Abstract
Drosera intermedia grows in acidic bogs in parts of valleys that are flooded in winter, and that often dry out in summer. It is also described as the sundew of the most heavily hydrated habitats in peatlands, and it is often found in water and even underwater. This sundew is the only one that can tolerate long periods of submersion, and more importantly produces a typical submerged form that can live in such conditions for many years. Submerged habitats are occupied by D. intermedia relatively frequently. The aim of the study was to determine the environmental conditions and architecture of individuals in the submerged form of D. intermedia. The features of the morphological and anatomical structure and chlorophyll a fluorescence of this form that were measured were compared with analogous ones in individuals that occurred in emerged and peatland habitats. The submerged form occurred to a depth of 20 cm. Compared to the other forms, its habitat had the highest pH (4.71–4.92; Me = 4.71), the highest temperature and substrate hydration, and above all, the lowest photosynthetically active radiation (PAR; 20.4–59.4%). This form differed from the other forms in almost all of the features of the plant’s architecture. It is particularly noteworthy that it had the largest main axis height among all of the forms, which exceeded 18 cm. The number of living leaves in a rosette was notable (18.1 ± 8.1), while the number of dead leaves was very low (6.9 ± 3.8). The most significant differences were in the shape of its submerged leaves, in which the length of the leaf blade was the lowest of all of the forms (0.493 ± 0.15 mm; p < 0.001) and usually the widest. The stem cross-sectional area was noticeably smaller in the submerged form than in the other forms, the xylem was less developed and collaterally closed vascular bundles occurred. Our analysis of the parameters of chlorophyll fluorescence in vivo revealed that the maximum quantum yield of the primary photochemistry of photosystem II is the highest for the submerged form (Me = 0.681), the same as the maximum quantum yield of the electron transport (Me φE0 = 0.183). The efficiency of energy use per one active reaction center of photosystem II (RC) was the lowest in the submerged form (Me = 2.978), same as the fraction of energy trapped by one active RC (Me = 1.976) and the non-photochemical energy dissipation (DI0/RC; Me = 0.916). The ET0/RC parameter, associated with the efficiency of the energy utilization for electron transport by one RC, in the submerged plant reached the highest value (Me = 0.489). The submerged form of D. intermedia clearly differed from the emerged and peatland forms in its plant architecture. The submerged plants had a thinner leaf blade and less developed xylem than the other forms, however, their stems were much longer. The relatively high photosynthetic efficiency of the submerged forms suggests that most of the trapped energy is utilized to drive photosynthesis with a minimum energy loss, which may be a mechanism to compensate for the relatively small size of the leaf blade.
Similar content being viewed by others
Background
Drosera intermedia Hayne (oblong-leaved sundew) is a sundew whose name comes from the Latin intermedius and refers to the intermediate plant and leaf size between the small D. rotundifolia and the large D. anglica. It has a wide distribution range throughout Europe and is also present in North America and the northern part of South America [1,2,3], and East Africa [4]. In acidic peatlands and valley bogs, it is a plant that is primarily found in characteristic valleys and depressions where the water level is extremely high [5]. Usually, this sundew is associated with open and very wet parts of peatlands, although it is also often found on the mineral shores of lakes [6].
In Poland, D. intermedia is in the endangered (EN) category and has been under protection by law since 1946 [7, 8]. Like other sundews, D. intermedia is receding from natural habitats primarily due to their land reclamation, but also because of their uncontrolled harvesting for medicinal and ornamental purposes [5, 9, 10].
Species of the genus Drosera are a rich source of bioactive substances [11,12,13] that can be used for medicinal purposes [14,15,16,17,18]. Studies have shown that D. intermedia extracts are more effective than the commercial products that are created from them [19], which unfortunately poses a major threat to sundews in the wild. One opportunity to improve their situation is the ever-improving in vitro culture methods for Drosera [20,21,22] including D. intermedia [22,23,24]. Relative to other species, D. intermedia tolerates longer periods of drought, and also total submersion (Fig. 1) although this ultimately results in a loss of its ability to capture insects.
The purpose of this work was to compare the environmental conditions and architecture of individuals of this unique submerged form of oblong-leaved sundew with those that are found in other habitats within peatlands. Submerged habitats are occupied by D. intermedia relatively frequently, and their populations are quite numerous in some places (Fig. 2). The prerequisite for the occurrence of this form is the presence of dystrophic lakes and/or smaller ponds in peatland with a characteristic mossy blanket overlying the surface of the water. Natural dystrophic lakes are specific habitats protected under the European Natura 2000 network (habitat number 3160). These water bodies are very acidic and poor in plant nutrients. Their water has a high humic acid content and is usually stained dark brown through exposure to peat. The unique morphometric conditions of these dystrophic lakes as well as the physicochemical conditions of their waters [25,26,27] force the plants to adapt to very specific habitats, which are characterized by an extremely low level of nutrients, low light intensity, limited oxygen and carbon dioxide availability, etc. Therefore, in addition to determining the morphological structure of the sundews, cross sections of the stems and leaves were taken to compare the structure of the plants in different habitats, and the photosynthetic efficiency of plants was analyzed in order to determine the condition of the plants.
Results
Environmental conditions in D. intermedia habitats
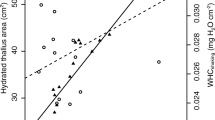
The submerged form of D. intermedia occurred in unique habitats within the studied peatbogs. The submerged habitats statistically differed in the studied traits from other habitats (emerged and peatland). First of all, they were extremely poor in dissolved mineral substances (conductivity was 22.89 ± 5.61µS cm− 1), which is similar to habitats of the emerged form (29.61 ± 11.76 µS cm− 1) and were distinguished by nearly a three-fold lower conductivity than habitats in peatlands (p < 0.001; Table A. 1). Moreover, they were characterized by the highest pH (p < 0.001; 4.71–4.92; Me = 4.71; Table 1) as well as the highest temperature and substrate hydration (p < 0.001), but the lowest PAR (p < 0.001; Fig. 3) by far. Depending on the depth of the immersion of the plants, the PAR ranged from 20.4 to 59.4% (37.9 ± 16.5%) of what reached the water surface.
Individual architectures of sundews
The submerged form of D. intermedia clearly differed in architecture from its emerged and peatland forms in almost all of the plant characteristics (Fig. 4; Table 2), except for the length of the roots and inflorescences and the number of flowers per inflorescence (Fig. 5). The size of its rosette was 5.04 ± 1.68 cm (Me = 4.63 cm), which was significantly smaller than the aquatic form (p < 0.001) but similar to the peatland form (p = 0.87; see Additional file– Table A.1). It is worth noting that the highest main axis height among all of its forms (p < 0.001) was more than 18 cm. In this form, inflorescences only occurred occasionally (p < 0.001), and were often from the previous growing season.
The number of living leaves was appreciable, and averaged 18.1 ± 8.1 per rosette, which was similar to the other forms (Fig. 6). The average leaf length was intermediate (3.15 ± 0.77) but lower than that found in the water form (3.98 ± 0.84; p < 0.001) and higher than that of the peatland form (2.71 ± 0.55; p = 0.035). The very low number of dead leaves (6.9 ± 3.8) as well as the appearance of the leaves themselves, which were clearly different than in the other forms was interesting (Figs. 7 and 8). Moreover, the length of the leaf blade was the lowest of all forms (0.493 ± 0.15 mm; p < 0.001) while the width of leaf blade was only lower than the that of the aquatic form (0.236 ± 0.048 mm; p < 0.001).
Differences in the individual architectures of the rosettes and inflorescences of D. intermedia from the submerged – S, emerged – E and peatland – P habitats. Different letters (a, b, c) represent significant differences at a p < 0.05 probability level according to the RIR Tukey’s post hoc test using the Compact Letter Display (CLD) methodology
Differences in the individual leave architectures of the submerged form of D. intermedia (S) and the other forms (E – emerged; P – peatland). Different letters (a, b, c) represent significant differences at a p < 0.05 probability level according to the RIR Tukey’s post hoc test using the Compact Letter Display (CLD) methodology
Stem and leave anatomy
In all of the plant forms, the stem consisted of a large cortex and a central cylinder (stele) with vascular bundles (Fig. 9A-F). In all of the examined plants, the cross sections revealed a primary structure (Fig. 9A-F). The epidermal cells had thin cell walls except for the external cell walls. In the submerged form, the stems were covered with filamentous algae. The stem cross-sectional area was noticeably smaller in the submerged form than in the other forms (Table 3); it is worth noting that the emerged form stood out as having the largest stem area of 1.461 ± 0.069 mm2. The cylinder cross-sectional area like the stem area was the smallest in the submerged form at 0.157 ± 0.017 mm2 and hardly varied.
Free hand sections and cross sections of the submerged (A,D,G and J), emerged (B, E, H, and K) and peatland (C, F, I, and L) forms of D. intermedia. Freehand sections of the stems with a visible epidermis (ep), parenchyma (p) and stele (st). (A-F). Details of the stems and steles (st) surrounded by the endodermis (en) and parenchyma cells (p). Transverse sections of the petioles (G-I) with the major vascular bundle (vb) in the center. Transverse sections of the leaves (J-L) with the adaxial (ad) and abaxial (ab) architecture. The petioles and leaves were visualized using calcofluor and propidium iodide
The parenchyma cells of the cylinders had thin cell walls. There were small intercellulars. In the peatland form, there were many sclerenchyma cells in the internal part of the cylinder (Fig. 9F), which had thin, but lignified cell walls. In the submerged and emerged forms, there were no or only a few sclerenchyma cells in the internal part of the cylinder. The cortex and cylinder were separated by an endodermis. The endodermis cells had Casparian strips. In the emerged and peatland forms, a concentric-leptocentric type of vascular bundles occurred. In the submerged form, the xylem was less developed and collaterally closed vascular bundles occurred (but a few vessels also occurred on the outer part of the phloem).
There were no differences in the structure of the leaf anatomy (Fig. 9G-L), and the leaf blade thickness had no statistically significant differences between the studied forms, although it was the lowest in the submerged form. However, there were highly significant differences in the petiole cross-sectional area (Table 3). The submerged form was characterized by intermediate values, which were significantly higher than in the emerged form (p = 0.003), but lower than in the peatland form (p = 0.002).
Photosynthetic performance
An analysis of the parameters of chlorophyll a fluorescence in vivo revealed that the maximum quantum yield of the primary photochemistry of photosystem II (φP0) differed significantly (p = 0.0317; Fig. 10, see Additional file– Table A.1) among all three forms of D. intermedia, and reached the highest median value for the submerged form (0.681) and the lowest one for the peatland form (0.605; Table 4; Fig. 10). The same tendency (not statistically significant, p = 0.144) was observed in the maximum quantum yield of the electron transport (φE0), which reached a median value of 0.183 in the submerged form as well as for the efficiency of the trapped exciton moving the electron further than the QA- (Ψ0) (p = 0.0818; mean value 0.260 for the submerged form).
The parameters that describe the efficiency of energy utilization per analyzed cross-section (CS) of the leaf indicated that the submerged form absorbed more light energy (median ABS/CS reached 1705.0) than the emerged and peatland forms (p = 0.0222; Fig. 10; Table 4). The amount of trapped energy that was absorbed by the leaf (TR0/CS) was also higher in the submerged form (median 1150.0) compared to the two other forms, although the effect was not statistically significant (p = 0.0787). A similar tendency was observed for the median value of the ET0/CS parameter, which describes the fraction of the energy that was utilized for electron transport by the leaves of the submerged form and reached 312.0 and was the highest among the all of the D. intermedia forms (p = 0.227). The analyzed plant forms differed significantly (p = 0.0067) in the efficiency of energy dissipation in a non-photochemical way (DI0/CS), wherein the peatland form had a much lower median value (516.0) than the submerged and emerged forms (533.0-534.0).
In the next step, the parameters that are associated with the efficiency of energy utilization per one active reaction center of photosystem II (RC) were analyzed. It was found that the ABS/RC parameters differed significantly (p = 0.0028) among the three D. intermedia forms with the lowest median value being found in the submerged form (2.978; Fig. 10; Table 4). The fraction of energy that was trapped by one active RC (TR0/RC) was also lowest in the submerged form (1.976) and was significantly different (p = 0.0045) from the parameter values that were found in the emerged and peatland forms (2.141–2.152). An opposite tendency was observed for the median value of the ET0/RC parameter, which is associated with the efficiency of the energy utilization for electron transport by one RC. In the submerged form, it reached the highest value (0.489), however, the differences between the plant forms were not significant (p = 0.324). The non-photochemical energy dissipation (DI0/RC) was lowest (0.916) in the submerged form and was significantly different (0.0072) from the emerged and peatland forms (Fig. 10; Table 4).
Differences in the photosynthetic parameters values of the submerged form of D. intermedia (S), and the other habitats (E – emerged; P – peatland). Different letters (a, b, c) represent significant differences at p < 0.05 probability level according to the RIR Tukey’s post hoc test using the Compact Letter Display (CLD) methodology. All of the values are given in arbitrary fluorescence units. The meanings of the abbreviations are given in the text
Discussion
Drosera intermedia and D. rotundifolia generally occupy similar habitats, and they often occur together, e.g., on the mineral shores of lakes. However, D. rotundifolia has a much wider ecological range than other Drosera species and often occurs in fertile bogs such as swamp forests and swamp birches as well as in anthropogenically transformed (mainly drained) peatlands where D. intermedia and D. anglica no longer usually occur. On the other hand, D. intermedia is able to survive in extreme habitats, including very wet and flooded areas [5], thereby forming dense populations that float freely on the surface of the water. What is most interesting is that this sundew can live completely submersed under water. However, it should be noted that this type of submerged habitat is characteristic only for specific lake-peatland complexes, which include natural dystrophic water bodies (habitat number 3160 in the Natura 2000 network). The numerous ponds and lakes that are found here are distinguished by their specific hydrochemical conditions [26], and most importantly, by the unique structure of the banks, which are occupied by peat sloughs that overlap the water surface [27]. It is the part of the submerged environment that is primarily built by the Carex limosa root system and bryophytes of the genus Sphagnum, which hosts the submerged form of D. intermedia. The sundew mainly occurs here in communities of submerged bryophytes such as Sphagnum denticulatum (Fig. 11), S. cuspidatum, or Warnstorfia exannulata.
The environmental conditions in which the submerged form of D. intermedia lives stand out due to their extreme characteristics among the habitats that are occupied by this species. They were characterized by the highest pH and temperature, but extremely low conductivity, and despite significant differences from the emerged and peatland habitats, these values were within the range of variation of these characteristics. On the other hand, an essential peculiarity of the submerged habitats was the very low PAR, which varied from only 20 to 60% of the PAR that fell on the surface of the bog (see Table 1). For the other habitats where D. intermedia occurred, the PAR was higher than 80% (cf. Fig 1). Similar conditions of a very high PAR are found in the habitats where other Drosera species live [6].
Habitat flooding creates stress in plants known as either submergence stress. In nature, these stresses are important factors dictating the species composition of the ecosystem. On agricultural land, they cause economic damage associated with long-term social consequences. This contributes to breed superior cultivars to guarantee a superior yield in the face of a flooding event [28]. Flooding disrupt the movement of oxygen from the air to plant tissues [29], producing hypoxia in plants [29, 30], limits the flow of light to the plant [31], and increases the vulnerability to pathogen attack [32]. Plants respond to flooding by changes in gene expression [29, 33, 34]. These changes coordinate morphological and metabolic adaptations to the stress [33, 35]. The genes recognized as key regulators for flooding and low-oxygen tolerance were found, among others, in Arabidopsis [36, 37] and also in rice [38, 39], specifically in deepwater rice [40]. The specific conditions of submerged habitat caused the architecture of the submerged form of D. intermedia to differ from those that were found in the other habitats (cf. Figs 3 and 4) primarily by the absence of (or short) inflorescences. Further, the submerged form had a relatively low number of dead leaves, which was similar to aquatic form (live: dead ratio was about 2.5), while in the peatland form, a relatively high number of dead lives was observed (live: dead ratio was about 1.4) (cf. Table 2). It can be assumed that in aquatic and submerged environments the low number of dead leaves results from a faster decay or may be associated with the balance between the costs and benefits of leaf construction and maintenance [41] as well as the need for rapid growth in the competition for light with both other plants and the fact that they are growing up to the water surface where light conditions are much better. This last assumption is supported by the fact that stems from the fact that most of the submerged individuals were significantly elongated, which is characteristic for terrestrial plants that grow in low-light conditions or that are partially/completely submerged [42,43,44]. Control of shoot elongation (i.e., the elongation of leaves, petioles, and inter nodes) and adventitious root formation are plant adaptations to avoid O2 insufficiency under flooding stress [45, 46]. The enhanced internode elongation under flooded conditions are also indicated in Rumex and Rorippa [47]. Ethylene is the first signal to initiate these responses in plants, whereas other hormones like auxin, gibberellin, and abscisic acid and their interactions are also involved in the flooding response [48].
The adaptation of the submerged form to low light was also visible in the photosynthetic parameters. The maximum quantum yield of the primary photochemistry of photosystem II and the maximum quantum yield of electron transport reached the highest values in the submerged form and were the lowest in the peatland form (see Fig. 10; Table 4). The photosynthetic parameters, which were recalculated per analyzed cross-section of a leaf, indicated that the submerged form could absorb, trap, and utilize the light energy more efficiently than the aquatic and peatland forms could. Our study further showed that the submerged form was distinguished by the lowest efficiency of energy utilization and trapping per one active reaction center of photosystem II. However, it had the highest efficiency of the energy utilization for electron transport by one RC, which suggests that most of the trapped energy is utilized to drive photosynthesis with a minimum energy loss, which has also been suggested for submerged Rumex palustris, R. crispus, and Phalaris arundinacea leaves [43, 49]. Indeed, the non-photochemical energy dissipation parameters were relatively low in the submerged form (see Fig. 10; Table 4). The photosynthesis efficiency of Hygrophila difformis under submersion stress was investigated by Horiguchi et al. [50], who have shown that this plant acclimated to the submerged condition by increasing its photosynthetic rate. The above results suggest that submerged form of D. intermedia cope with submergence by means of strategy based partially on the improvement of photosynthesis underwater, as it was shown for many Rumex, Ranunculus, Oryza and Scirpus species (for review see Striker [51] and others [48, 52]).
The efficient utilization of light energy could be considered to be a mechanism to compensate for the relatively small size of the leaf blade – the trap (cf. Fig 4), which may in part explain why our results differ from the literature data [53], which has reported that the terrestrial forms of aquatic plants have smaller leaves than the individuals living in the water. It should be also considered that in many aquatic plants the reduced leaf blades of the terrestrial forms is an effective adaptive mechanism for reducing water loss through transpiration [54], which may not be entirely applicable to sundews as they occur in highly hydric habitats. In the case of submerged sundews, the production of smaller leaves could be a result of low environmental fertility and the necessity to diversify resources between growth and the stress defense [41, 55, 56].
Due to the specificity of the occupied habitat, the submerged form can also be distinguished by its anatomical structure. The submerged plants have a thinner leaf blade than the other forms, which is a common trend that is observed for plants that develop both floating and submerged leaves. For example, it was demonstrated that the floating leaves of Potamogeton natans, P. polygonifolius, P. gramineus, P. zizii, and P. perfoliatus were much thicker than the submerged ones [57]. The same trend was described for submerged and terrestrial leaves of Rumex palustris [43, 58] and Ranunculus flabellaris [59]. One of the reasons for the above-described changes might be a slow gas diffusion in the water, which leads to an oxygen/carbon dioxide deficiency inside the leaf that, in turn, forces changes that lead to an improved gas exchange [43, 60, 61]. Because the need for long-distance transport of water and inorganic substances in submerged plants is extremely limited, the xylem in the submerged forms was less developed than in the other forms, and there were also collaterally closed vascular bundles. In the emerged and peatland forms, there was a concentric-leptocentric type of vascular bundles. The cross-sections of the stem and leaves indicated many similarities between the submerged form and the emerged form and significant differences from the peatland form. The morphological changes and metabolic changes such as aerenchyma formation, adventitious root formation and shoot/internodes elongation are involved in plants to survive under flooded condition [62,63,64,65]. Also, aquatic leaves compared to those formed aerially can become narrower and more elongated e.g. Potamogeton nodosus. This can be combined to a low concentration of exogenous abscisic acid (ABA) [64].
Conclusions
-
Our work shows that among carnivorous plants, not only can species of the genera Utricularia and Aldrovanda, but Drosera can also be a completely aquatic plant and can survive in these conditions for a long period of time (several vegetation seasons).
-
The submerged form of D. intermedia clearly differed from emerged and peatland forms in its plant architecture. The submerged plants had a thinner leaf blade and less developed xylem than the other forms, however, their stems were much longer. These are common adaptations found in submerged plants, indicating the high plasticity of D. intermedia and the persistence of its populations even in habitats with highly disturbed water levels.
-
The relatively high photosynthetic efficiency of the submerged forms suggests that most of the trapped energy is utilized to drive photosynthesis with a minimum energy loss, which may be a mechanism to compensate for the relatively small size of the leaf blade. This is of great importance in the ecological success of this Drosera species in highly hydrated habitats, and may contribute to its persistence in flooded areas due to climate change.
-
In conclusions, all of the features of the submerged form of D. intermedia in the analyzed material indicate that the main environmental factor that determines them is the low photosynthetically active radiation and the limited rate of gas exchange in submerged habitats. The observed adaptations of the submerged form allow us to assume that D. inetrmedia will be relatively resistant to climatic changes, especially rapid weather changes, as it occurs both on drained and mineral substrates, but can also occur in completely flooded organic habitats.
Methods
Collection of the samples and methods for analysing the environmental conditions
The material for the study was collected from three well-preserved high and intermediate peatlands, including one Nature Reserve in August 2022 (Fig. 12; Table 5). For the study, individuals of D. intermedia were collected from the three types of habitats/environments that were found at the study sites, which differ in ecological conditions:
-
1.
submerged (where the sundew stem and leaves were completely under water);
-
2.
emerged (sundews floated freely on the surface of the water or grew on Sphagnum mosses that were submerged in the water; the sundew leaves were elevated above the water, while the stem was slightly submerged or was just above the surface of the water), and.
-
3.
peatland (sundews grew directly on the peat or on a carpet of Sphagnum mosses; the sundew stem and leaves were completely above the surface of the water).
The research methodology was in line with what was presented by Banaś et al. [6]. At each site, the measurement areas were designated in which the environmental conditions were determined, and the architecture of the intermediate sundew individuals was measured. For this purpose, plants were collected and the fluorescence of chlorophyll a in vivo was immediately measured using fluorometer for each individual. Then, all of the individuals from the site were carefully spread out on a special scaled backing and photographed for the subsequent measurements of their architectural features using a graphics program. After the photos were taken, the plants were planted in the same location from which they had been taken. Using CorelDRAW X6 from the photos, the plant architecture was measured, including primarily the rosette width, main axis height, length of roots (was usually the length of stem with adventitious roots), number of inflorescences and length of inflorescence, number of flowers, number of live and dead leaves, average length of a leaf, and the width and length of the leaf blade. The results were obtained for 151 individuals of Drosera intermedia using this procedure. The study was conducted after obtaining the relevant permits of the Regional Directorate for Environmental Protection in Gdansk. We adopted the species designation and nomenclature from Lowrie et al. 2017 [66]. Typical specimens of each form were preserved as herbarium material in the Herbarium Universitatis Gedanensis UGDA (herbarium number sheets: UGDA.0279037; UGDA.0279038, UGDA.0279039, UGDA.0279051, UGDA.0279052, UGDA.0279058 and UGDA.0279059), where the species identification was confirmed by Dr. Ryszard Markowski.
Using field meters, the environmental characteristics such as (1) PAR, (2) substrate temperature, (3) substrate pH, and (4) substrate conductivity were measured at all of the sites. A small sample of the substrate (about 250 ml) was collected from each site for laboratory analysis to determine its hydration and organic matter content.
The characterization of the environmental conditions at each measuring site was based on a determination of the photosynthetically active radiation (PAR), which was measured with a LI-250 Light Meter for an average of five measurements of the sundews, which was then converted for the plant site as % of light that reached the object/plant site under full illumination or that reached the water surface in the case of the submerged plants – measurements were taken at the depth of the sundews (usually 10–20 cm below the surface). The pH and temperature of the substrate were measured using a WTW 320/SET1 pH meter with a SENT IX 97T measuring electrode, while conductivity was measured using a WTW Cond 3210 SET 2 conductivity meter. Substrate hydration was calculated as a percentage from the difference in the weight between the fresh substrate and the substrate that had been dried to a constant weight at 105 °C in a Binder FD115 laboratory dryer, and the organic matter content of the substrate was calculated as a percentage of the difference in weight between the dry substrate and the substrate that had been roasted in a SEL 96 C type muffle furnace at 550 °C for five hours.
Chlorophyll a fluorescence in vivo was measured using a Handy Pea fluorometer (Hansatech Ltd., King’s Lynn, UK). Measurements were taken on the second or third youngest leaf of each plant. Before a measurement, the leaves were adapted to the darkness for 15 min. using dark-adaptation leaf-clips with a closed shutter-plate. Immediately prior to a measurement the shutter-plate was opened, and a saturated light pulse (10 s, 3.000 mmol photons m− 2 s− 1) was applied. The following parameters were calculated based on the fluorescence signal: the maximum yield of the primary photochemistry (φP0 = FV/FM); the maximum yield of electron transport (or the probability that the absorbed photon would move the electron into the electron transport chain) (φE0); the efficiency of the trapped exciton moving the electron further than the QA- (Ψ0); the specific energy fluxes per active reaction center (RC) for the energy absorption (ABS/RC), energy trapping (TR0/RC), electron transport driving (ET0/RC), and energy dissipation (DI0/RC); the specific energy fluxes per excited cross section of a leaf (CS) for energy absorption (ABS/CS), energy trapping (TR0/CS), electron transport driving (ET0/CS), and energy dissipation (DI0/CS) [67,68,69].
The FV/FM ratio—recognized as a reliable measure of the photochemical activity of the photosynthetic apparatus under optimal conditions of plant growth should be approximately 0.85 relative units [70]. A decrease in the value of this parameter indicates the occurrence of stress, which can lead to the phenomenon of photoinhibition. Very low values of this parameter (about 0.2–0.3) indicate irreversible changes in the structure of PSII. However, FV/FM was found to be highly insensitive to effects occurring under moderate drought stress in many plants grown in field conditions and was almost completely insensitive to nitrogen treatment [70]. Chlorophyll fluorescence is widely used as a plant response indicator under many abiotic constraints, but it should be remembered that it can also indicate the contribution of biotic factors such as pathogen infection and parasitism effect [71,72,73].
Statistical methods that were used to develop the results
The results of both the environmental conditions and plant measurements that were obtained were summarized in a Microsoft Excel spreadsheet, where each individual was assigned the characterized environmental conditions at the site and physiological parameters, respectively. Using Statistica 13.1, the arithmetic mean and median, standard deviation, minimum and maximum value of all of the plant traits and environmental conditions were calculated. Graphs were also created to compare the habitats and traits of the different forms of sundew; the differences were determined according to ANOVA and the Tukey’s RIR post-hoc test for unequal abundances. Moreover, ANOVA and the Tukey’s RIR post-hoc test for equal abundances were used to analyze cross sections of the stems and leaves.
Microscopy methods
Material was collected from each form of Drosera to make the stem and leaf cross sections. This material was fixed and preserved in 70% ethanol. Later, the stems were hand sliced using a razor blade. The sections were treated with alum carmine and iodine green and mounted in glycerol. The sections were examined using a Nikon Eclipse E400 light microscope with UV-2 A filter. The ImageJ 1.54f (Wayne Rasband and contributors, National Institutes of Health, USA) was used to distinguish the areas that were measured on the cross sections of stems and leaves that had been taken from three different but typical specimens of each form of D. intermedia.
Additionally, the leaves and petioles were fixed overnight in 8% (w/v) paraformaldehyde (PFA) with 0.25% (v/v) glutaraldehyde (GA) in a PIPES buffer at 4 °C, embedded in Steedman’s wax, and sectioned [74]. The chromatin of the nuclei was visualized with 1 µg/mL propidium iodide (Sigma Aldrich, St. Louis, MO, USA), and the cell walls were stained with 0.1% Calcofluor White M2R (Sigma Aldrich, St. Louis, MO, USA) [75]. The specimens were visualized using a Leica STELLARIS 5 WLL confocal microscope with the Lightning deconvolution module. The presented images are maximum projections from the Z-stacks that were taken to improve the spatial resolution.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Rivadavia F, Kondo K, Kato M, Hasebe M. Phylogeny of the sundews, Drosera (Droseraceae) based on chloroplast rbcL and nuclear 18S ribosomal DNA sequences. Am J Bot. 2003;90:123–30.
Fleischmann A, Gonella PM. Typification and authorship of Drosera intermedia (Droseraceae). Taxon. 2020;69:153160. https://doi.org/10.1002/tax.12158.
Gonella PM. October. Droseraceae in Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. Available online: https://floradobrasil2020.jbrj.gov.br/FB7425 (accessed on 21 2023).
Gonella PM, Caram SV, Dutra VF. Flora of Espírito Santo, Brazil Flora of Espírito Santo: Droseraceae. Rodriguésia. 2022;73:e00312021. https://doi.org/10.1590/2175-7860202273016.
Crowder AA, Pearson MC, Grubb PJ, Langlois PH. Biological flora of the British Isles. J Ecol. 1990;78:233–67.
Banaś K, Ronowski R, Marciniak P. Effects of Environmental conditions on the Individual Architectures and Photosynthetic performances of three species in Drosera. Int J Mol Sci. 2023;24:9823. https://doi.org/10.3390/ijms24129823.
Dz.U. z 1946 r. nr 70, poz. 384 – Rozporządzenie Ministra Oświaty z dnia 29 sierpnia 1946 r. wydane w porozumieniu z Ministrem Rolnictwa i Reform Rolnych i z Ministrem Leśnictwa w sprawie wprowadzenia gatunkowej ochrony roślin (in Polish).
Dz.U. z 2014 r. poz. 1409 – Rozporządzenie Ministra Środowiska z dnia 9 października 2014 r. w sprawie ochrony gatunkowej roślin (in Polish).
Kawiak A, Królicka A, Łojkowska E. Direct regeneration of Drosera from leaf explants and shoot tips. Plant Cell Tiss Org. 2003;75:175–8. https://doi.org/10.1023/A:1025023800304.
Kawiak A, Łojkowska E. In vitro cultures of Drosera aliciae as a source of a cytotoxic naphthoquinone: ramentaceone. Biotechnol Lett. 2011;33:2309–16.
Milella L, Caruso M, Galgano F, Favati F, Padula MC, Martelli G. Role of the cultivar for choosing Clementine fruits with high level of health-promoting compounds. J Agric Food Chem. 2011;59:5293–8.
Padula MC, Lepore L, Milella L, Ovesna J, Malafronte N, Martelli G, De Tommasi N. Cultivar based selection and genetic analysis of strawberry fruits with high levels of health promoting compounds. Food Chem. 2013;140:639–46.
Wójciak M, Feldo M, Stolarczyk P, Płachno BJ. Carnivorous Plants from Nepenthaceae and Droseraceae as a source of secondary metabolites. Molecules. 2023;28:2155. https://doi.org/10.3390/molecules28052155.
Paper DH, Karall E, Kremser M, Krenn L. Comparison of the antiinflammatory effects of Drosera rotundifolia and Drosera madagascariensis in the HET-CAM assay. Phytother Res. 2005;19(4):323–6. https://doi.org/10.1002/ptr.1666.
Fukushima K, Nagai K, Hoshia Y, Masumoto S, Mikami I, Takahashib Y, Oikeb H, Kobori M. Drosera rotundifolia and Drosera tokaiensis suppress the activation of HMC-1 human mast cells. J Ethnopharmacol. 2009;125:90–6.
Babula P, Adam V, Havel L, Kizek R. Noteworthy secondary metabolites naphthoquinones - their occurrence, pharmacological properties and analysis. Curr Pharm Anal. 2009;5(1):47–68. https://doi.org/10.2174/157341209787314936.
MacKinnon A. Edible and Medicinal Plants of Canada. Edmonton: Lone Pine Publishing; 2009. p. 448.
Egan PA, van der Kooy F. Phytochemistry of the carnivorous sundew genus Drosera (Droseraceae) - future perspectives and ethnopharmacological relevance. Chem Biodivers. 2013;10(10):1774–90. https://doi.org/10.1002/cbdv.201200359.
Gerschler S, Guenther S, Schulze C. Antibiofilm Activity of Sundew Species against Multidrug-Resistant Escherichia coli strains. Int J Mol Sci. 2022;23:13720. https://doi.org/10.3390/ijms232213720.
Jimenez VM, Guevara E, Masis E. Effect of macronutrients and sucrose concentration on in vitro grow of Drosera capensis L. (Droseraceae) plants, and evaluation of six substrates for acclimatization. Propag Ornam Plants. 2011;5:47–68.
Swart PA, Kulkarni MG, Bairu MW, Finnie JF, Van Staden J. Micropropagation of Romulea Sabulosa Schltr. Ex Beg. - a potential ornamental plant. Sci Hort. 2012;135:151–6.
Rejthar J, Viehmannova I, Cepkova PH, Fernández E, Milella L. In vitro propagation of Drosera intermedia as influenced by cytokinins, pH, sucrose, and nutrient concentration. Emir J Food Agric. 2014;26(6):558–64. https://doi.org/10.9755/ejfa.v26i6.18022.
Grevenstuk T, Coelho N, Gonçalves S, Romano A. In vitro propagation of Drosera intermedia in a single step. Biol Plant. 2010;54:391–4.
Laslo V, Vicaş S, Agud E, Zăpârţan M. Methods of conservation of the plant germplasm. In vitro techniques, Analele Universităţii din Oradea, Fascicula Protecţia Mediului. 2011;17:697–708.
Banaś K. Morphology of peatland lakes. Limnol Rev. 2010;10:3–14.
Banaś K. The hydrochemistry of peatland lakes as a result of the morphological characteristics of their basins. Oceanol Hydrobiol St. 2013;42:28–39.
Banaś K, Gos K, Szmeja J. Factors controlling vegetation structure in peatland lakes. Aquat Bot. 2012;96:42–7.
Mickelbart MV, Hasegawa PM, Bailey-Serres J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat Rev Genet. 2015;16:237–51. https://doi.org/10.1038/nrg3901.
Lee SC, Mustroph A, Sasidharan R, Vashisht D, Pedersen O, Oosumi T, et al. Molecular characterization of the submergence response of the Arabidopsis thaliana Ecotype Columbia. New Phytol. 2011;190:457–71. https://doi.org/10.1111/j.1469-8137.2010.03590.x.
Sasidharan R, Bailey-Serres J, Ashikari M, Atwell BJ, Colmer TD, Fagerstedt K, et al. Community recommendations on terminology and procedures used in flooding and low oxygen stress research. New Phytol. 2017;214:1403–7. https://doi.org/10.1111/nph.14519.
Jackson MB, Ram PC. Physiological and molecular basis of susceptibility and tolerance of rice plants to complete submergence. Ann Bot. 2003;91:227–41.
Hsu FC, Chou MY, Peng HP, Chou SJ, Shih MC. Insights into hypoxic systemic responses based on analyses of transcriptional regulation in Arabidopsis. PLoS ONE, 2011;6e28888.
Mustroph A, Lee SC, Oosumi T, Zanetti ME, Yang H, Ma K, Yaghoubi-Masihi A, Fukao T, Bailey-Serres J. Cross-kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol. 2010;152:1484–500.
Juntawong P, Girke T, Bazin J, Bailey-Serres J. Translational dynamics revealed by genome-wide profiling of ribosome footprints in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 111, 2014; E203–E212. https://doi.org/10.1073/pnas.1317811111.
Bailey-Serres J, Voesenek LACJ. Flooding stress: Acclimations and genetic diversity. Annu Rev Plant Biol. 2008;59:313–39.
Bui LT, Giuntoli B, Kosmacz M, Parlanti S, Licausi F. Constitutively expressed ERF-VII transcription factors redundantly activate the core anaerobic response in Arabidopsis thaliana. Plant Sci. 2015;236:37–43. https://doi.org/10.1016/j.plantsci.2015.03.008.
Gasch P, Fundinger M, Müller JT, Lee T, Bailey-Serres J, Mustroph A. Redundant ERF-VII transcription factors bind an evolutionarily conserved cis-motif to regulate hypoxia-responsive gene expression in Arabidopsis. Plant Cell. 2016;28:160–80.
Fukao T, Xu KN, Ronald PC, Bailey-Serres J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell. 2006;18:2021–34.
Xu KN, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ. Sub1A is anethylene-response-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–8.
Hattori Y, Nagai K, Furukawa S, Song XJ, Kawano R, Sakakibara H, Wu JZ, Matsumoto T, Yoshimura A, Kitano H, et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature. 2009;460:1026–30.
Kikuzawa K. A cost-benefit analysis of leaf habit and leaf longevity of trees and their geographical pattern. Amer Nat. 1991;138:1250–63.
Ballaré CL, Scopel AL, Sánchez RA. Photocontrol of stem elongation in plant neighbourhoods: effects of photon fluence rate under natural conditions of radiation. Plant Cell Environ. 1991;14(1):57–65.
Mommer L, Pons TL, Wolters-Arts M, Venema JH, Visser EJW. Submergence-induced morphological, anatomical, and biochemical responses in a terrestrial species affect gas diffusion resistance and photosynthetic performance. Plant Physiol. 2005;139:497–508.
Mommer L, Lenssen JPM, Huber H, Visser EW, Kroon HD. Ecophysiological determinants of plant performance under flooding: a comparative study of seven plant families. J Ecol. 2006;94:1117–29.
Voesenek LACJ, Bailey-Serres J. Flood adaptive traits and processes: an overview. New Phytol. 2015;206:57–73.
Watanabe K, Takahashi H, Sato S, Nishiuchi S, Omori F, Malik AI, Colmer TD, Mano Y, Nakazono M. A major locus involved in the formation of the radial oxygen loss barrier in adventitious roots of teosinte Zea nicaraguensis is located on the short-arm of chromosome 3. Plant Cell Environ. 2017;40:304–16.
van Veen H, Akman M, Jamar DC, Vreugdenhil D, Kooiker M, van Tienderen P, Voesenek LACJ, Schranz ME, Sasidharan R. Group VII ethylene response factor diversification and regulation in four species from flood-prone environments. Plant Cell Environ. 2014;37:2421–32.
Qi X, Hu Z, Chen X, Zhang M, Nakazono M. Involvement of Phytohormones in flooding stress tolerance in plants. In: Ahammed GJ, Yu J, editors. Plant hormones and Climate Change. Singapore: Springer; 2023. https://doi.org/10.1007/978-981-19-4941-8_11.
Vervuren PJA, Beurskens SMJH, Blom CWPM. Light acclimation, CO2 response and long-term capacity of underwater photosynthesis in three terrestrial plant species. Plant Cell Environ. 1999;22(8):959–68.
Horiguchi G, Nemoto K, Yokoyama T, Hirotsu N. Photosynthetic acclimation of terrestrial and submerged leaves in the amphibious plant Hygrophila Difformis. AoB Plants. 2019;11(2):plz009.
Striker GG. Flooding stress on plants: anatomical, morphological and physiological responses. Botany. 2012;1(1):3–28.
Nakamura M, Noguchi K. Tolerant mechanisms to O2 deficiency under submergence conditions in plants. J Plant Res. 2020;133:343–71.
Li Z, Yu D, Xu J. Adaptation to water level variation: responses of a floating-leaved macrophyte Nymphoides peltata to terrestrial habitats. Ann Limnol – Int J Lim. 2011;47:97–102.
Oikawa S, Hikosaka K, Hirose T. Leaf lifespan and lifetime carbon balance of individual leaves in a stand of an annual herb, Xanthium canadense. New Phytol. 2006;172:104–16.
Chapin FSIII, Autumn K, Pugnaire F. Evolution of suites of traits in response to environmental stress. Amer Nat. 1993;142:S78–92.
Moriuchi KS, Winn AA. Relationships among growth, development and plastic response to environment quality in a perennial plant. New Phytol. 2005;166:149–58.
Frost-Christensen H, Sand-Jensen K. Comparative kinetics of photosynthesis in floating and submerged Potamogeton leaves. Aquat Bot. 1995;51(1–2):121–34.
Voesenek LACJ, Benschop JJ, Bou J, Cox MCH, Groeneveld HW, Millenaar FF, Vreeburg RAM, Peeters AJM. Interactions between plant hormones regulate submergence-induced shoot elongation in the flooding tolerant dicot Rumex palustris. Ann Bot. 2003;91:205–11.
Bruni NC, Young JP, Dengler NC. Leaf developmental plasticity of Ranunculus flabellaris in response to terrestrial and submerged environments. Can J Bot. 1996;74:823–37.
Sand-Jensen K, Frost-Christensen H. Plant growth and photosynthesis in the transition zone between land and stream. Aquat Bot. 1999;63:23–35.
Mommer L, Pedersen O, Visser EJW. Acclimation of a terrestrial plant to submergence facilitates gas exchange under water. Plant Cell Environ. 2004;27:1281–7.
Jia W, Ma M, Chen J, Wu S. Plant Morphological, physiological and anatomical adaption to flooding stress and the underlying Molecular mechanisms. Int J Mol Sci. 2021;22:1088. https://doi.org/10.3390/ijms22031088.
Aslam A, Mahmood A, Ur-Rehman H, Li C, Liang X, Shao J, Negm S, Moustafa M, Aamer M, Hassan MU. Plant adaptation to flooding stress under changing Climate conditions: Ongoing breakthroughs and Future challenges. Plants (Basel). 2023;12(22):3824. https://doi.org/10.3390/plants12223824.
van Veen H, Sasidharan R. Shape shifting by amphibious plants in dynamic hydrological niches. New Phytol. 2021;229(1):79–84. https://doi.org/10.1111/nph.16347.
Fukao T, Barrera-Figueroa BE, Juntawong P, Peña-Castro JM. Submergence and waterlogging stress in plants: a review highlighting Research opportunities and Understudied aspects. Front Plant Sci. 2019;10:340. https://doi.org/10.3389/fpls.2019.00340.
Lowrie A, Robinson AS, Nunn R, Rice B, Bourke G, Gibson R, McPherson S, Fleischmann A. In: Robinson AS, editor. Drosera of the World, volume 2 - Oceania, Asia, Europe, North America. Poole, Dorset, England: Redfern Natural History Productions; 2017.
Strasser RJ, Tsimilli-Michael M, Srivastava A. Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou G, Govindjee, editors. Chlorophyll a fluorescence: a signature of photosynthesis. Dordrecht: Springer; 2004. pp. 321–62.
Aksmann A, Tukaj Z. Intact anthracene inhibits photosynthesis in algal cells: a fluorescence induction study on Chlamydomonas reinhardtii cw92 strain. Chemosphere. 2008;74(1):26–32.
Aksmann A, Pokora W, Baścik-Remisiewicz A, Dettlaff-Pokora A, Tukaj Z. High hydrogen peroxide production and antioxidative enzymes expression in the Chlamydomonas reinhardtii cia3 mutant with an increased tolerance to cadmium and anthracene. Phycol Res. 2016;64:300–11. https://doi.org/10.1111/pre.12147.
Kalaji MH, Goltsev VN, Żuk-Gołaszewska K, Zivcak M, Brestic M. Chlorophyll fluorescence: understanding crop performance — basics and applications. 1st ed. CRC Press, Taylor & Francis Group; 2017. p. 222.
Shen H, Hong L, Chen H, Ye WH, Cao HL, Wang ZM. The response of the invasive weed Mikania micrantha to infection density of the obligate parasite Cuscuta campestris and its implications for biological control of M. Micrantha. Bot Stud. 2011;52:89–97.
Walters DR. Photosynthesis in attacked plants and crops. In: Walters DR, editor. Physiological responses of plants to attack. Crop & Soil Systems Research Group SRUC Edinburgh. UK: Wiley-Blackwell; 2015. p. 248.
Ennami M, Mansi MJ, Briache FZ, Oussible N, Gaboun F, Ghaouti L, Belqadi L, Ghanem ME, Aberkani K, Westwood J, Mentag R. Growth-defense tradeoffs and source-sink relation explain the responses of susceptible and resistant faba bean and lentil genotypes to infection by Orobanche crenata. J Crop Prot. 2020;127. https://doi.org/10.1016/j.cropro.2019.104924.
Płachno BJ, Kapusta M, Stolarczyk P, Bogucka-Kocka A. Spatiotemporal distribution of homogalacturonans and hemicelluloses in the Placentas, Ovules and female gametophytes of Utricularia nelumbifolia during pollination. Cells. 2022;11:475. https://doi.org/10.3390/cells11030475.
Błażejewska K, Kapusta M, Zielińska E, Tukaj Z, Chincinska IA. Mature Luffa leaves (Luffa cylindrica L.) as a Tool for Gene expression analysis by Agroinfiltration. Front Plant Sci. 2017;8:228. https://doi.org/10.3389/fpls.2017.00228.
Funding
This research was, in part, supported by the Ministry of Science and Higher Education of Poland within the statutory activities of the Department of Plant Ecology, Faculty of Biology, University of Gdansk [531-D040-D245-23] and the Department of Plant Cytology and Embryology, Institute of Botany, Faculty of Biology, Jagiellonian University in Kraków [N18/DBS/000002].
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception: K.B., R.R., P.M.; design of the work: K.B., R.R., P.M.; fieldwork: K.B., R.R., P.M., B.J.P.; laboratory work: K.B., B.J.P., M.K., R.R., P.M.; data analysis: K.B., A.A.; interpretation of the data: K.B., A.A., B.J.P., R.R. All of the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The sundew collection and environmental studies were conducted in accordance with the permit of the Regional Directorate for Environmental Protection in Gdansk: RDOŚ-Gd-WZG.6400.98.2022.SK.2, while the studies in the Lisia Kępa Nature Reserve were conducted in accordance with the permit RDOŚ-Gd-WOC.6205.36.2022.JK.2. Typical specimens of each form were preserved as herbarium material in the Herbarium Universitatis Gedanensis UGDA (herbarium number sheets: UGDA.0279037; UGDA.0279038, UGDA.0279039, UGDA.0279051, UGDA.0279052, UGDA.0279058 and UGDA.0279059), where the species identification was confirmed by Dr. Ryszard Markowski.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Banaś, K., Aksmann, A., Płachno, B.J. et al. Individual architecture and photosynthetic performance of the submerged form of Drosera intermedia Hayne. BMC Plant Biol 24, 449 (2024). https://doi.org/10.1186/s12870-024-05155-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-05155-9