Abstract
Background
Brassica napus, a hybrid resulting from the crossing of Brassica rapa and Brassica oleracea, is one of the most important oil crops. Despite its significance, B. napus productivity faces substantial challenges due to heavy metal stress, especially in response to cadmium (Cd), which poses a significant threat among heavy metals. Natural resistance-associated macrophage proteins (NRAMPs) play pivotal roles in Cd uptake and transport within plants. However, our understanding of the role of BnNRAMPs in B. napus is limited. Thus, this study aimed to conduct genome-wide identification and bioinformatics analysis of three Brassica species: B. napus, B. rapa, and B. oleracea.
Results
A total of 37 NRAMPs were identified across the three Brassica species and classified into two distinct subfamilies based on evolutionary relationships. Conservative motif analysis revealed that motif 6 and motif 8 might significantly contribute to the differentiation between subfamily I and subfamily II within Brassica species. Evolutionary analyses and chromosome mapping revealed a reduction in the NRAMP gene family during B. napus evolutionary history, resulting in the loss of an orthologous gene derived from BoNRAMP3.2. Cis-acting element analysis suggested potential regulation of the NRAMP gene family by specific plant hormones, such as abscisic acid (ABA) and methyl jasmonate (MeJA). However, gene expression pattern analyses under hormonal or stress treatments indicated limited responsiveness of the NRAMP gene family to these treatments, warranting further experimental validation. Under Cd stress in B. napus, expression pattern analysis of the NRAMP gene family revealed a decrease in the expression levels of most BnNRAMP genes with increasing Cd concentrations. Notably, BnNRAMP5.1/5.2 exhibited a unique response pattern, being stimulated at low Cd concentrations and inhibited at high Cd concentrations, suggesting potential response mechanisms distinct from those of other NRAMP genes.
Conclusions
In summary, this study indicates complex molecular dynamics within the NRAMP gene family under Cd stress, suggesting potential applications in enhancing plant resilience, particularly against Cd. The findings also offer valuable insights for further understanding the functionality and regulatory mechanisms of the NRAMP gene family.
Similar content being viewed by others
Background
Crops face the challenge of overcoming the adverse effects of abiotic stress, which can ultimately lead to decreased productivity [1, 2]. Cadmium (Cd), a highly toxic heavy metal, exerts its detrimental effects by binding to thiol groups in proteins, inhibiting enzyme activity, disrupting protein function, and interfering with the absorption of essential elements [3]. Consequently, these actions have profound implications for the physiological and biochemical functions of crops. Compounding this issue, cadmium readily accumulates in crops, posing a significant threat to human health through the food chain [4, 5]. Even low doses of Cd, when experienced through prolonged exposure, can have severe health implications [6]. Cadmium, classified as a nonessential element, lacks specialized transporters within plant systems. Its absorption primarily occurs through transporters designed for other metals, such as natural resistance-associated macrophage proteins (NRAMPs) [7]. NRAMPs, crucial proton/metal transporters in plants [8, 9], are involved in transporting various essential elements, such as zinc (Zn), iron (Fe), and manganese (Mn), as well as some nonessential elements, such as Cd or arsenic (As) [9, 10]. Consequently, NRAMPs play a pivotal role in maintaining metal homeostasis and detoxifying heavy metals in plant systems [11]. NRAMPs exhibit highly conserved domains and are widely distributed across genomes from bacteria to humans [12]. Extensive research on the NRAMP gene family has been conducted in plants such as Arabidopsis thaliana [13,14,15], Oryza sativa L [10, 16, 17]. , and Medicago truncatula [18].
NRAMP gene family transporters primarily facilitate the transport of divalent metal cations, such as Fe2+, Mn2+, and Cd2+, exhibiting variations in ion selectivity among different NRAMPs. For example, in A. thaliana, AtNRAMP1 is involved in high-affinity transport for Mn uptake in roots [15] and acts as a transporter for Fe [13]. Moreover, OsNRAMP1 exhibits wide affinity and is capable of transporting Fe, Cd, Mn, and As [9, 16]. Interestingly, rice OsNRAMP4 (also known as Nrat1) may enhance rice aluminum (Al) tolerance by reducing Al levels in the cell wall, where it is capable of transporting Al [18]. Unfortunately, the specific ion preferences and evolutionary relevance of NRAMP transporters have not been determined.
Gene expression patterns are intricately linked to physiological functions. For instance, AtNRAMP1 in A. thaliana, MtNRAMP1 in M. truncatula, MhNRAMP1 in M. hupehensis and OsNRAMP1 in Oryza sativa L. exhibit localization on the root plasma membrane [15, 18,19,20]. Under conditions of iron deficiency, AtNRAMP1 transcripts accumulate primarily in roots and exhibit minimal accumulation in leaves [13]. Notably, MtNRAMP1 exhibits the highest expression in both roots and nodules [18]. Overexpression of MhNRAMP1 leads to increased transport of Cd from roots to leaves and heightens the susceptibility of yeast, tobacco, and apple callus tissues to Cd [19]. Additionally, the knockout of OsNRAMP1 significantly diminishes the uptake of Cd and Mn in rice roots, subsequently impacting their accumulation in shoots and grains [16]. These findings underscore the pivotal relationship between the tissue-specific expression of these genes and their physiological functions in roots. Furthermore, the subcellular location of a protein is intimately linked with its function. For instance, both AtNRAMP3 and AtNRAMP4 are localized to the vacuolar membrane [21] and play indispensable roles in maintaining Mn homeostasis [22]. A double mutant of A. thaliana, nramp3nramp4, accumulates notably greater amounts of Mn in leaf mesophyll cell vacuoles than does the wild type. Notably, OsNRAMP4 (Nrat1) localizes to the plasma membrane of all cells, excluding the epidermal cells of the root tip [23]. This demonstrated the transport of trivalent Al ions in yeast but not other divalent ions, such as Mn2+, Fe2+, or Cd2+ [23]. Knockout of OsNRAMP4 diminishes rice’s Al intake and intensifies Al binding to cell walls, consequently enhancing Al sensitivity [23]. OsNRAMP5 is located on the root plasma membrane [24] and is actively involved in facilitating the cellular uptake of Cd [25, 26]. In conclusion, the variability in ion selectivity and expression patterns among NRAMPs underscores the intricate nature of the physiological functions governed by these genes. Moreover, this complexity is likely compounded by considerations of protein structure and responses to both internal and external stimuli.
Oilseed rape (Brassica napus, 2n = 38, AACC) is an allopolyploid species resulting from interspecific hybridization between turnip (Brassica rapa, 2n = 20, AA) and Mediterranean cabbage (Brassica oleracea, 2n = 18, CC) approximately 7,500 years ago [27]. In contrast to B. rapa and B. oleracea, B. napus has an enlarged NRAMP gene family, suggesting probable diversification in the functional aspects of NRAMPs within this particular lineage. However, the functional aspects of NRAMPs in Brassica species have not been fully explored. Although NRAMPs have been extensively studied in A. thaliana, O. sativa L., and M. truncatula, the existing knowledge concerning their functional roles in these plants falls short of providing a comprehensive understanding of their physiological impacts. To elucidate the intricate functions of NRAMPs in Brassica species, further research is warranted, particularly in B. napus, considering its pivotal role within the Brassica species. Exploring the evolutionary relationships, functional differentiation, tissue distribution, and responses of gene family members to internal and external cues at the broader family level is crucial for addressing this knowledge gap. Therefore, this study conducted a comprehensive analysis of NRAMPs in B. napus. Through analysis of publicly available data, 18, 9, and 10 NRAMPs were identified in B. napus, B. rapa, and B. oleracea, respectively. This study involved a thorough investigation of the evolutionary relationships, conserved motifs, domains, gene structures, chromosomal positions, cis-regulatory elements, and expression profiles of NRAMPs within Brassica species. Furthermore, qRT‒PCR analysis was used to examine the influence of different concentrations of Cd on the expression patterns of the BnNRAMPs. Consequently, this research contributes valuable resources toward a thorough comprehension of the evolutionary mechanisms involving BnNRAMPs. This study provides valuable insights that may contribute to unraveling the broader physiological functions exhibited by the NRAMP gene family.
Results
Identification and evolutionary analysis of the Brassica species NRAMP gene family
Within the genomes of the three Brassica species, a total of 37 NRAMPs were identified (Table 1), comprising 18, 9 and 10 NRAMPs in B. napus, B. oleracea and B. rapa, respectively. Of the 18 BnNRAMPs, 9 were distributed in the A subgenome, while the remaining 9 were distributed in the C subgenome.
Physicochemical property analysis of NRAMP proteins is valuable for predicting their structure, function, protein interactions, and evolutionary relationships. Among the 37 identified NRAMP proteins in Brassica species, all of the proteins exhibited hydrophobic properties, reflecting their role as transporters. The majority of the NRAMP proteins were stable (with an instability coefficient < 40), except for five unstable proteins (13.51%). Subfamily I (NRAMP1s/6s) had smaller average sequence lengths and molecular weights (511 aa and 55.39 kD, respectively) compared to the larger sequence lengths and molecular weights (523 aa and 57.58 kD, respectively) observed in the subfamily II (NRAMP2s/3s/4s/5s). The remaining subfamily I proteins were alkaline (average theoretical isoelectric point of 8.64), and those in subfamily II were acidic (average theoretical isoelectric point of 5.61). Despite substantial differences in isoelectric points between the two subgroups, the majority of the NRAMP protein regions were hydrophobic, suggesting minimal differences in the actual charge properties of the proteins.
Protein localization within the cell is intricately linked to protein function; therefore, predicting the cellular localization of a protein is indispensable for investigating gene function. Subcellular localization prediction using WoLFPSORT indicated that all NRAMP proteins in the three Brassica species were localized on the plasma membrane, except for BrNRAMP1.2, which was located in vacuoles. This finding suggested that the primary function of Brassica species NRAMP proteins may involve regulating ion homeostasis inside and outside the cell. However, predictions from the Cell-PLoc 2.0 tool placed BrNRAMP1.2 on the plasma membrane, indicating that more accurate subcellular localization requires further experimental validation. Overall, the predicted subcellular location consolidates the transporter activity of NRAMPs, yet BrNRAMP1.2 may perform unique functions in metal ion homeostasis.
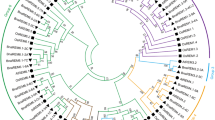
Evolutionary analysis is highly beneficial for studying gene functions, interspecies evolutionary relationships, genetic diversity and variations. To determine the evolutionary relationships between the A. thaliana, B. napus, B. rapa, and B. oleracea NRAMP gene families, we constructed a phylogenetic tree. The results revealed that all the Brassica species NRAMPs clustered well with their homologous genes in A. thaliana (Fig. 1). Based on phylogenetic relationships, the NRAMP genes in Brassica species were categorized into two subfamilies: NRAMP1s/6s constituted subfamily I, whereas NRAMP2s/3s/4s/5s comprised subfamily II.
Phylogenetic tree of B. napus, B. rapa, B. oleracea, and A. thaliana. In this diagram, the light orange leaf background delineates subfamily I (NRAMP1s/6s), while the deep sky-blue leaf background signifies subfamily II (NRAMP2s/3s/4s/5s). Branches are color-coded for clarity: NRAMP6s are represented by black, NRAMP1s by red, NRAMP5s by blue, NRAMP2s by purple, NRAMP3s by gold, and NRAMP4s by green. With respect to leaf label decoration, different shapes indicate distinct NRAMPs of various plant species: triangles denote AtNRAMPs, stars denote BnNRAMPs, circles denote BoNRAMPs, and rectangles denote BrNRAMPs. Moreover, it is pertinent to emphasize that bootstrap values ranging from 80 to 100 are distinctly marked in a dark red shade, those between 50 and 80 are indicated in blue, while bootstrap values falling within the range of 0 to 50 are not rendered for display
In the genomes of B. rapa, B. oleracea, and B. napus, the homologous genes to AtNRAMP1 were found as 3 (BrNRAMP1.1/1.2/1.3), 3 (BoNRAMP1.1/1.2/1.3), and 6 (BnNRAMP1.1/1.2/1.3/1.4/1.5/1.6), respectively. Similarly, homologous genes to AtNRAMP2 were observed as 2 (BrNRAMP2.1/2.2), 2 (BoNRAMP2.1/2.2), and 4 (BnNRAMP1.1/1.2/1.3/1.4), while genes homologous to AtNRAMP4/5/6 were detected as 1 (BrNRAMP4; BrNRAMP5; BrNRAMP6), 1 (BoNRAMP4; BoNRAMP5; BoNRAMP6), and 2 (BnNRAMP4.1/4.2; BnNRAMP5.1/5.2; BnNRAMP6.1/6.2) ,respectively, across these genomes. The number of genes homologous to AtNRAMP1/2/4/5/6 in the B. napus genome equals the sum of such homologous genes found in the genomes of B. rapa and B. oleracea. Moreover, the analysis revealed the presence of 1 (BrNRAMP3), 2 (BoNRAMP3.1, BoNRAMP3.2), and 2 (BnNRAMP3.2, BnNRAMP3.2) orthologous genes to AtNRAMP3 in the B. rapa, B. oleracea, and B. napus genomes, respectively. The phylogenetic analysis depicted in Fig. 1 reveals the presence of two distinct BnNRAMP3 genes, one originating from BrNRAMP3 and the other from BoNRAMP3.1. Notably, BoNRAMP3.2 in B. oleracea has no orthologous genes in B. napus, suggesting that this gene was lost during the evolution of B. napus.
Chromosomal localization and collinearity analysis of the Brassica species NRAMP gene family
Chromosomal localization revealed that 18 BnNRAMPs were distributed across 12 out of the 19 chromosomes in the B. napus genome, with 9 in each A and C subgenome (Fig. 2A, Additional file 1). In B. rapa, 9 BrNRAMPs are located on 6 chromosomes out of 10, and in B. oleracea, 10 BoNRAMPs are positioned on 7 chromosomes out of 9 (Fig. 2B, Additional file 1). The number of NRAMP genes on each chromosome ranged from 1 to 3, indicating that there was no apparent correlation with chromosome length. The dispersed arrangement of NRAMP genes on chromosomes suggested that these genes did not form gene clusters.
Collinearity analysis serves as a pivotal tool for comprehensively exploring genome architecture and evolution, facilitating the elucidation of genetic relationships and evolutionary trajectories among diverse biological species. An examination of collinearity within B. napus revealed 37 NRAMP syntenic gene pairs (Fig. 3).
Furthermore, intergenomic collinearity analysis involving A. thaliana, B. rapa, and B. oleracea revealed 50 NRAMP syntenic gene pairs (Fig. 4A), while 115 NRAMP syntenic gene pairs were identified in the collinearity analysis among B. napus, B. rapa, and B. oleracea (Fig. 4B).
Collinearity relationships of NRAMPs among B. napus and three ancestral plants. (A) Relationships of collinearity among the A. thaliana, B. rapa, and B. napus genomes. (B) Collinearity relationships among the B. rapa, B. napus, and B. oleracea genomes. The gray lines in the background indicate colinear blocks among Brassica species, while the blue lines represent collinear NRAMP gene pairs
In plant genomes, tandem repeats and segmental duplications have been instrumental in expanding gene family members and facilitating the emergence of novel functions during evolutionary processes [28]. To elucidate the evolutionary scenarios within the NRAMP gene families of B. napus, B. rapa, and B. oleracea, we investigated tandem repeats and segmental duplication events. Surprisingly, no tandem repeat genes were observed in B. napus, B. rapa, or B. oleracea. Among the 37 Brassica species NRAMP genes studied, all were found to have originated from whole-genome duplication or segmental duplication events (Additional file 2). These findings strongly indicate the pivotal role of segmental duplication in the evolutionary trajectory of NRAMP genes.
The evaluation of positive selection pressure on recurrent events relies on nonsynonymous (Ka) and synonymous (Ks) substitution rates. This study computed the Ka/Ks ratios between the NRAMP genes in B. napus and those in B. rapa and B. oleracea. The Ka/Ks values ranged from 0.15 to 0.60, with an average of 0.31. Notably, all the NRAMP genes exhibited Ka/Ks values less than 1 (Additional file 3), suggesting that the evolution of NRAMP genes in B. napus occurred under the influence of purifying selection.
Conserved motifs, domains, and gene structure analysis of the Brassica species NRAMP gene family
To predict protein function and discover the relationship between protein structure and function, conserved motif analysis was performed. An examination of the conservation patterns within the protein sequences of the Brassica species NRAMP gene family revealed several conserved motifs, and the distributions of the top 10 highly conserved motifs are shown in Fig. 5A. All NRAMP proteins contained motifs 1, 2, 5, and 7 within the central region of their protein sequences, indicating a high level of conservation of these motifs within the NRAMP gene family of Brassica species. This finding underscores the importance of these motifs for the NRAMP gene family. The number, type, and distribution of motifs within the NRAMP gene family in Brassica species exhibit considerable variation. With the exception of BrNRAMP1.2 (7 motifs), BoNRAMP1.2 (9 motifs), and BrNRAMP6 (8 motifs), the remaining NRAMP proteins feature 10 motifs. This finding underscores the unique functional characteristics of these NRAMPs compared to those of other NRAMPs. Among the NRAMP family proteins, all members of subfamily I (NRAMP1/6) harbor motif 6, while members of subfamily II (NRAMP2/3/4/5) lack this motif. Furthermore, except for NRAMP6s, all the other NRAMPs contained motif 8. Notably, both NRAMP1s and NRAMP2s/3s/4s/5s harbor motif 8, yet the positioning of motif 8 varies between these two subclasses of NRAMPs. In NRAMP1s, motif 8 spans amino acids 450–600, whereas in NRAMP2s/3s/4s/5s, motif 8 is located between amino acids 250–350. Hence, Motif 6 and motif 8 could be pivotal contributors to the functional distinctions observed between subfamilies I and II within the NRAMP gene family. Conducted conserved domain analysis of the 37 Brassica species NRAMP protein sequences using the Pfam and NCBI databases revealed that all the NRAMP proteins possess a conserved domain labelled “Nramp,” representing the hallmark domain of the NRAMP gene family (Fig. 5B). Additionally, except for motif 8 of NRAMP1s, all the other motifs reside within this conserved domain.
A comparison of gene structures within the Brassica species NRAMP gene family (Fig. 5C) revealed significant variations in gene length and the number of introns. Genes with high sequence similarity exhibit a similar number of exons, as well as similar lengths of exons and introns. The maximum sequence length among the Brassica species NRAMP gene family sequences was 6474 bp (BnNRAMP6.1), while the minimum was 1762 bp (BoNRAMP3.1). The number of exons in the Brassica species NRAMP gene family ranged from 3 to 13. Intriguingly, the sequences of subfamily I genes (averaging 3285 bp) are longer than those of subfamily II genes (averaging 2208 bp). Similarly, the number of exons in subfamily I genes (averaging 11) exceeded that in subfamily II genes (averaging 4). Interestingly, the coding sequence (CDS) lengths of either subfamily I or subfamily II are not distinguishable from each other, with approximately 30 nt more in clade I. The larger exon numbers in subfamily I are likely counteracted by the smaller average size of exons, indicating elasticity in alternative splicing. This scenario results in enrichment of transcripts and proteins, suggesting a dynamic regulatory mechanism at the posttranscriptional level.
Cis-acting element analysis of the Brassica species NRAMP gene family promoter regions
Understanding cis-regulatory elements is pivotal in predicting gene functions and unravelling the intricate mechanisms governing gene expression regulation. The NRAMP gene family is significantly impacted by diverse abiotic stresses. To assess the potential functions of NRAMP genes within Brassica species, a comprehensive analysis and screening of cis-regulatory elements in the promoter regions—comprising the 2000 upstream base pairs of NRAMP genes—were conducted. Our investigation revealed several cis-regulatory elements intricately associated with regulating abiotic stresses and hormonal responses, as depicted in Fig. 6. Notably, substantial variability exists within the NRAMP gene family concerning both the quantity and types of cis-regulatory elements. In terms of quantity, BoNRAMP3.2 exhibited the highest abundance of cis-regulatory elements (48 elements), while BnNRAMP2.1 had the lowest (12 elements). In terms of diversity, BnNRAMP1.2 demonstrated the most expansive repertoire (15 elements), whereas BrNRAMP2.1 showed the most limited set (8 elements). Empirical studies suggest a potential correlation between abscisic acid (ABA) and a reduction in Cd uptake via the downregulation of NRAMP expression [5]. Our detailed analysis revealed that, with the exception of 7 genes—BnNRAMP1.6/2.1/2.4/3.2, BoNRAMP1.3/3.1, and BrNRAMP2.1—the promoters of most Brassica species NRAMP genes (30 elements for ABA, 32 elements for MeJA) contain response elements for ABA and methyl jasmonate (MeJA). Furthermore, a subset of Brassica species NRAMP gene promoters lacking ABA response elements shows responsiveness to other plant hormones, such as gibberellin (GA), salicylic acid (SA), and auxin. This finding suggested the potential induction of Brassica species NRAMP genes by hormones, particularly ABA and MeJA. Considering the significant correlation of ABA and MeJA with abiotic and biotic stress [3], these compounds may influence NRAMP family gene expression, potentially impacting metal ion absorption under adverse environmental conditions. Moreover, the NRAMP gene promoters of 37 Brassica species contain light-responsive elements, indicating the potential of light to modulate NRAMP gene expression due to its role in maintaining element homeostasis, which is crucial for photosynthesis-related elements such as Fe, Mn, magnesium (Mg), calcium (Ca), and copper (Cu). Additionally, aerobic respiration in plant cells, which is crucial for ion uptake, requires adequate energy supplied by aerobic respiration. The promoter regions of most Brassica species NRAMP genes (31 genes) contain anaerobic responsive elements (AREs), further supporting their association with plant tolerance to abiotic stress, particularly anaerobic conditions. Importantly, 15 NRAMP gene promoters harboured defence and stress response elements, 26 harboured MYB transcription factor response elements, 17 had SA response elements, 16 had cold response elements, and 14 had drought-induced response elements. These findings underscore the diverse and potential multifaceted roles of the NRAMP gene family in various biological and abiotic stress responses.
Analysis of the expression patterns of BnNRAMPs based on transcriptome data
The expression patterns of genes represent a pivotal aspect of elucidating gene function. To explore the roles of the NRAMP genes further, we constructed a heatmap displaying NRAMP gene expression patterns utilizing publicly available data from the BnIR website (https://yanglab.hzau.edu.cn/). Notably, BnNRAMP1.2 exhibited minimal expression in roots, whereas BnNRAMP1.3/1.6 exhibited slight expression in 2 mm buds (Fig. 7A). In contrast, BnNRAMP5.1/5.2 demonstrated minimal expression in 4 mm buds and pollen. Intriguingly, BnNRAMP1.1 exhibited elevated expression in stamens, petals, sepals, seeds (at 14–34 days postflowering), and siliques (at 26–54 days postflowering). Similar expression patterns were observed for BnNRAMP1.4. Moreover, BnNRAMP4.1 exhibited expression across multiple tissues, including buds, petals, leaves, roots, seeds, and siliques, with notably greater expression in leaves. Concurrently, BnNRAMP6.2 exhibited increased expression in seeds between 14 and 50 days after flowering, followed by nearly undetectable expression levels from 54 to 62 days after flowering. This finding delineates the functional involvement of this gene across diverse tissues and developmental stages, underscoring the significance of ion uptake throughout the life cycle of B. napus. Expression analyses revealed that BnNRAMPs, particularly BnNRAMP1.2/1.3/1.5/1.6/5.1/5.2/6.1/6.2, were scarcely detected under drought, cold, or heat stress (Fig. 7B). Conversely, the expression of BnNRAMP4.1/4.2 in leaves and roots decreased during exposure to these adverse conditions. Overall, the BnNRAMP genes function in coordination to withstand diverse environmental conditions. The assessment of B. napus NRAMP gene responses to various hormone treatments revealed a lack of notable sensitivity across different types of hormones (Fig. 7C). Notably, under hormonal treatment, BnNRAMP1.3/1.5/1.6/5.1/5.2/6.2 exhibited low and unresponsive expression levels. Conversely, BnNRAMP4.1/4.2 displayed increased expression within 0.5–1 h after treatment with indole-3-acetic acid (IAA), GA, ABA, and jasmonic acid (JA). However, their expression levels decreased, reaching or falling below those of the control group at 3–6 h posttreatment. Interestingly, unlike those of other hormones, the expression of BnNRAMP4.1/4.2 substantially increased within 0.5–1 h following treatment with brassinolide (BL), followed by a pronounced decrease at 3–6 h. This finding underscores the heightened sensitivity of BnNRAMPs, particularly BnNRAMP4.1/4.2, to BL compared to that of other hormones.
Analysis of expression patterns in BnNRAMPs. (A) Tissue-specific expression patterns of BnNRAMPs. (B) Expression patterns of BnNRAMPs under stress conditions. (C) Expression patterns of BnNRAMPs under hormone treatments. In Fig. 7A, the notations ‘N + D’ and ‘N + DAF’ represent the Nth day and the Nth day after flowering, respectively. Within Fig. 7C, the abbreviations correspond to specific phytohormones: IAA, indole-3-acetic acid; GA, gibberellic acid; ABA, abscisic acid; JA, jasmonic acid; and BL, brassinolide. The expression of the BnNRAMPs was normalized and represented as transcripts per kilobase of exon model per million mapped reads (TPM) values, and the log2(TPM + 1) was used to construct the heatmap diagram
Analysis of the expression patterns of BnNRAMPs under cadmium stress
In this study, the impact of varying concentrations of CdCl2·5/2H2O (25 µmol/L and 50 µmol/L) on the expression of BnNRAMPs in B. napus seedlings was investigated. Our findings revealed distinct expression patterns among BnNRAMPs under Cd stress conditions (Fig. 8). Most BnNRAMPs exhibited decreased expression levels in response to Cd treatment compared to those in the control group. The observed decrease in expression levels with increasing Cd concentrations suggested a dose-dependent effect, presumably influenced by cadmium-induced toxicity impacting the regulatory mechanisms of these transporters. Similarly, BnNRAMP6.1/6.2 presented reduced expression levels under Cd treatment, consistent with the overall trend. Nevertheless, a marginal upregulation in expression was noted for BnNRAMP6.1/6.2 at relatively high Cd concentrations (50 µmol/L), suggesting a nuanced response under elevated stress conditions. However, compared to the low Cd treatment, the expression levels of BnNRAMP6.1/6.2 were relatively higher under the 50 µmol/L Cd treatment, indicating a potentially finer regulation in B. napus under high cadmium concentrations. Notably, BnNRAMP5.1/5.2 exhibited a divergent response mechanism compared to that of other BnNRAMPs under Cd stress. In contrast to the general trend, BnNRAMP5.1/5.2 exhibited increased expression in response to 25 µmol/L Cd, implying heightened sensitivity to lower Cd levels and a potential role in responding to reduced ionic strength. Nevertheless, after 50 µmol/L Cd treatment, phytotoxicity was evident in B. napus seedlings, leading to a subsequent decrease in the expression of BnNRAMP5.1/5.2, highlighting the intricate interplay between Cd stress levels and gene expression regulation. These observations shed light on the intricate regulatory mechanisms governing BnNRAMP expression under Cd stress, emphasizing the need for further exploration into the specific molecular responses of these transporters in B. napus under varying stress conditions.
Differential responses of BnNRAMP expression under cadmium stress in B. napus seedlings. Figure 8 (A-I) shows the BnNRAMPs. The expression level of each gene in the control plants at 0 µmol/L was normalized to 1.0. Error bars represent the mean values of three replicates ± SEM (standard error of the mean). Different lowercase letters indicate significant differences according to Duncan’s multiple range test (p < 0.05)
Discussion
Elucidation of the mechanisms governing Cd absorption and transport is imperative. Although the association between NRAMP transporters and Cd absorption has been documented in certain species, such as A. thaliana and O. sativa L [14, 17, 29]. , advancements in this area of research remain notably limited. Additionally, investigations elucidating the involvement of NRAMP transporters in the uptake and translocation of Cd in B. napus are scarce. Hence, this study undertook a comprehensive analysis of the NRAMP gene family within Brassica species using bioinformatics methodologies. Our objective was to provide novel insights into the function of the NRAMP gene family within Brassica species.
In this investigation, a total of 37 NRAMP genes were discerned within the Brassica species genome and distributed across its varieties, such as Brassica napus (18 genes), B. rapa (9 genes), and B. oleracea (10 genes). Additionally, in other previously documented species, namely, A. thaliana [30], O. sativa L [31]. , Glycine max [32], and Arachis hypogaea [33], The presence and number of NRAMP genes were noted for 6 genes, 7 genes, 13 genes, and 15 genes. These results underscore the conserved origin of the NRAMP gene family across diverse plant species. However, the number of genes substantially varies among the abovementioned species. Therefore, despite their common ancestry, it is likely that environmental stresses were distinctly exerted on these species, resulting in different evolutionary pathways of the NRAMP family, which ultimately led to different scales of gene numbers. Based on phylogenetic analysis, the 37 Brassica species NRAMP genes were categorized into two distinct subfamilies: subfamily I, comprising NRAMP1s and NRAMP6s; and subfamily II, encompassing NRAMP2s, NRAMP3s, NRAMP4s, and NRAMP5s. This classification aligns with the established categorization of the NRAMP gene family observed in A. thaliana [30]. Owing to the whole-genome triplication of Brassica species [34, 35], the A. thaliana gene typically corresponds to three homologues in B. rapa or B. oleracea. However, except for NRAMP1s, the numbers of other NRAMPs in B. rapa and B. oleracea is less than three times. Contraction events may occur during the evolution process of BrNRAMPs and BoNRAMPs. In the course of hybridizing B. rapa with B. oleracea to create B. napus, the number of homologous genes corresponding to AtNRAMP1/2/4/5/6 within the B. napus genome was comparable to the cumulative number of homologous genes to AtNRAMP1/2/4/5/6 found in both B. rapa and B. oleracea. This observation serves to reassert the preservation and fidelity of the NRAMP gene family within Brassica species. However, phylogenetic analysis (Fig. 1) and chromosomal localization (Fig. 2) revealed that during evolution, the homologous gene originating from BoNRAMP3.2 was lost in B. napus. This result demonstrated a moderate contraction of the NRAMP gene family in B. napus during this interspecies hybridization. The distinct contraction within the NRAMP gene family of Brassica species might be attributed to different selection pressures. Indeed, purifying selection occurred during the evolution (with Ka/Ks values consistently less than 1) of B.napus, indicating decreased selection pressure, which may ultimately lead to moderate contraction of BnNRAMPs.
Conserved motif and domain analysis aids in predicting protein functions and understanding the relationship between protein structure and function. The results indicate that motifs 1, 2, 5, and 7 could be pivotal for normal NRAMP protein function, as they are universally present. These motifs likely encompass the metal ion binding sites that are crucial for NRAMP functionality and are thus possibly retained throughout evolution. However, the location and composition of motifs 6 and 8 across the NRAMP family showed greater diversity. Subfamily I (NRAMP1/6) possesses motif 6, which is absent in subfamily II (NRAMP2/3/4/5). Additionally, except for NRAMP6s, all the other NRAMPs contain motif 8. Motif 8 is positioned centrally within the NRAMP2s/3s/4s/5s sequences, whereas it resides in the C-terminal region among the NRAMP1s. These findings highlight motif 6 and 8 as potential contributors to functional disparities within the NRAMP family, warranting further investigation into their role in the physiological functions of NRAMPs. Motifs 6 and 8, located within the transmembrane domain of NRAMP proteins, constitute a channel that facilitates the entry of divalent cations into cells in conjunction with motifs 1, 3, and 10 [36]. Therefore, variations in motifs 6 and 8 may lead to differences in the transport activity of these NRAMPs. Additionally, in the NRAMP of Deinococcus radiodurans, the substitution of threonine for histidine at position 230 results in a significant reduction in the uptake of Cd2+, while the uptake of Mn2+ and Fe2+ remains largely unaffected [37]. Therefore, motif 2 may be closely associated with the absorption of Cd ions.
The analysis of cis-regulatory elements is imperative for comprehending the mechanisms regulating gene expression and predicting gene functions. Most promoters of BnNRAMPs contain response elements for ABA, MeJA, GA, SA, or auxin, indicating potential hormonal regulation pathways. Studies have shown that plant hormones such as ABA, JA and SA are involved in plant responses to different metal stresses [38]. However, expression analysis revealed an overall subdued response of BnNRAMPs to these hormones and stress treatments. Similarly, research indicates that under exogenous ABA treatment, alterations in the A. thaliana NRAMP genes are not prominently observed [39]. Minimal changes in expression levels after hormone and stress treatments might be due to the fine-tuning of NRAMP gene expression and potential spatial regulation, altering NRAMP distribution without significant effects on overall expression levels. Additionally, the tissue-specific expression patterns of BnNRAMPs suggest that, apart from BnNRAMP1.3/1.6/5.1/5.2, the remaining BnNRAMPs do not exhibit noticeable tissue specificity. Similarly, the Solanum tuberosum StNRAMP5 also shows tissue specificity [40]. However, StNRAMP5 in S. tuberosum has a broader tissue specificity compared to BnNRAMP5.1/5.2, implying that NRAMP5 may exert different functions in B. napus and S. tuberosum.
Apart from those of BnNRAMP1.1/1.4/4.1/4.2, the basal expression levels of the remaining BnNRAMPs are relatively low. Similar occurrences have been observed for species such as Arachis hypogaea L [33]. , Solanum tuberosum [40], Phaseolus vulgaris [41], and Morus notabilis [42], among others. Consequently, the relatively low expression levels of the NRAMP gene family may be a universal phenomenon. Notably, the comparatively low basal expression levels of BnNRAMPs might contribute to the minor changes observed in BnNRAMPs expression under the different hormonal and stress treatments mentioned above.
The expression patterns of BnNRAMPs in B. napus under Cd stress showed that BnNRAMPs had different response mechanisms to Cd. As the Cd concentration increased, there was a corresponding decrease in the expression levels of most BnNRAMPs (Fig. 8). Similar trends were observed in earlier investigations involving Spirodela polyrhiza. Compared to those in the control (0 h), the expression levels of SpNRAMP1, SpNRAMP2, and SpNRAMP3 were downregulated at 6 h, 12 h, or 24 h of exposure to 50 µmol/L Cd [7]. However, in related Glycine max L. studies, an opposite trend was observed. The majority of GmNRAMPs (6 out of 10) showed upregulation, while a minority (3) exhibited downregulation [32]. This could be attributed to the use of higher Cd concentrations (100 µM) and shorter treatment durations (24 h) in soybean compared to other species. Alternatively, it may signify inherent expression differences among NRAMPs across different species. In the present study, the expression levels of BnNRAMP6.1/6.2 also decreased under Cd treatment, but contrary to the general trend, a marginal increase in expression was noted in BnNRAMP6.1/6.2 at higher Cd concentrations (50 µmol/L) compared to those at lower concentrations (25 µmol/L). This finding suggested a potential differential regulatory mechanism for BnNRAMP6.1/6.2 under heightened stress conditions. In prior B. napus studies, the expression of BnNRAMP6b significantly increased after 4 h of exposure to 80 µmol/L Cd stress [43]. Similarly, under Cd stress treatment for 6–24 h, the expression levels of the NRAMP gene family in potato leaves exhibited an initial decrease followed by an increase [40]. Furthermore, within this investigation, BnNRAMP5.1/5.2 exhibited a divergent response mechanism under Cd stress compared to that of other BnNRAMPs. BnNRAMP5.1/5.2 expression increased at lower Cd concentrations (25 µmol/L). This suggests that BnNRAMP5.1/5.2 is more sensitive to lower Cd levels, leading to increased expression in environments with low ionic strength. However, at a higher Cd concentration (50 µmol/L), B. napus seedlings might have experienced significant physiological toxicity, resulting in decreased expression of BnNRAMP5.1/5.2. However, a different scenario emerges in rice, where OsNRAMP5 serves as the primary transporter for cadmium uptake and transport [24, 25, 44]. The expression of OsNRAMP5 decreases at lower Cd concentrations (10 µmol/L) [17]. This discrepancy may stem from varying sensitivities to Cd among different species, with rapeseed exhibiting greater cadmium tolerance compared to rice. These findings suggest intricate regulatory mechanisms governing BnNRAMPs expression under Cd stress, emphasizing the necessity for further exploration of the specific molecular responses of these transporters in B. napus under diverse stress conditions.
It is important to acknowledge the limitations of this study. While we conducted a comprehensive analysis of the NRAMP family at the bioinformatics level, this paper did not involve extensive experimental validation. As a result, many conclusions may lack robust support. Future research efforts can focus on identifying beneficial allelic variants of NRAMP for agriculture. For instance, the rice OsNRAMP5-Q337K mutant has shown the ability to accumulate less Cd while obtaining sufficient Mn [45], which holds significant implications for the development of low-cadmium-accumulating rice varieties applicable to agricultural production. Additionally, analysis of cis-regulatory elements has revealed probable regulation of the NRAMP gene family by various plant hormones, underscoring the importance of elucidating the intricate regulatory mechanisms imposed by these hormones. Furthermore, in addition to the NRAMP family, other families, such as zinc-regulated transporter-like proteins (ZIPs), heavy metal ATPases (HMA transporters), and metal tolerance or transporter proteins (MTPs), play crucial roles in mediating the absorption, transportation, and chelation of metal ions, notably Cd, within plant systems [42]. The synergistic interactions among these transporter proteins within plants highlight the imperative need to comprehend the intricate mechanisms governing their interactions. It is noteworthy that it is preferable to conduct research on NRAMP genes within the native species rather than solely focusing on the function of this gene in A. thaliana. This is because NRAMP may have different functions in different species. For example, rice OsNRAMP4 has been reported to be involved in the transport of Al ions, but there is no corresponding evidence in A. thaliana. We have successfully generated NRAMP gene knockout mutants in B. napus. Looking ahead, we anticipate shedding light on the specific role of the NRAMP gene in the absorption and transport of metal ions, particularly cadmium ions. This holds substantial importance in the context of developing rapeseed varieties with lower cadmium accumulation.
Conclusions
This study involved a thorough exploration of the NRAMP gene family across Brassica species at the genome level. The investigation identified 37 NRAMP genes, 18 of which were in B. napus, 9 in B. rapa, and 10 in B. oleracea; these genes were classified into two subfamilies. Computational collinearity analysis suggested that these genes might have originated from either whole-genome duplication or segmental duplication events. All NRAMP proteins are hydrophobic, with the majority characterized as stable proteins. Subfamily I exhibited alkaline traits, while subfamily II exhibited acidic properties. Conservative motif analysis highlighted motif 6 and motif 8 as the probable primary contributors to the divergence between the two subfamilies. Subcellular localization assays indicated that, except for the potential vacuole localization of BrNRAMP1.2, the remaining NRAMP proteins were predominantly localized on the plasma membrane. Evolutionary and chromosomal analyses suggested that contraction occurred within the NRAMP gene family during the evolutionary progression of B. napus. The majority of NRAMP gene family members exhibited negligible tissue specificity across various tissues of B. napus. The cis-acting element analysis suggests that the NRAMP gene family in B. napus may be regulated by plant hormones, especially ABA and MeJA. Transcriptomic expression analysis indicates that hormones such as ABA, MeJA, and BL have an inductive effect on the expression of BnNRAMP4.1/4.2. However, with increasing treatment time, the expression levels of BnNRAMP4.1/4.2 decrease. Under Cd treatment, expression analysis of B. napus reveals that the expression of most BnNRAMP genes may be negatively regulated by Cd, while BnNRAMP5.1/5.2 and BnNRAMP6.1/6.2 may have a complex regulatory mechanism distinct from other NRAMP genes. This study presents a comprehensive genome-wide identification and analysis of the gene structure of the NRAMP gene family within the Brassica species. Additionally, it reveals the adverse regulatory impact of Cd ions on NRAMP gene expression, as evidenced by expression level analysis. The findings from this study carry substantial reference value for subsequent functional explorations within the NRAMP gene family.
Materials and methods
Plant materials and growth conditions
In this study, our aim was to investigate the impact of Cd on the germination stage of B. napus seedlings and the response of NRAMP genes to Cd during this period. Therefore, in designing the experiment, we referenced previous research methods on the germination stage of B. napus seedlings [46, 47]. We utilized seeds from the inbred line of the B. napus variety Zhongshuang 11 as the primary material. The seeds selected exhibited full grains and uniform texture and underwent a sterilization process involving treatment with 70% ethanol (1 min), followed by triple rinsing with distilled water. Subsequently, these sterilized seeds were placed within seed germination boxes (6.3 × 6.3 × 9 cm) layered with four sheets of filter paper, accommodating 50 seeds per box. The irrigation process involved the application of 10 ml of a CdCl2·5/2H2O solution at concentrations of 0 µmol/L, 25 µmol/L, or 50 µmol/L. The germination process commenced in darkness at a controlled environment of 23 °C with a relative humidity of 70% for an initial period of 2 days. A cultivation period of 5 days was maintained under the following specified light conditions: light intensity, 300 µmol·m− 2 s− 1; temperature, 25 °C during the day; temperature, 22 °C at night; photoperiod, 16 h light and 8 h dark; and relative humidity, 70% [48]. Each treatment was replicated three times to ensure reliability and reproducibility. Sampling was conducted on the 7th day of the experiment. Immediately upon collection, the samples were subjected to rapid freezing in liquid nitrogen and subsequently stored at -80 °C for subsequent analyses and experimentation.
Identification and evolutionary analysis of the NRAMP gene family in Brassica species
The protein sequences belonging to the NRAMP gene family were obtained from the Arabidopsis thaliana genome database (https://www.arabidopsis.org/). Homologous protein sequence alignments were performed against three Brassica species genome databases: Brassica napus multi-omics information resource (BnIR) (https://yanglab.hzau.edu.cn/BnIR/genome_data), Brassica. oleracea genome database (http://brassicadb.cn/download_genome/Brassica_Genome_data/Braol_JZS_V2.0), and the Brassica. rapa genome database (http://brassicadb.cn/download_genome/Brassica_Genome_data/Brara_Chiifu_V3.5), employing an E-value threshold of < 1e-10. Initial selection of NRAMP gene candidates was based on sequence similarity. Subsequent validation of candidate gene protein sequences was accomplished by utilizing the InterPro database (https://www.ebi.ac.uk/interpro/) to retrieve the hidden Markov model (HMM) (PF01566) specific to the NRAMP gene family. The application of the HMM confirmed the identification of NRAMP genes specific to the Brassica species.
To elucidate the physicochemical properties of the NRAMP gene family proteins, the ProtParam tool (https://web.ExPASy.org/protparam/) was utilized for analysis. Multiple sequence alignment was performed for the identified NRAMPs within both the Brassica species and A. thaliana NRAMP gene sets using MUSCLE (https://www.ebi.ac.uk/Tools/msa/muscle/). Additionally, a maximum likelihood estimation-based phylogenetic tree was constructed utilizing IQ-Trees. The resulting tree underwent visual enhancement through the use of Evolview (https://www.evolgenius.info/evolview-v2/).
Subcellular localization prediction of NRAMP family proteins was conducted utilizing two online tools, WoLFPSORT (https://wolfpsort.hgc.jp/) and Cell-PLoc 2.0 (http://www.csbio.sjtu.edu.cn/bioinf/plant/).
Chromosomal localization and collinearity analysis of the Brassica species NRAMP gene family
TBtools [49] was used to chromosomally map the NRAMPs by utilizing the gene location data sourced from the Brassica species gff3 annotation file.
Collinearity analysis between the intragenomic and intergeneric genomes of B. napus was carried out using MCScanX, delineating homologous gene pairs within the B. napus genome as well as across Brassica species genomes. For the analysis of nonsynonymous (Ka) to synonymous (Ks) substitution rates, the simple Ka/Ks calculator function within TBtools [49] was employed.
Conserved motifs, domains, and gene structure analysis of the Brassica species NRAMP gene family
The MEME Suite (https://meme-suite.org/meme/) was used to analyse conserved motifs present within the NRAMP gene family. Information regarding conserved domains within the NRAMP gene family was sourced from the Conserved Domains Database (CDD) and Resources (https://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). The visualization of gene structure within the NRAMP gene family was accomplished using TBtools [49]. Additionally, the examination of cis-acting elements within the NRAMP gene family was performed utilizing the online tool PlantCARE (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
Analysis of the expression patterns of BnNRAMPs based on transcriptome data
We retrieved transcriptome data pertaining to B. napus from the BnIR (https://yanglab.hzau.edu.cn/BnIR/expression_zs11), encompassing tissue specific, stress-responsive, and hormone-induced expression profiles. The visualization of B. napus NRAMP gene expression data was conducted using the heatmap function available in TBtools [49].
Analysis of the expression patterns of BnNRAMPs under cadmium stress
Oligonucleotide primers targeting the BnNRAMPs were designed utilizing Primer Premier 5 (Additional file 4). Total RNA was isolated with the FlaPure Plant Total RNA Extraction Kit sourced from Genesand Biotech Co., Ltd., based in Beijing, China. Subsequently, first-strand cDNA synthesis was accomplished using All-In-One 5X RT MasterMix manufactured by Applied Biological Materials, Inc., located at V6V 2J5, Canada. Quantitative real-time polymerase chain reaction (qRT‒PCR) analysis was performed utilizing the Bio-Rad CFX96 touch real-time PCR system (Bio-Rad, Hercules, CA, USA). qPCR was conducted using BlasTaq™ 2X qPCR MasterMix (also provided by Applied Biological Materials, Inc.) at V6V 2J5, Canada. The qPCR protocol involved an initial denaturation step at 95 °C for 3 min, followed by 39 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. A melting curve analysis was generated over a temperature range of 65 to 95 °C. For normalization, BnACTIN2 (NM_001315560.1, LOC106390277) was utilized as the internal control. Relative expression levels of the BnNRAMP genes were determined using the 2−∆∆Ct method [50]. The data are presented as the mean ± standard error of the mean (SEM). Statistical analysis was performed using GraphPad Prism 9.0 software, employing one-way analysis of variance (ANOVA).
Data availability
The datasets supporting the conclusions of this article are included within the article and its additional files. In this study, genomic data and annotation files for Arabidopsis thaliana, Brassica napus, Brassica rapa, and Brassica oleracea were obtained from TAIR (https://www.arabidopsis.org/), BnIR (https://yanglab.hzau.edu.cn/BnIR/genome_data), BRAD (http://brassicadb.cn/download_genome/Brassica_Genome_data/Braol_JZS_V2.0), and BRAD (http://brassicadb.cn/download_genome/Brassica_Genome_data/Brara_Chiifu_V3.5). Public transcriptome data can be accessed from BnIR (https://yanglab.hzau.edu.cn/BnIR/expression_zs11). The Zhongshuang11 seeds used in this research were provided by the Crop Research Institute, Hunan Academy of Agricultural Sciences.
Abbreviations
- aa:
-
Amino acid
- ABA:
-
Abscisic acid
- Al:
-
Aluminum
- ANOVA:
-
One-way analysis of variance
- AREs:
-
Anaerobic responsive elements
- As:
-
Arsenic
- BL:
-
Brassinolide
- BnIR:
-
Brassica napus multi-omics information resource
- Ca:
-
Calcium
- Cd:
-
Cadmium
- CDD:
-
Conserved Domains Database
- CDS:
-
Coding sequence
- Cu:
-
Copper
- Fe:
-
Iron
- GA:
-
Gibberellin
- HMA:
-
Heavy metal ATPases
- HMM:
-
Hidden Markov Model
- IAA:
-
Indole-3-acetic acid
- kDa:
-
KiloDalton
- MeJA:
-
Methyl Jasmonate
- Mg:
-
Magnesium
- Mn:
-
Manganese
- MTPs:
-
Metal tolerance or transporter proteins
- MW:
-
Molecular weight
- MYB:
-
Myeloblastosis
- NRAMP:
-
Natural resistance-associated macrophage proteins
- PI:
-
Isoelectric point
- qRT‒PCR:
-
Quantitative reverse transcription Polymerase Chain Reaction
- SA:
-
Salicylic Acid
- SEM:
-
Standard Error of the Mean
- TAIR:
-
The Arabidopsis Information Resource
- TPM:
-
Transcripts per kilobase of exon model per million mapped reads
- ZIP:
-
Zinc-regulated transporter-like proteins
- Zn:
-
Zinc
References
Zhang H, Zhu J, Gong Z, Zhu JK. Abiotic stress responses in plants. Nat Rev Genet. 2022;23(2):104–19.
Zhao Y, Wang J, Huang W, Zhang D, Wu J, Li B, Li M, Liu L, Yan M. Abscisic-acid-regulated responses to Alleviate Cadmium toxicity in plants. Plants (Basel) 2023;12(5).
Li D, Xu X, Hu X, Liu Q, Wang Z, Zhang H, Wang H, Wei M, Wang H, Liu H, et al. Genome-wide analysis and Heavy Metal-Induced expression profiling of the HMA Gene Family in Populus trichocarpa. Front Plant Sci. 2015;6:1149.
Lu Q, Weng Y, You Y, Xu Q, Li H, Li Y, Liu H, Du S. Inoculation with abscisic acid (ABA)-catabolizing bacteria can improve phytoextraction of heavy metal in contaminated soil. Environ Pollut. 2020;257:113497.
Zhang W, Wang Z, Song J, Yue S, Yang H. Cd2 + uptake inhibited by MhNCED3 from Malus hupehensis alleviates Cd-induced cell death. Environ Exp Bot. 2019;166.
Baba H, Tsuneyama K, Yazaki M, Nagata K, Minamisaka T, Tsuda T, Nomoto K, Hayashi S, Miwa S, Nakajima T, et al. The liver in itai-itai disease (chronic cadmium poisoning): pathological features and metallothionein expression. Mod Pathol. 2013;26(9):1228–34.
Chen Y, Zhao X, Li G, Kumar S, Sun Z, Li Y, Guo W, Yang J, Hou H. Genome-wide identification of the Nramp Gene Family in Spirodela polyrhiza and expression analysis under cadmium stress. Int J Mol Sci. 2021;22(12).
Zhang J, Zhang M, Song H, Zhao J, Shabala S, Tian S, Yang X. A novel plasma membrane-based NRAMP transporter contributes to cd and zn hyperaccumulation in Sedum Alfredii Hance. Environ Exp Bot. 2020;176.
Tiwari M, Sharma D, Dwivedi S, Singh M, Tripathi RD, Trivedi PK. Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ. 2014;37(1):140–52.
Wang T, Li Y, Fu Y, Xie H, Song S, Qiu M, Wen J, Chen M, Chen G, Tian Y, et al. Mutation at different sites of Metal Transporter Gene OsNramp5 affects cd Accumulation and related agronomic traits in Rice (Oryza sativa L). Front Plant Sci. 2019;10:1081.
Ihnatowicz A, Siwinska J, Meharg AA, Carey M, Koornneef M, Reymond M. Conserved histidine of metal transporter AtNRAMP1 is crucial for optimal plant growth under manganese deficiency at chilling temperatures. New Phytol. 2014;202(4):1173–83.
Nevo Y, Nelson N. The NRAMP family of metal-ion transporters. Biochim Biophys Acta. 2006;1763(7):609–20.
Curie C, Alonso JM, Jean MLE, Ecker JR, Briat J-F. Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem J. 2000;347(3):749–55.
Thomine S, Wang R, Ward JM, Crawford NM, Schroeder JI. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc Natl Acad Sci U S A. 2000;97(9):4991–6.
Cailliatte R, Schikora A, Briat JF, Mari S, Curie C. High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell. 2010;22(3):904–17.
Chang JD, Huang S, Yamaji N, Zhang W, Ma JF, Zhao FJ. OsNRAMP1 transporter contributes to cadmium and manganese uptake in rice. Plant Cell Environ. 2020;43(10):2476–91.
Ishimaru Y, Takahashi R, Bashir K, Shimo H, Senoura T, Sugimoto K, Ono K, Yano M, Ishikawa S, Arao T, et al. Characterizing the role of rice NRAMP5 in Manganese, Iron and Cadmium Transport. Sci Rep. 2012;2:286.
Tejada-Jimenez M, Castro-Rodriguez R, Kryvoruchko I, Lucas MM, Udvardi M, Imperial J, Gonzalez-Guerrero M. Medicago truncatula natural resistance-associated macrophage Protein1 is required for iron uptake by rhizobia-infected nodule cells. Plant Physiol. 2015;168(1):258–72.
Zhang W, Yue S, Song J, Xun M, Han M, Yang H. MhNRAMP1 from Malus hupehensis exacerbates cell death by accelerating cd uptake in Tobacco and Apple Calli. Front Plant Sci. 2020;11:957.
Takahashi R, Ishimaru Y, Nakanishi H, Nishizawa NK. Role of the iron transporter OsNRAMP1 in cadmium uptake and accumulation in rice. Plant Signal Behav. 2014;6(11):1813–6.
Lanquar V, Lelievre F, Bolte S, Hames C, Alcon C, Neumann D, Vansuyt G, Curie C, Schroder A, Kramer U, et al. Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 2005;24(23):4041–51.
Lanquar V, Ramos MS, Lelievre F, Barbier-Brygoo H, Krieger-Liszkay A, Kramer U, Thomine S. Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiol. 2010;152(4):1986–99.
Xia J, Yamaji N, Kasai T, Ma JF. Plasma membrane-localized transporter for aluminum in rice. Proc Natl Acad Sci U S A. 2010;107(43):18381–5.
Chang JD, Huang S, Konishi N, Wang P, Chen J, Huang XY, Ma JF, Zhao FJ. Overexpression of the manganese/cadmium transporter OsNRAMP5 reduces cadmium accumulation in rice grain. J Exp Bot. 2020;71(18):5705–15.
Tang L, Dong J, Qu M, Lv Q, Zhang L, Peng C, Hu Y, Li Y, Ji Z, Mao B, et al. Knockout of OsNRAMP5 enhances rice tolerance to cadmium toxicity in response to varying external cadmium concentrations via distinct mechanisms. Sci Total Environ. 2022;832:155006.
Ishikawa S, Ishimaru Y, Igura M, Kuramata M, Abe T, Senoura T, Hase Y, Arao T, Nishizawa NK, Nakanishi H. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc Natl Acad Sci U S A. 2012;109(47):19166–71.
Chalhoub B, Denoeud F, Liu S, Parkin IA, Tang H, Wang X, Chiquet J, Belcram H, Tong C, Samans B, et al. Plant genetics. Early allopolyploid evolution in the post-neolithic Brassica napus oilseed genome. Science. 2014;345(6199):950–3.
Liu Z, Coulter JA, Li Y, Zhang X, Meng J, Zhang J, Liu Y. Genome-wide identification and analysis of the Q-type C2H2 gene family in potato (Solanum tuberosum L). Int J Biol Macromol. 2020;153:327–40.
Cailliatte R, Lapeyre B, Briat JF, Mari S, Curie C. The NRAMP6 metal transporter contributes to cadmium toxicity. Biochem J. 2009;422(2):217–28.
Maser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJ, Sanders D, et al. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001;126(4):1646–67.
Mani A, Sankaranarayanan K. In Silico Analysis of Natural Resistance-Associated macrophage protein (NRAMP) family of transporters in Rice. Protein J. 2018;37(3):237–47.
Qin L, Han P, Chen L, Walk TC, Li Y, Hu X, Xie L, Liao H, Liao X. Genome-wide identification and expression analysis of NRAMP Family genes in soybean (Glycine Max L). Front Plant Sci. 2017;8:1436.
Tan Z, Li J, Guan J, Wang C, Zhang Z, Shi G. Genome-wide identification and expression analysis reveals roles of the NRAMP gene family in Iron/Cadmium interactions in peanut. Int J Mol Sci 2023, 24(2).
Bian X, Cao Y, Zhi X, Ma N. Genome-wide identification and analysis of the Plant Cysteine Oxidase (PCO) Gene Family in Brassica napus and its role in abiotic stress response. Int J Mol Sci 2023, 24(14).
Cheng F, Wu J, Wang X. Genome triplication drove the diversification of Brassica plants. Hortic Res. 2014;1:14024.
Bozzi AT, Gaudet R. Molecular mechanism of Nramp-Family Transition Metal Transport. J Mol Biol. 2021;433(16):166991.
Bozzi AT, Bane LB, Weihofen WA, McCabe AL, Singharoy A, Chipot CJ, Schulten K, Gaudet R. Conserved methionine dictates substrate preference in Nramp-family divalent metal transporters. Proc Natl Acad Sci U S A. 2016;113(37):10310–5.
Ma X, Yang H, Bu Y, Zhang Y, Sun N, Wu X, Jing Y. Genome-wide identification of the NRAMP gene family in Populus trichocarpa and their function as heavy metal transporters. Ecotoxicol Environ Saf. 2023;261:115110.
Pan W, You Y, Shentu JL, Weng YN, Wang ST, Xu QR, Liu HJ, Du ST. Abscisic acid (ABA)-importing transporter 1 (AIT1) contributes to the inhibition of cd accumulation via exogenous ABA application in Arabidopsis. J Hazard Mater. 2020;391:122189.
Tian W, He G, Qin L, Li D, Meng L, Huang Y, He T. Genome-wide analysis of the NRAMP gene family in potato (Solanum tuberosum): identification, expression analysis and response to five heavy metals stress. Ecotoxicol Environ Saf. 2021;208:111661.
Ishida JK, Caldas DGG, Oliveira LR, Frederici GC, Leite LMP, Mui TS. Genome-wide characterization of the NRAMP gene family in Phaseolus vulgaris provides insights into functional implications during common bean development. Genet Mol Biol. 2018;41(4):820–33.
Fan W, Liu C, Cao B, Qin M, Long D, Xiang Z, Zhao A. Genome-wide identification and characterization of four gene families putatively involved in Cadmium Uptake, translocation and sequestration in Mulberry. Front Plant Sci. 2018;9:879.
Meng JG, Zhang XD, Tan SK, Zhao KX, Yang ZM. Genome-wide identification of Cd-responsive NRAMP transporter genes and analyzing expression of NRAMP 1 mediated by miR167 in Brassica napus. Biometals. 2017;30(6):917–31.
Yang M, Zhang Y, Zhang L, Hu J, Zhang X, Lu K, Dong H, Wang D, Zhao FJ, Huang CF, et al. OsNRAMP5 contributes to manganese translocation and distribution in rice shoots. J Exp Bot. 2014;65(17):4849–61.
Zhao FJ, Chang JD. A weak allele of OsNRAMP5 for safer rice. J Exp Bot. 2022;73(18):6009–12.
Zhou T, Yue CP, Zhang TY, Liu Y, Huang JY, Hua YP. Integrated ionomic and transcriptomic dissection reveals the core transporter genes responsive to varying cadmium abundances in allotetraploid rapeseed. BMC Plant Biol. 2021;21(1):372.
Zhou ZS, Song JB, Yang ZM. Genome-wide identification of Brassica napus microRNAs and their targets in response to cadmium. J Exp Bot. 2012;63(12):4597–613.
Zhang F, Xiao X, Wu X. Physiological and molecular mechanism of cadmium (cd) tolerance at initial growth stage in rapeseed (Brassica napus L). Ecotoxicol Environ Saf. 2020;197:110613.
Chen C, Wu Y, Li J, Wang X, Zeng Z, Xu J, Liu Y, Feng J, Chen H, He Y, et al. TBtools-II: a one for all, all for one bioinformatics platform for biological big-data mining. Mol Plant. 2023;16(11):1733–42.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–8.
Acknowledgements
We extend our gratitude to Qian Liu, Shilin Ouyang, and Long Zhang for their invaluable assistance in bioinformatics analysis. We thank the public database for the raw data downloaded. We are very grateful to the editor and reviewers for critically evaluating the manuscript and providing constructive comments for its improvement.
Funding
This study was funded by the National Natural Science Foundation of China (32071965) and the Hunan Natural Science Foundation project (2022JJ50130).
Author information
Authors and Affiliations
Contributions
Conception, M.Y. and L.L.; methodology, M.Y., L.L., Q.X. and Q.Y. ; software, Y.Z., J.C. and Q.X.; visualization, Y.Z., W.T., Q.X., Y.G.; validation, L.D. and W.T.; data analysis, D.Z. and J.X.; writing—original draft preparation, Y.Z.; writing—review and editing, M.Y., L.L., Q.X. and Q.Y.; All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, Y., Xie, Q., Yang, Q. et al. Genome-wide identification and evolutionary analysis of the NRAMP gene family in the AC genomes of Brassica species. BMC Plant Biol 24, 311 (2024). https://doi.org/10.1186/s12870-024-04981-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-04981-1