with Cas9 and Cas12a increases the frequency and size of deletion at target sites. Sci China Life Sci. 2020;63:1918–27.
Bi H, Fei Q, Li R, Liu B, Xia R, Char SN, et al. Disruption of miRNA sequences by TALENs and CRISPR/Cas9 induces varied lengths of miRNA production. Plant Biotechnol J. 2020;18:1526–36.
Tsuzuki M, Futagami K, Shimamura M, Inoue C, Kunimoto K, Oogami T, et al. Early arising role of the microRNA156/529-SPL module in reproductive development revealed by the liverwort Marchantia polymorpha. Curr Biol. 2019;29:3307–14.
Wang S, Zong Y, Lin Q, Zhang H, Chai Z, Zhang D, et al. Precise, predictable multi-nucleotide deletions in rice and wheat using APOBEC-Cas9. Nat Biotechnol. 2020;38:1460–5.
Jacobs TB, LaFayette PR, Schmitz RJ, Parrott WA. Targeted genome modifications in soybean with CRISPR/Cas9. BMC Biotechnol. 2015;15:16.
Lin W, Gupta SK, Arazi T, Spitzer-Rimon B. MIR172d is required for floral organ identity and number in tomato. Int J Mol Sci. 2021;22:4659.
Deng F, Zeng F, Shen Q, Abbas A, Cheng J, Jiang W, et al. Molecular evolution and functional modification of plant miRNAs with CRISPR. Trends Plant Sci. 2022;27:890–907.
Mangrauthia SK, Maliha A, Prathi NB, Marathi B. MicroRNAs: potential target for genome editing in plants for traits improvement. Ind J Plant Physiol. 2017;22:530–48.
Okazaki Y, Saito K. Recent advances of metabolomics in plant biotechnology. Plant Biotechnol Rep. 2012;6:1–15.
Wen W, Li D, Li X, Gao Y, Li W, Li H, et al. Metabolome-based genome-wide association study of maize kernel leads to novel biochemical insights. Nat Commun. 2014;5:3438.
Celik I, Camci H, Kose A, Kosar FC, Doganlar S, Frary A. Molecular genetic diversity and association mapping of morphine content and agronomic traits in Turkish opium poppy (Papaver somniferum) germplasm. Mol Breeding. 2016;36:46.
Verma N, Jena SN, Shukla S, Yadav K. Genetic diversity, population structure and marker trait associations for alkaloid content and licit opium yield in India-wide collection of poppy (Papaver somniferum L.). Plant Gene. 2016;7:26–41.
Adamski J. Genome-wide association studies with metabolomics. Genome Med. 2012;4:34.
Bae SH, Oh JH, Lee J. Identification of interspecific and intraspecific single nucleotide polymorphisms in Papaver spp. Plant Breed Biotech. 2021;9:55–64.
Nieto MA, Huang RY, Jackson RA, Thiery JPEMT. Cell. 2016;2016(166):21–45.
Shu S, Wu HJ, Ge JY, Zeid R, Harris IS, Jovanović B, et al. Synthetic lethal and resistance interactions with BET bromodomain inhibitors in triple-negative breast cancer. Mol Cell. 2020;78:1096–113.
Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533:420–4.
Azameti MK, Dauda WP. Base editing in plants: Applications, challenges, and future prospects. Front Plant Sci. 2021;12:664–997.
Wu J, Chen C, Xian G, Liu D, Lin L, Yin S, et al. Engineering herbicide-resistant oilseed rape by CRISPR/Cas9-mediated cytosine base-editing. Plant Biotechnol J. 2020;18:1857–9.
Kang BC, Yun JY, Kim ST, Shin YJ, Ryu J, Choi M, et al. Precision genome engineering through adenine base editing in plants. Nat Plants. 2018;4:427–31.
Samanani N, Facchini PJ. Purification and characterization of norcoclaurine synthase. The first committed enzyme in benzylisoquinoline alkaloid biosynthesis in plants. J Biol Chem. 2002;277:33878–83.
De Sousa JPM, Oliveira NCSA, Fernandes PA. Rational engineering of (S)-norcoclaurine synthase for efficient benzylisoquinoline alkaloids biosynthesis. Molecules. 2023;28:4265.
Modrzejewski D, Hartung F, Lehnert H, Sprink T, Kohl C, Keilwagen J, et al. Which factors affect the occurrence of off-target effects caused by the use of CRISPR/Cas: A systematic review in plants. Front Plant Sci. 2020;11:574959.
Guo L, Winzer T, Yang X, Li Y, Ning Z, He Z, et al. The opium poppy genome and morphinan production. Science. 2018;362:343–7.
Das S, Kwon M, Kim JY. Enhancement of specialized metabolites using CRISPR/Cas gene editing technology in medicinal plants. Front Plant Sci. 2024;15:1279738.
Corsi GI, Qu K, Alkan F, Pan X, Luo Y, Gorodkin J. CRISPR/Cas9 gRNA activity depends on free energy changes and on the target PAM context. Nat Commun. 2022;13:3006.
Chen S. Minimizing off-target effects in CRISPR-Cas9 genome editing. Cell Biol Toxicology. 2019;35:399–401.
Facchini PJ, Bird DA. Developmental regulation of benzyl isoquinoline alkaloid biosynthesis in opium poppy plants and tissue cultures. In Vitro Cell Dev Bioi. 1998;34:69–79.
Royandezagh SD, Khawar KM, Osalou AR, Ozcan S. Agrobacterium mediated genetic transformation of Papaver somniferum L. using semi solid agar gelled primed seeds as explant. Bulg J Agric Sci. 2013;19:222–7.
Zhu H, Li C, Gao C. Applications of CRISPR–Cas in agriculture and plant biotechnology. Nat Rev Mol Cell Biol. 2020;21:661–77.
Idris SH, Mat Jalaluddin NS, Chang LW. Ethical and legal implications of gene editing in plant breeding: a systematic literature review. J Zhejiang Univ Sci B. 2023;24:1093–105.
Ayanoğlu FB, Elçin AE, Elçin YM. Bioethical issues in genome editing by CRISPR-Cas9 technology. Turk J Biol. 2020;44:110–20.
Rodriguez E. Ethical issues in genome editing using Crispr/Cas9 system. J Clin Res Bioethics. 2016;7:266.
Esvelt KM, Smidler AL, Catteruccia F, Church GM. Concerning RNA-guided gene drives for the alteration of wild populations. Elife. 2014;3: e03401.
Macnaghten P, Habets MGJL. Breaking the impasse: towards a forward-looking governance framework for gene editing with plants. Plants People Planet. 2020;2:353–65.
Clapp J, Ruder SL. Precision technologies for agriculture: digital farming, gene-edited crops, and the politics of sustainability. Glob Environ Polit. 2020;20:49–69.
Shinwari ZK, Tanveer F, Khalil AT. Ethical issues regarding CRISPR-mediated genome editing. Curr Issues Mol Biol. 2017;26:103–10.
Nielsen S, Murnion B, Dunlop A, Degenhardt L, Demirkol A, Muhleisen P, et al. Comparing treatment-seeking codeine users and strong opioid users: Findings from a novel case series. Drug Alcohol Rev. 2015;34:304–11.
Ebrahimi SA. Noscapine, a possible drug candidate for attenuation of cytokine release associated with SARS-CoV-2. Drug Dev Res. 2020;81:765–7.
Majnooni MB, Fakhri S, Bahrami G, Naseri M, Farzaei MH, Echeverría J. Alkaloids as potential phytochemicals against SARS-CoV-2: Approaches to the associated pivotal mechanisms. Evid Based Complement Alternat Med. 2021;2021:6632623.
Cheng TJ, Goodsell DS, Kan CC. Identification of sanguinarine as a novel HIV protease inhibitor from high-throughput screening of 2,000 drugs and natural products with a cell based assay. Lett Drug Des Discov. 2005;2:364–71.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
ZA conceptualization; ZA writing and preparation of the initial draft; ZA visualization, MRN supervision; MRN writing review and editing. All authors read and approve the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Aghaali, Z., Naghavi, M.R. Developing benzylisoquinoline alkaloid-enriched opium poppy via CRISPR-directed genome editing: A review. BMC Plant Biol 24, 700 (2024). https://doi.org/10.1186/s12870-024-05412-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-05412-x