Abstract
Background
Several WRKY transcription factors (TFs), including CaWRKY6, CaWRKY22, CaWRKY27, and CaWRKY40 are known to govern the resistance of pepper (Capsicum annuum L.) plants to Ralstonia solanacearum infestation (RSI) and other abiotic stresses. However, the molecular mechanisms underlying these processes remain elusive.
Methods
This study functionally described CaWRKY3 for its role in pepper immunity against RSI. The roles of phytohormones in mediating the expression levels of CaWRKY3 were investigated by subjecting pepper plants to 1 mM salicylic acid (SA), 100 µM methyl jasmonate (MeJA), and 100 µM ethylene (ETH) at 4-leaf stage. A virus-induced gene silencing (VIGS) approach based on the Tobacco Rattle Virus (TRV) was used to silence CaWRKY3 in pepper, and transiently over-expressed to infer its role against RSI.
Results
Phytohormones and RSI increased CaWRKY3 transcription. The transcriptions of defense-associated marker genes, including CaNPR1, CaPR1, CaDEF1, and CaHIR1 were decreased in VIGS experiment, which made pepper less resistant to RSI. Significant hypersensitive (HR)-like cell death, H2O2 buildup, and transcriptional up-regulation of immunological marker genes were noticed in pepper when CaWRKY3 was transiently overexpressed. Transcriptional activity of CaWRKY3 was increased with overexpression of CaWRKY6, CaWRKY22, CaWRKY27, and CaWRKY40, and vice versa. In contrast, Pseudomonas syringae pv tomato DC3000 (Pst DC3000) was easily repelled by the innate immune system of transgenic Arabidopsis thaliana that overexpressed CaWRKY3. The transcriptions of defense-related marker genes like AtPR1, AtPR2, and AtNPR1 were increased in CaWRKY3-overexpressing transgenic A. thaliana plants.
Conclusion
It is concluded that CaWRKY3 favorably regulates phytohormone-mediated synergistic signaling, which controls cell death in plant and immunity of pepper plant against bacterial infections.
Similar content being viewed by others
Background
Plants (being immobile) are vulnerable to several abiotic stresses, disease and insect infestations, and harsh environments, including high temperatures, water scarcity, and excessive salinity [1, 2]. Plants overcome these challenges by using coordinated responses, either through initiation of transcriptional reprogramming or regulation of diverse transcription factors (TFs). Nevertheless, the underlying mechanism responsible for this synchronization remains unspecified.
Plants use dual immunity to protect themselves against disease infestations [3, 4]. Pathogen associated molecular pattern (PAMP)-triggered immunity (PTI) (often referred as basal defense) is initiated by the identification of PAMPs on the cell surface by trans-membrane receptors (PRRs) (pattern recognition receptors). Effector-triggered immunity (ETI) is the second layer, and activated in response to pathogen-made effectors that are released into the host cell and work to dampen PTI by destroying different PTI-signaling components [5]. The ETI is severe, protracted, and coupled with localized cell death at infection site and systemic acquired resistance in most instances [6]. Despite these differences, PTI and ETI have several comparable signaling processes such as Ca+ 2 signaling, oxidative burst, activation of mitogen-activated protein kinase (MAPKs), and transcriptional control of defense-related genes through several TFs [6, 7]. A few of these elements have been connected to the way plants react to various stressful conditions. This raises the possibility that these elements have a regulatory function in regulating how plants react to diverse stress stimuli. Despite this, little is known about the regulators and underlying processes involved in the coordination of plant responses to various stressors.
One of the major families of plant TFs is thought to be made up of WRKY proteins. In addition to unique zinc-finger like motifs, members of WRKY family always include one or two WRKY domains, each of which is 60 amino acids in length and identified by the conserved amino acid sequence WRKYGQK at its N-terminus [8, 9]. There are three distinct classes of WRKY TFs based on the presence or absence of certain WRKY domains and the structure of zinc finger motifs [9]. The binding site for WRKY TFs is the W-box (TTGACC/T) found in the promoter region of the respective target genes. The WKRY TFs are essential for plant growth, development, and responsiveness to biotic and abiotic stresses in numerous plant species [10, 11]. Several essential plant functions are either inhibited or repressed by genes belonging to the WRKY family. Previous evidence suggests that several WRKY TFs may coordinately control a single biological process [12, 13]. On the other hand, it is possible that a single WRKY TF regulates many biological activities in plants, even if they seem to be at odds with one another [14].
The WRKY gene family plays a crucial role in the regulation of many abiotic stresses including drought, waterlogging, heat, cold, and salinity [15]. Plant stress responses are controlled by WRKY TFs, which regulate their own expression and interact with other TFs [16]. The WRKY3 transcription factor is essential for mediating plant defense responses against pathogens. The large proportion of WRKY genes respond to pathogens, elicitors, and defense-related phytohormones like salicylic acid (SA) or jasmonic acid (JA), which indicates the important role of WRKY gene family in plant immunity [17]. The WRKY3 acts as a positive regulator to defend the plant against necrotrophic fungal infections in Arabidopsis [18]. However, integration of WRKY TFs with other TFs in networks that control a wide range of plant biological processes is not well known.
Plant hormones (or phytohormones) naturally occur in plants and play a vital role in several functions, including immunological response, defensive signaling, and growth. Historically, SA, JA, and ethylene (ETH) have been associated with defense responses to pathogen infestation [19]. Nevertheless, plant immunity is not regulated by a single hormone; rather, an intricate network of antagonistic and synergistic interactions involving many plant hormones is involved in the regulation of plant immunity [19]. Pathogens employ numerous strategies to enhance infection by exploiting phytohormone pathways, which demonstrate the importance of phytohormones in plant-pathogen interactions. Pathogens may alter physiological processes like stomatal opening and aging by influencing channels that carry phytohormone signals. In this way, the pathogens infiltrate the plants easily and induce disease symptoms by reducing or blocking the synthesis of phytohormones in their hosts [20, 21]. Pathogen may manipulate phytohormone pathways by directly altering hormone levels, inhibiting their synthesis, or generating hormone mimics [22]. Pathogens may significantly influence the interactions between plants and other pathogens by modifying phytohormone pathways [23]. This alteration has consequences for other processes, including the equilibrium between pathogenesis and mutualism, the manifestation of disease signs, and colonization [23]. The modulation of WRKY transcription factors via phytohormone signaling pathways underscores the intricate regulatory processes involved in plants’ responses to diverse environmental stimuli, such as pathogen infections and stress [12, 24].
Pepper (Capsicum annuum L.) is a member of Solanaceae and widely used for culinary and medicinal purposes [25, 26]. The Solanaceae members are very susceptible to bacterial infections, which causes severe production losses. Ralstonia solanacearum causes a disastrous disease in pepper known as bacterial wilt. It can adapt to environmental changes and evade the host immune responses by penetrating the root system and multiplying in the xylem tissue [27,28,29]. Pepper growth and development are significantly impacted by high temperatures and high humidity (HTHH). Although low levels of HTHH may not directly harm pepper plants, they may worsen bacterial wilt disease by fostering the rapid development of R. solanacearum and impairing plant immunity [30, 31]. The evolution in pepper may have been impacted by the strong relationship between HTHH and R. solanacearum infection. Several earlier studies reported that CaWRKY6, CaWRKY22, CaWRKY27 and CaWRKY40 positively control pepper’s response to combined R. solanacearum inoculation (RSI) and HTHH, revealing a tight link between RSI and HTHH [13, 30, 32]. The underlying processes, nevertheless, have not yet been fully analyzed.
This study was aimed at describing CaWRKY3 (an additional member of the group I WRKY TF family). For this purpose, induction of CaWRKY3 was observed under R. solanacearum infestation and foliar application of SA, MeJA, and ETH. Furthermore, the impact of CaWRKY3 silencing with virus-induced gene silencing was on pepper immunity was tested. It was hypothesized that foliar application of phytohormones and R. solanacearum infestation would increase the transcription of CaWRKY3. It was further hypothesized that silencing CaWRKY3 would result in immunity loss of pepper against R. solanacearum infestation. The results would help to understand the role of CaWRKY3 in pepper immunity against R. solanacearum and underlying molecular mechanisms/regulations.
Results
Cloning and sequencing analysis of CaWRKY3
Genomic study (http://passport.pepper.snu.ac.kr) identified a member of the WRKY family (LOC107860501) that has not been studied before in pepper. The CaWRKY3 was selected for functional characterization and its role in pepper immunity against bacterial pathogen infection, and presence of subset of immunity related cis elements, including TGA element, GARE-motif, CGTCA-motif, TATA-box, and W-box in promoter region of CaWRKY3 indicate its potential role in Capsicum immunity (Fig. 1A).
We used gene-specific primers to clone a 1500-base-pair (bp) cDNA fragment encoding the whole open reading frame (ORF) of CaWRKY3 (Table S1). It was classified as belonging to group I based on its deduced amino acid sequence, which consisted of a total of 500 amino acid residues and had one conserved WRKY domain (Fig. 1A). The size of the anticipated protein is 55 kDa and a projected pI is 7.56. CaWRKY3 shares 97%, 93%, 82% and 66% amino acid similarity with StWRKY3, SsWRKY3, SaWRKY3 and NtWRKY3 (Fig. 1B).
Structural and promoter analysis of CaWRKY3. Occurrence of defense related cis-elements in promoter region of CaWRKY3 “A” in the translational start codon ATG is considered as position + 1 (A). Multiple alignment of CaWRKY3 deduced amino acid sequence with proteins from Solanum tuberosum StWRKY3 (NM_001318664.1), Solanum acranum SaWRKY3 (KU674829.1), Nicotiana benthamiana NbWRKY15 (AB711136.1), Tamarix hispida ThWRKY8 (JX416197.1) and Diospyros kaki DkWRKY13 (MK737969.1) (B) Green shade = 50–75% similarity; red shade = 75–100% similarity, and black shade = 100% similarity. Analysis A and B were assayed by using DNAMAN5
Transcriptional expression levels of CaWRKY3 after R. solanacearum inoculation and treatment with different phytohormones
Since promoter region of CaWRKY3 contains several immune-related cis elements, it may have a role in developing immunity against RSI. Compared to mock-treated leaves, leaves exposed to R. solanacearum had higher transcriptional expression levels of CaWRKY3 (Fig. 2A). These elevated transcriptional expression levels of CaWRKY3 persisted between 0 and 48 h after treatment (hpt). This highest level of expressions was noted at 48 hpt (Fig. 2A).
The response of plants to biotic and abiotic stressors is heavily influenced by signaling pathways controlled by plant hormones such as SA, MeJA, and ETH. The role of phytohormones in the regulation of CaWRKY3 was investigated by spraying SA, MeJA, and ETH on pepper plants. The data was subsequently put through a quantitative RT-PCR analysis. The qRT-PCR research revealed that the relative transcriptional expression levels of CaWRKY3 rose from 0 to 48 h following foliar spraying with 1 mM SA in contrast to mock-treated plants. Most transcriptional expressions were still detectable 24 h after treatment (Fig. 2B).
According to qRT-PCR, CaWRKY3 had higher relative transcriptional expression levels from 0 to 48 h in pepper plants sprayed with 100 µM MeJA in contrast to mock-treated plants. At 48 hpt, top levels of transcriptional expressions were seen (Fig. 2C).
Transcriptional expression levels of CaWRKY3 in pepper increased from 0 to 48 h following the foliar application of 100 µM ETH, compared to mock-treated plants. There was a peak in this relative transcriptional expression at 24 h after treatment (Fig. 2D).
Relative transcriptional levels of CaWRKY3 in pepper leaves treated with R. solanacearum infections and phytohormones quantified by qRT-PCR analysis. Expression levels of CaWRKY3 in pepper leaves treated with R. solanacearum (A); foliar spray of 1 mM SA (B), foliar spray of 100 μm MeJA (C), and foliar spray of 100 μm ETH (D). The abundance of RNA synthesis in RSI-treated leaves in comparison to MgCl2-control plants (mock) was noted to evaluate the effects of RSI on pepper transcription, where the relative expression level was set to 1. Each treatment consisted of different mock plants. The error bars indicate standard errors of means. Different letters above the bars indicate statistically significant differences between the means of three biological replicates determined by the Fisher protected LSD test
CaWRKY3 silencing by VIGS damaged resistance of peppers to the bacterial pathogen R. solanacearum and decreased transcriptional abundance of marker genes linked to plant defence
The role of CaWRKY3 in plant immunity against R. solanacearum was studied by silencing of CaWRKY3 by VIGS. A total 50 CaWRKY3-silenced (TRV:CaWRKY3) and 50 CaWRKY3-unsilenced (TRV:00) plants were used in the VIGS experiment. Six CaWRKY3-silenced pepper plants were randomly chosen to assess the efficiency of gene silencing by root infiltration with virulent strain of R. solanacearum. Results exhibited that transcriptional abundance of CaWRKY3 was decreased by ∼ 30% in R. solanacearum infected CaWRKY3-silenced plants in comparison to CaWRKY3-unsilnced plants exhibiting effective silencing of CaWRKY3 by VIGS (Fig. 3A and B).
The CaWRKY3-silenced plants expressed notably higher vulnerability to Ralstonia infection, whereas intensity of 3,3′-Diaminobenzidine (DAB) and trypan blue was clearly visible in CaWRKY3-silenced pepper plants leaves (Fig. 3C). Electrical conductivity (EC) is marker of ion leakage, and it was measured to check damage of cell membrane and plant cell death after RSI. Results showed that R. solanacearum infested CaWRKY3-unsilenced plants showed significantly high ion leakage as compared to R. solanacearum infested CaWRKY3-silenced plants at 48 hpi (Fig. 3D).
Relative disease index assay was carried out to check the intensity of disease levels up to 10 dpi of R. solanacearum in CaWRKY3-silenced and CaWRKY3-unsilenced plants (Table S2). Very strong disease signs were identified in CaWRKY3-silenced pepper plants as compared to CaWRKY3-unsilnced pepper plants at 10 dpi (Fig. 3E). To check phenotype, 4 CaWRKY3-silenced and 4 CaWRKY3-unsilenced plants were chosen at random and then treated with R. solanacearum in plant roots. Prominent wilting disease symptoms were observed in CaWRKY3-silenced pepper plants, whereas very visible disease signs were observed in CaWRKY3-unsilnced pepper plants at 10 dpi (Fig. 3F).
Results demonstrated that R. solanacearum infected CaWRKY3-unsilenced plants showed significantly high ion leakage as compared to R. solanacearum challenged CaWRKY3-silenced pepper plants at 48 hpi (Fig. 3D). Relative disease index assay was carried out to check the intensity of disease levels up to 10 dpi of R. solanacearum in CaWRKY3-silenced and CaWRKY3-unsilenced pepper plants (Table S2). Very strong disease signs were identified in CaWRKY3-silenced pepper plants as compared to CaWRKY3-unsilnced pepper plants at 10 dpi (Fig. 3E). To check phenotype, 4 plants of CaWRKY3-silenced and 4 plants of CaWRKY3-unsilenced were randomly selected and then treated with R. solanacearum in plant roots. Prominent wilting disease symptoms were observed in CaWRKY3-silenced pepper plants whereas, very feeble disease signs were identified in CaWRKY3-unsilnced pepper at 10 dpi (Fig. 3F).
In a quantitative real-time polymerase chain reaction (qRT-PCR) experiment, we looked at the relative expression of many defense-related genes. The transcriptional abundance of multiple defense-related marker genes was reduced in CaWRKY3-silenced plants in contrast to CaWRKY3-unsilenced plants (Fig. 3G).
CaWRKY3-silencing reduced resistance of pepper to Ralstonia solanacearum and down-regulated the immunity-related marker genes. The qRT-PCR analysis of CaWRKY3 transcripts accumulation in unsilenced (TRV:00) and CaWRKY3-silenced plants (TRV:CaWRKY3) and CaWRKY3-un-silenced plants (TRV:00) (A), R. solanacearum growth in silenced and un-silenced pepper plants at 0 and 3 days after infection (B), DAB and Trypan blue staining in R. solanacearum-infected CaWRKY3-silenced and CaWRKY3-un-silenced leaves (full leaves are provided in Figure S2) (C), electrolyte leakage in CaWRKY3-silenced and CaWRKY3-un-silenced plants (D), disease index of silenced and unsilenced plants (E), phenotypic response of silenced and unsilenced plants (F), qRT-PCR expression of defense-associated marker genes (G)
Transient over-expression of CaWRKY3 instigated cell death resembling HR, synthesis of H2O2, and transcriptional expression of defense-related marker genes is increased
The CaWRKY3 has a favorable regulatory role in pepper immunity to R. solanacearum inoculation, as indicated by VIGS experiemnt investigating the loss of function of the gene. In order to provide more evidence for this hypothesis, transient over-expression tests of CaWRKY3 were conducted by injecting healthy pepper leaves with either 35 S:00 (EV) or 35 S:CaWRKY3-containing GV3101 Agrobacterium cells. These injections were done to test whether CaWRKY3 could be overexpressed temporarily. The impact of CaWRKY3 transient over-expression on HR-like cell death, H2O2 generation, and transcriptional control of defense-related marker genes was investigated. The results of the Western blot demonstrated that CaWRKY3 was successfully expressed (Fig. 4A). The experiments with trypan blue and DAB staining verified the formation of H2O2 and the presence of HR-like cell death in the plant leaves. Following the temporary overexpression of CaWRKY3, DAB staining in dark brown and extensive trypan blue were seen in plant leaves. These stains served as indications of HR-like cell death and H2O2 production (Fig. 4B and C). Electrical conductivity experiment was carried out to check the intensity of plasma membrane damage induced by transient over-expression of CaWRKY3. Our data exhibited that electrolyte leakage was significantly high in pepper leaves transiently over-expressing CaWRKY3 as compared to pepper leaves transiently over-expressing empty vector at 24 and 48 h of Agro-infiltration (Fig. 4D and E).
The CaWRKY3-transiently over-expressing peppers plants showed HR-like cell death, accumulation of reactive oxygen species and up-regulation of defense-related marker genes. CaWRKY3 over-expression confirmation by western blot (A), HR-like cell detected by phenotype UV light exposure (B), HR-like cell detected by DAB and Trypan Blue staining (C), ion conductivity to estimate the cell death response in leaf discs (D), and qRT–PCR analysis of the transcriptional expression levels of immunity-associated marker genes (E)
Relationship between CaWRY3, CaWRKY6, CaWRKY22, CaWRKY27, and CaWRKY40
Previous studies have suggested that CaWRKY40 was expressionally and functionally associated to various WRKY TFs, including CaWRKY6 and CaWRKY22. We also found previously that various WRKY TFs were expressionally and functionally interconnected. This notion indicates that CaWRKY3 might also be related to various WRKY TFs responsible for pepper immunity to RSI (Fig. 5A and B).
Relationship between CaWRKY3, CaWRKY6, CaWRKY22, CaWRKY27, and CaWRKY40. Transcriptional expression of CaWRKY3 in pepper leaves transiently over expressing CaWRKY6, CaWRKY27 and CaWRKY40 (A), and transcriptional expression of CaWRKY6, CaWRKY27 and CaWRKY40 in pepper leaves transiently over-expressing CaWRKY3 (B)
CaWRKY3-OX (Over-expression) increased the resistance of transgenic Arabidopsis plants to bacterial infection and up-regulated the levels of immunity-related marker genes transcription
Production of CaWRKY3 transgenic plants in Arabidopsis and over-expressing the complete precursor under the control of the 35 S promoter allowed researchers to learn more about the role of CaWRKY3 in plant defense in vivo. This was made feasible by the extreme difficulty of maintaining metamorphosis in pepper plants. The goal was to learn CaWRKY3 functions in real-life plant defense. The genomic DNA was isolated and analyzed from CaWRKY3-OX transgenic Arabidopsis plants and wild plants using PCR. This was done in a variety of homozygous T3 Arabidopsis lines to verify that CaWRKY3-OX transgenic expression was successful (Col-0). Only two CaWRKY3-OX plants had their CaWRKY3 gene amplified by PCR; however, none of the wild-type Arabidopsis plants amplified the gene (Fig. 6A). In typical growing circumstances, CaWRKY3-OX Arabidopsis plants showed no observable phenotypic differences from wild-type plants. Needle-free syringe injections of P. syringe pv tomato DC3000 were used to test the wild type and CaWRKY3-OX strains of Arabidopsis for resistance to the bacterial pathogen. Standard chlorotic signals were found on infected leaves of wild type plants three days after inoculation, but not on infected leaves of CaWRKY3-OX plants (Fig. 6B).
CaWRKY3 over-expression in transgenic Arabidopsis plants enhanced the resistance against Pseudomonas syringae pv tomato (Pst) DC3000 inoculation. CaWRKY3 gene amplification by qRT-PCR (A), wild type and CaWRKY3-OX strains of Arabidopsis (B), disease index in wild type and CaWRKY3-OX strains of Arabidopsis (C), ion conductivity to estimate the cell death response in leaf discs (D), and transcriptional expression of SA-dependent PR genes involved in immunity (E)
To determine whether disease symptoms were caused by bacterial multiplication in plants, researchers looked at the growth of the bacterial pathogen in plant leaves at 0 and 3 days after the first infection. In comparison to CaWRKY3-OX plants, wild type plants produced bacterial pathogens more rapidly (as measured by higher cfu levels at time intervals of 0 and 3 days). These findings were apprehended via a comparison of the two plant types (Fig. 6C). Increased plant resistance to bacterial infection may be attributable to higher CaWRKY3 expression levels, as shown by our findings. To monitor plasma membrane damage and cell mortality due to the HR reaction, an electrolyte leakage experiment was performed. At 0, 12, and 24 h after infection with P. syringe pv tomato Pst DC3000, compared to wild type plants, the CaWRKY3-OX leaves showed higher amounts of electrolyte ion leakage (Fig. 6D).
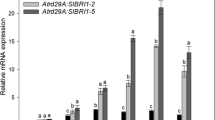
We compared the CaWRKY3-OX transgenic Arabidopsis plants to the wild type plants in terms of the transcriptional expression of SA-dependent PR genes involved in immunity using qRT-PCR. The CaWRKY3-OX plants showed greater transcriptional abundance of immunity-related marker genes compared to wild type plants 48 h after being treated with P. syringe pv tomato Pst DC3000. This was true irrespective of the genotypes of the plants (Fig. 6E). The enhanced disease resistance of CaWRKY3-OX against bacterial pathogen infection may be due to SA-dependent immunological mechanism, as shown by the qRT-PCR experiment.
Discussion
The WRKY TF family is abundant in plant cells. The members of this family have been demonstrated to play important roles in the control of plant immunity in Arabidopsis and rice. Previous research has shown that WRKY proteins found in a wide range of plant species have high degrees of structural similarity but vastly different functional properties [33, 34]. However, the function of WRKY TFs in immunological responses of non-model plants, such as pepper, has received less attention. The CaWRKY3 (a member of WRKY TF family group I) was the focus of our investigation as we defined its functional properties. According to our findings, CaWRKY3 functions as a positive regulator of pepper immunity to RSI together with CaWRKY6, CaWRKY22, CaWRKY27, and CaWRKY40, which are also members of the WRKY transcriptional web.
The promoter region of CaWRKY3 contains immunity-sensitive cis-elements such as the CGTCA-motif, p-box, TATA box, and W-box, lending credence to the hypothesis that it plays a role in regulating pepper immunity. Further supporting the role of CaWRKY3 in pepper immunity are the results of qRT-PCR reaction, which demonstrated that CaWRKY3 transcriptional levels were up-regulated with R. solanacerum infection. Genes are known to have a part in the body’s response to stress if their expression level rises in such circumstance [27]. We hypothesized that CaWRKY3 would regulate pepper immunity in a manner that would make the plant more resistant to R. solanacearum. Results of the VIGS experiment for CaWRKY3 silencing, transient over-expression assay for gain-of-function analysis, and persistent transgenic overexpression of CaWRKY3 in Arabidopsis all supported our hypothesis.
The loss of function of CaWRKY3 by VIGS assay increased the vulnerability of pepper plants to RSI. This decreased immunity was accompanied by increase in growth of inoculated bacterial pathogen R. solanacearum and down regulation of HR-related CaHIR1 [35] pathogenesis-related CaPR1 [36] and SA-related CaNPR1 [37] and JA-related DEF1 [38]. On the other hand, pepper plants transiently over-expressing CaWRKY3 showed cell death resembling HR, H2O2 synthesis, and up-regulation of immunity related marker genes, including CaHIR1, CaNPR1, CaPR1, and CaDEF1. These results of CaWRKY3-silencing and gain-of-function experiments significantly suggest that CaWRKY3 functions as a positive regulator of pepper’s immunity and HR-like cell death against bacterial pathogen infection.
Furthermore, transgenic Arabidopsis CaWRKY3-OX plants exhibited higher resistance compared to plants of the wild type. This data suggested that CaWRKY3 positively regulate pepper plant immunity and play a critical part in the defense of plants against bacterial pathogen. Previous studies exhibited that WRKY3 is a positive regulator of plant defense against F. oxysporum in Lilium regale and transcriptional levels of JA-biosynthesis, SA-signal transduction-related marker genes were up-regulated in LrWRKY3 transgenic tobacco lines [33, 34]. It can be suggested that CaWRKY3 is up-regulated upon inoculation of R. solanacearum.
The CaWRKY3 was constantly detected to be triggered by foliar spraying of phytohormones including SA, JA, and ETH. The SA, JA and ETH-dependent defense related marker genes such as CaPR1 [36], CaNPR1 [37], CaDEF1 [38] and CaHIR1 [35] were down-regulated upon silencing of CaWRKY3, while these all defense associated marker genes were up-regulated in CaWRKY3-transiently over-expressing pepper plants showing that CaWRKY3 is involved in SA, JeA and ETH synergistically mediated defense signaling, hence leads to PTI.
Genome-wide studies showed the involvement of many WRKY TFs in plant immunity [12, 39]. By functional genomics studies WRKY11 [40], WRKY17 [41], WRKY18 [42], WRKY22 [13], WRKY25 [43], WRKY28 [44], WRKY33 [45], WRKY38 [46], WRKY45 [47], WRKY46 [48], WRKY53 [49], WRKY62 [50], WRKY70 [51], and WRKY75 [52] have been identified as having functional characteristics in Arabidopsis’ immunity, these were either positive regulator or negative regulator of plant immunity. These WRKY TFs have been recommended to assimilate into a transcriptional network consisted of + ve and –ve feedback loops and feed forward modules [16].
However, the formulation of these WRKY TFs networks in various plants species is poorly understood. Our previous experiments data exhibited that CaWRKY6, CaWRKY22, CaWRKY27, CaWRKY30 and CaWRKY40 are positive regulator of pepper’s immunity against RSI [11, 32, 53,54,55], CaWRKY58 was found to be negative regulator in pepper’s resistance to RSI.
Conclusions
The CaWRKY3 was functionally characterized in the current study. The findings indicated that CaWRKY3 functions as a positive regulator of pepper immunity to RSI together with CaWRKY6, CaWRKY22, CaWRKY27, and CaWRKY40, which are also members of the WRKY transcriptional web. An increase in CaWRKY3 transcription was seen after temporary over-expression of previously identified CaWRKY6, CaWRKY22, CaWRKY27, and CaWRKY40, and the same was observed after transient over-expression of CaWRKY3, showing the existence of WRKY TF networks and positive feedback.
Methods
Plant material and growth circumstances
We procured pepper (‘Mexi’) and tobacco (Nicotiana tabacum L.) seeds from the Ayub Agriculture Research Institute (AARI), Faisalabad, Pakistan. Soil composed of peat, moss, and perlite [2/1(v/v)] was used to plant the seeds in plastic containers.
Vectors construction
The gateway cloning method was used for vector creation. To generate vectors for VIGS, we chose a 288 bp fragment from the 3’-untranslated region (UTR) of CaWRKY3 and validated its specificity by BLASTing it against the genome sequences of CM334 (http://peppergenome.snu.ac.kr/).
Pathogens and inoculation procedures
Plants infected with R. solanacearum in South Punjab produced a strain of the bacterium that is very pathogenic (Pakistan). Tetrazolium chloride was used to purify the exudates from the stem and stem vascular tissue of these diseased plants. Sucrose, peptone, and agar (SPA) medium was used to cultivate a highly infectious R. solancearum strain overnight at 200 rpm and 28 °C in a temperature-control shaker (200 g potatoes, 20 g sucrose, 3 g beef extract, 5 g tryptone, and 1 L of double-distilled ddH2O). R. solanacearum culture was centrifuged for 10 min at 6500 rpm and 28 °C. After discarding the supernatant, the pellet was dissolved in sterile, distilled 10 mM MgCl2. When using bacteria, a concentration of 0.8 log (108 cfu mL-1) was used (at an optical density of 600 nm, or OD600). To investigate how RSI affects CaWRKY3 transcriptions levels and pepper plants’ resistance to RSI, a needleless syringe was used to inject 10 ml of R. solanacearum into the upper third of the leaves of pepper plants. At certain periods, leaves from treated plants were collected for DAB and trypan blue staining and RNA extraction. CaWRKY3 knocked-down pepper plants were subjected to root injury (through glass road cuts) and R. solanacearum infiltration to evaluate phenotypic changes after RSI. The plants infected with R. solanacearum were grown in a greenhouse at a temperature of 28 degrees Celsius, with a light intensity of 60 to 70 micromoles per square meter per second, a humidity level of 70%, and a photoperiod of 16 h of light and 8 h of darkness. The potentially lethal pathogen Pseudomonas syringae pv. Tomato DC3000 (Pst Dc3000) was also used. Overnight, the bacterial pathogen was cultivated at 200 rpm and 28 °C in SPA medium supplemented with the necessary antibiotics for treating Arabidopsis plants. This pathogen culture solution was centrifuged for 10 min at 28 °C and 200 rpm. The pellet was centrifuged and then suspended at a concentration of 105 cfu mL-1 in distilled water with 10 mM MgCl2. A needleless syringe was used to inject Pst Dc3000 into the leaves of an Arabidopsis plant that was five to six weeks old. After 18 h in the humid chamber, the pathogen-treated Arabidopsis plants were transferred to the growth room. Phenotyping, electrolyte conductivity testing, and RNA isolation all required periodic sample collection.
Foliar application of phytohormones
Pepper plants that were in the 4-leaf stage were sprayed with 1 mM salicylic acid (SA), 100 µM methyl jasmonate (MeJA), and 100 µM ethylene (ETH) to investigate the function of these phytohormones. The analytical grade SA, MeJA and ETH were obtained from Merck. Sterile solution of ddH2O was used to treat the mock plants. At various time periods, leaf samples of pepper plants treated with phytohormones, and mock plants were collected for subsequent experiments.
VIGS (virus-induced gene silencing) experiment
A VIGS approach based on the Tobacco Rattle Virus (TRV) was applied to silence CaWRKY3 in pepper. A. tumefaciens strain ‘GV3101’ with PYL192 and PYL279- or PYL279 was grown over night in a thermocontrol shaker at 200 rpm and 28 °C. After that, cultured A. tumefaciens was centrifuged for 10 min at 6800 rpm and 28 °C. The liquid supernatant was discarded, and the solid pellet at the bottom was dissolved in induction media [10 mM MES, 10 mM MgCl2, 200 M acetosyringone, (pH 5.6)] and diluted to an optical density of 0.6 (OD600). A. tumefaciens strains containing PYL192 and PYL279, PYL 279-CaWRKY3, and PYL192-PDS were respectively combined in a ratio of 1:1. A syringe without a needle was used to inject this solution into the cotyledons of pepper seedlings that are two weeks old. After that, treated pepper plants were placed in a dark at 16 °C in the growth room. with 45% relative humidity for 56 h. After 56 h, the plants were transferred to the growth chamber, where they were subjected to a 16-hour light/8-hour dark cycle, 25 ± 2 °C temperatures, 70% relative humidity and 60–70 mol photons m-2s-1 light intensity.
CaWRKY3 transient over-expression assay
A. tumefaciens strain ‘GV3101’ was grown overnight to an OD600 of 1 on LB medium bearing necessary antibiotics in a temperature-control shaker at 28 °C and 200 rpm. It included 35 S: CaWRKY3-flag and 35 S:00 (empty vector). After centrifuging the culture of A. tumefaciens, the solid pellet was dissolved in induction media removed [10 mM MES, 10 mM MgCl2, 200 M acetosyringone, (pH 5.6)] after the supernatant liquid was removed and adjusted to an optical density (OD) value of 0.8. A needleless syringe was used to inject this into the vigorous pepper plants’ leaves. Plants were then placed in a growing chamber and samples were taken at different intervals to be used in further trials.
Histochemical staining
The leaves of CaWRKY3-transiently over-expressed plants, plants with and without CaWRKY3 silencing were observed, as well as leaves that were infected with R. solanacearum were histochemically stained using trypan blue and 3-diaminobenzidine (DAB) staining. After treating the pepper leaves, they were placed in a solution of chloral hydrate (2.5 g of chloral hydrated dissolved in 1 mL of distilled water) after being heated in a solution of trypan blue staining (10 mL lactic acid, 10 mL glycerol, 10 mL phenol, 40 mL ethanol, and 10 mL ddH2O) for 30 min. The leaves were re-boiled three times for 20 min each to remove the remaining discoloration. Finally, samples were stored in glycerol at a 70% concentration. By soaking treated pepper leaves in a 1 mg/ml DAB solution overnight at room temperature, the DAB staining test was performed. Absolute ethanol, glycerol, and lactic acid at a ratio of 1:1:3 (vol/vol/vol) solution was used to remove the DAB stain from the pepper leaves, and the leaves were then preserved in a 95% absolute ethanol solution. Photos of dyed leaves with trypan blue, and diaminobenzidine were taken by microscope (Leica, Wetzlar, Germany).
RNA isolation and real-time qRT-PCR
The TRIzol reagent technique was used to extract the total RNA from the pepper leaves (Invitrogen, Carlsbad, California, USA), and it was then subjected to reverse transcription using a Prime Script RT-PCR Kit (TAKARA, Dalian, China). Quantitative real-time polymerase chain reaction (qRT-PCR) was used to investigate the relative transcriptional levels of certain marker genes. This was accomplished by utilizing a SYBR premix Ex Taq II system (TAKARA Perfect Real Time) in conjunction with a Bio-Rad Real time PCR system (Bio-Rad, Foster City, California, United States). Real-time quantitative PCR and data processing were performed in the same manner as described earlier [13]. The primers used in qRT-PCR are given in Table S3. The original gel images are given in Fig. S1.
Ion Conductivity
We examined electrical conductivity (ion leakage) in previously described manner with some minor adjustments [11]. Six discs of leaf tissue using a hole-puncher, the 4 mm-diameter pieces were taken, incubated in 10 ml of ddH2O after being sterilized by washing them three times in ddH2O. These discs were incubated for 1 h at room temperature while being gently shaken at 60 rpm. Conductivity meter was used to measure electrical conductivity (Zurich, Switzerland: Mettler Toledo 326).
Immunoblotting
Using a protein extraction buffer, the whole protein in pepper leaves was extracted, as reported elsewhere [29, 56]. After extraction, the protein was left to react with anti-HA agarose at a temperature of 4 °C for overnight (Thermo Fisher Scientific; Waltham, Massachusetts; United States). Using a magnetic crack, beads were collected. They were then washed three times in tris-buffer saline (TBS) and tween 20 (0.05%). Anti-HA peroxidase (Abcam, Cambridge, UK) and immunoblotting were both employed to investigate the eluted protein.
Transgenic Arabidopsis plants that overexpress CaWRKY3-OX
Using the BP method, CaWRKY3’s full-length cDNA was cloned into the vector pDONR207. This construct was then ligated into the PK7WG2 target vector. Then, the vector PK7WG2 bearing the CaWRKY3-35 S promoter of the cauliflower mosaic virus was introduced into the ‘GV3101’ strain of Agrobacterium tumefaciens. By using the floral dip approach, transgenic Arabidopsis plants that overexpress CaWRKY3-OX were created using an agrobacterium-mediated transformation process [57, 58]. To create separate transgenic lines, seeds from these CaWRKY3-OX over-expressing transgenic Arabidopsis seedlings were collected and seeded in Petri plates using MS agar medium containing 50 g ML-1 kanamycin. PCR was used to verify that the CaWRKY3 cDNA was successfully inserted into the transgenic Arabidopsis plants’ genome.
Data availability
All data are within the manuscript and supplementary files.
References
Kuromori T, Fujita M, Takahashi F, Yamaguchi-Shinozaki K, Shinozaki K. Inter‐tissue and inter‐organ signaling in drought stress response and phenotyping of drought tolerance. Plant J. 2022;109:342–58.
Hussain A, Noman A, Arif M, Farooq S, Khan MI, Cheng P, et al. A basic helix-loop-helix transcription factor CabHLH113 positively regulate pepper immunity against Ralstonia solanacearum. Microb Pathog. 2021;156:104909.
Hannan Parker A, Wilkinson SW, Ton J. Epigenetics: a catalyst of plant immunity against pathogens. New Phytol. 2022;233:66–83.
Noman A, Hussain A, Adnan M, Khan MI, Ashraf MF, Zainab M, et al. A novel MYB transcription factor CaPHL8 provide clues about evolution of pepper immunity against soil borne pathogen. Microb Pathog. 2019;137:103758.
DeFalco TA, Zipfel C. Molecular mechanisms of early plant pattern-triggered immune signaling. Mol Cell. 2021;81:3449–67.
Liu D, Luo D, He P. ROS around RIPK. Mol Plant. 2021;14:1607–9.
Noman A, Liu Z, Aqeel M, Zainab M, Khan MI, Hussain A, et al. Basic leucine zipper domain transcription factors: the vanguards in plant immunity. Biotechnol Lett. 2017;39:1779–91.
Tang Y, Guo J, Zhang T, Bai S, He K, Wang Z. Genome-wide analysis of WRKY gene family and the dynamic responses of key WRKY genes involved in Ostrinia furnacalis attack in Zea mays. Int J Mol Sci. 2021;22:13045.
Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206.
Sheikh AH, Hussain RMF, Tabassum N, Badmi R, Marillonnet S, Scheel D et al. Possible role of WRKY transcription factors in regulating immunity in Oryza sativa ssp. indica. Physiol Mol Plant Pathol. 2021;114:101623.
Shen L, Liu Z, Yang S, Yang T, Liang J, Wen J, et al. Pepper CabZIP63 acts as a positive regulator during Ralstonia solanacearum or high temperature–high humidity challenge in a positive feedback loop with CaWRKY40. J Exp Bot. 2016;67:2439–51.
Wani SH, Anand S, Singh B, Bohra A, Joshi R. WRKY transcription factors and plant defense responses: latest discoveries and future prospects. Plant Cell Rep. 2021;40:1071–85.
Hussain A, Li X, Weng Y, Liu Z, Ashraf MF, Noman A, et al. CaWRKY22 acts as a positive regulator in pepper response to Ralstonia solanacearum by constituting networks with CaWRKY6, CaWRKY27, CaWRKY40, and CaWRKY58. Int J Mol Sci. 2018;19:1426.
Li Q, Huang C, Liu C, Jia X, Wen W, Li L, et al. Exploring the role and expression pattern of WRKY transcription factor in the growth and development of Bletilla striata based on transcriptome. Gene Rep. 2023;30:101730.
Li W, Pang S, Lu Z, Jin B. Function and mechanism of WRKY Transcription Factors in Abiotic stress responses of plants. Plants. 2020;9:1515.
Jiang J, Ma S, Ye N, Jiang M, Cao J, Zhang J. WRKY transcription factors in plant responses to stresses. J Integr Plant Biol. 2017;59:86–101.
Chen X, Li C, Wang H, Guo Z. WRKY transcription factors: evolution, binding, and action. Phytopathol Res. 2019;1:13.
Lai Z, Vinod K, Zheng Z, Fan B, Chen Z. Roles of Arabidopsis WRKY3 and WRKY4 transcription factors in plant responses to pathogens. BMC Plant Biol. 2008;8.
Shigenaga AM, Argueso CT. No hormone to rule them all: interactions of plant hormones during the responses of plants to pathogens. Semin Cell Dev Biol. 2016;56:174–89.
Kazan K, Lyons R. Intervention of Phytohormone pathways by Pathogen effectors. Plant Cell. 2014;26:2285–309.
Ma K-W, Ma W. Phytohormone pathways as targets of pathogens to facilitate infection. Plant Mol Biol. 2016;91:713–25.
Han X, Kahmann R. Manipulation of Phytohormone pathways by effectors of Filamentous Plant pathogens. Front Plant Sci. 2019;10.
Di X, Takken FLW, Tintor N. How phytohormones shape interactions between plants and the soil-borne fungus fusarium oxysporum. Front Plant Sci. 2016;7.
Phukan UJ, Jeena GS, Shukla RK. WRKY transcription factors: molecular regulation and stress responses in plants. Front Plant Sci. 2016;7.
Araújo LM, Neves LG, Sousa DA, Zeviani WM, Silva L da, Marostega R. TN. Biochemical descriptors: importance of the genetic divergence study in peppers. Hortic Bras. 2019;37:210–4.
Ifnan Khan M, Zhang Y, Liu Z, Hu J, Liu C, Yang S, et al. CaWRKY40b in pepper acts as a negative regulator in response to Ralstonia solanacearum by directly modulating defense genes including CaWRKY40. Int J Mol Sci. 2018;19:1403.
Ahmed W, Yang J, Tan Y, Munir S, Liu Q, Zhang J, et al. Ralstonia solanacearum, a deadly pathogen: revisiting the bacterial wilt biocontrol practices in tobacco and other Solanaceae. Rhizosphere. 2022;21:100479.
Hussain A, Khan MI, Albaqami M, Mahpara S, Noorka IR, Ahmed MAA, et al. CaWRKY30 positively regulates Pepper immunity by targeting CaWRKY40 against Ralstonia solanacearum Inoculation through Modulating Defense-related genes. Int J Mol Sci. 2021;22:12091.
Shi L, Li X, Weng Y, Cai H, Liu K, Xie B, et al. The < scp > ca Pti1 – <scp > ca ERF3 module positively regulates resistance of Capsicum annuum to bacterial wilt disease by coupling enhanced immunity and dehydration tolerance</scp >. Plant J. 2022;111:250–68.
Ashraf MF, Yang S, Wu R, Wang Y, Hussain A, Noman A, et al. Capsicum annuum HsfB2a positively regulates the response to Ralstonia solanacearum infection or high temperature and high humidity forming transcriptional cascade with CaWRKY6 and CaWRKY40. Plant Cell Physiol. 2018;59:2608–23.
Yang S, Shi Y, Zou L, Huang J, Shen L, Wang Y, et al. Pepper CaMLO6 negatively regulates Ralstonia solanacearum resistance and positively regulates high temperature and high humidity responses. Plant Cell Physiol. 2020;61:1223–38.
Cai H, Yang S, Yan Y, Xiao Z, Cheng J, Wu J, et al. CaWRKY6 transcriptionally activates CaWRKY40, regulates Ralstonia solanacearum resistance, and confers high-temperature and high-humidity tolerance in pepper. J Exp Bot. 2015;66:3163–74.
Wang Z, Deng J, Liang T, Su L, Zheng L, Chen H, et al. Lilium regale Wilson WRKY3 modulates an antimicrobial peptide gene, LrDef1, during response to Fusarium oxysporum. BMC Plant Biol. 2022;22:1–17.
Hichri I, Muhovski Y, Žižková E, Dobrev PI, Gharbi E, Franco-Zorrilla JM, et al. The Solanum lycopersicum WRKY3 transcription factor SlWRKY3 is involved in salt stress tolerance in tomato. Front Plant Sci. 2017;8:1343.
Mazzoni C, Palermo V, Torella M, Falcone C. HIR1, the co-repressor of histone gene transcription of Saccharomyces cerevisiae, acts as a multicopy suppressor of the apoptotic phenotypes of the LSM4 mRNA degradation mutant. FEMS Yeast Res. 2005;5:1229–35.
Beatrice C, Linthorst JM, Cinzia F, Luca R. Enhancement of PR1 and PR5 gene expressions by chitosan treatment in kiwifruit plants inoculated with Pseudomonas syringae Pv. Actinidiae. Eur J Plant Pathol. 2017;148:163–79.
Nova B, Syukur S, Jamsari J. Molecular cloning and characterization of Npr1 ankyrin domain from Capsicum annum L. Pharm Lett. 2017;9:148–55.
Liu X, Yang W, Li Y, Li S, Zhou X, Zhao Q, et al. The intergenic region of the maize defensin-like protein genes Def1 and Def2 functions as an embryo-specific asymmetric bidirectional promoter. J Exp Bot. 2016;67:4403–13.
Pandey SP, Somssich IE. The role of WRKY transcription factors in plant immunity. Plant Physiol. 2009;150:1648–55.
Ali MA, Azeem F, Nawaz MA, Acet T, Abbas A, Imran QM, et al. Transcription factors WRKY11 and WRKY17 are involved in abiotic stress responses in Arabidopsis. J Plant Physiol. 2018;226:12–21.
Lacroix B, Citovsky V. A mutation in negative regulator of basal resistance WRKY17 of Arabidopsis increases susceptibility to Agrobacterium-mediated genetic transformation. F1000Res. 2013;2:33.
Abeysinghe JK, Lam K, Ng DW. Differential regulation and interaction of homoeologous WRKY 18 and WRKY 40 in Arabidopsis allotetraploids and biotic stress responses. Plant J. 2019;97:352–67.
Ramos RN, Martin GB, Pombo MA, Rosli HG. WRKY22 and WRKY25 transcription factors are positive regulators of defense responses in Nicotiana Benthamiana. Plant Mol Biol. 2021;105:65–82.
Lin-tao W, Gui-mai Z, Jian-mei W, Xu-feng L, Xu S, Yi Y. Arabidopsis WRKY28 transcription factor is required for resistance to necrotrophic pathogen, Botrytis Cinerea. Afr J Microbiol Res. 2011;5:5481–8.
Birkenbihl RP, Diezel C, Somssich IE. Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection. Plant Physiol. 2012;159:266–85.
Kim K-C, Lai Z, Fan B, Chen Z. Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. Plant Cell. 2008;20:2357–71.
Shimono M, Koga H, Akagi AYA, Hayashi N, Goto S, Sawada M, et al. Rice WRKY45 plays important roles in fungal and bacterial disease resistance. Mol Plant Pathol. 2012;13:83–94.
Ding ZJ, Yan JY, Li CX, Li GX, Wu YR, Zheng SJ. Transcription factor WRKY 46 modulates the development of Arabidopsis lateral roots in osmotic/salt stress conditions via regulation of ABA signaling and auxin homeostasis. Plant J. 2015;84:56–69.
Zentgraf U, Laun T, Miao Y. The complex regulation of WRKY53 during leaf senescence of Arabidopsis thaliana. Eur J Cell Biol. 2010;89:133–7.
Mao P, Duan M, Wei C, Li Y. WRKY62 transcription factor acts downstream of cytosolic NPR1 and negatively regulates Jasmonate-Responsive Gene expression. Plant Cell Physiol. 2007;48:833–42.
Shim JS, Jung C, Lee S, Min K, Lee Y, Choi Y, et al. A t MYB 44 regulates WRKY 70 expression and modulates antagonistic interaction between salicylic acid and jasmonic acid signaling. Plant J. 2013;73:483–95.
Guo P, Li Z, Huang P, Li B, Fang S, Chu J, et al. A tripartite amplification loop involving the transcription factor WRKY75, salicylic acid, and reactive oxygen species accelerates leaf senescence. Plant Cell. 2017;29:2854–70.
Dang F, Wang Y, She J, Lei Y, Liu Z, Eulgem T, et al. Overexpression of CaWRKY27, a subgroup IIe WRKY transcription factor of Capsicum annuum, positively regulates tobacco resistance to Ralstonia solanacearum infection. Physiol Plant. 2014;150:397–411.
DANG F, WANG Y, Yu LU, Eulgem T, Lai YAN, LIU Z, et al. CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection. Plant Cell Environ. 2013;36:757–74.
Yu S, AI C, JING S. Research progress on functional analysis of rice WRKY genes. Rice Sci. 2010;17:60–72.
Litovchick L, Immunoblotting. Cold Spring Harb Protoc. 2020;2020:pdb–top098392.
Bent A. Arabidopsis thaliana floral dip transformation method. Agrobacterium Protocols. 2006;:87–104.
Cheng X, Huang C, Zhang X, Lyu Y. Establishment of transgenic marigold using the floral dip method. Acta Physiol Plant. 2019;41:1–8.
Acknowledgements
The current study was funded by the Key Scientific and Technological Innovation Projects of Guangdong Forestry Bureau (grant no. 2020KJCX009) and National Natural Science Foundation of China (grant no. 32000086). The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R470), King Saud University, Riyadh, Saudi Arabia.
Funding
The current study was funded by the Key Scientific and Technological Innovation Projects of Guangdong Forestry Bureau (grant no. 2020KJCX009) and National Natural Science Foundation of China (grant no. 32000086). The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R470), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.H. and Y.S.; methodology, A.H.; software, S.F., T.V.; validation, S.F., M.Q., T.V., A.Q., and S.M.A.; formal analysis, S.F., and R.A.R; investigation, A.H.; resources, A.H.; data curation, A.H.; writing—original draft preparation, A.H., and S.F.; writing—review and editing, M.Q., A.Q., S.M.A., R.A.R., T.V. and Y.S.; visualization, S.F.; supervision, A.H.; project administration, Y.S.; funding acquisition, Y.S., A.H. and S.M.A. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hussain, A., Qayyum, A., Farooq, S. et al. Pepper immunity against Ralstonia solanacearum is positively regulated by CaWRKY3 through modulation of different WRKY transcription factors. BMC Plant Biol 24, 522 (2024). https://doi.org/10.1186/s12870-024-05143-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-05143-z