Abstract
Background
Salinity stress is a major limiting factor for plant growth, particularly in arid and semi-arid environments. To mitigate the detrimental effects of salinity stress on vegetable production, selenium (Se) biofortification and grafting onto tolerant rootstocks have emerged as effective and sustainable cultivation practices. This study aimed to investigate the combined effects of Se biofortification and grafting onto tolerant rootstock on the yield of cucumber grown under salinity stress greenhouse conditions. The experiment followed a completely randomized factorial design with three factors: salinity level (0, 50, and 100 mM of NaCl), foliar Se application (0, 5, and 10 mg L-1 of sodium selenate) and grafting (grafted and non-grafted plants) using pumpkin (Cucurbita maxima) as the rootstock. Each treatment was triplicated.
Results
The results of this study showed that Se biofortification and grafting significantly enhanced salinity tolerance in grafted cucumbers, leading to increased yield and growth. Moreover, under salinity stress conditions, Se-Biofortified plants exhibited increased leaf relative water content (RWC), proline, total soluble sugars, protein, phenol, flavonoids, and antioxidant enzymes. These findings indicate that Se contributes to the stabilization of cucumber cell membrane and the reduction of ion leakage by promoting the synthesis of protective compounds and enhancing antioxidant enzyme activity. Moreover, grafting onto pumpkin resulted in increased salinity tolerance of cucumber through reduced Na uptake and translocation to the scion.
Conclusion
In conclusion, the results highlight the effectiveness of Se biofortification and grafting onto pumpkin in improving cucumber salinity tolerance. A sodium selenate concentration of 10 mg L-1 is suggested to enhance the salinity tolerance of grafted cucumbers. These findings provide valuable insights for the development of sustainable cultivation practices to mitigate the adverse impact of salinity stress on cucumber production in challenging environments.
Similar content being viewed by others
Background
Salinity stress is widely recognized as a major constraint for agricultural productivity in tropical and subtropical regions. It leads to a decrease in crop productivity and product quality, thereby posing significant challenges to global food security [1]. Approximately 20% of arable land worldwide is severely damaged by salinity, with the remaining half being affected to varying degrees. There are two forms of soil saline process. Primary or natural salinity and secondary or human salinity. Various hydrological, geomorphic and climatic factors are the primary causes of saline soil. Secondary soil salinity is caused by low water levels, poor water irrigation, over irrigation with inadequate drainage, overuse of ground-water in coastal areas and unsolvable industrial wastewater, and Sewage with high soluble salt. In particular, secondary salinization can stem from different human driven processes [2]. High salinity levels in the soil can result in osmotic balance disruption, limiting water intake and transpiration and consequently yield [3]. The impact of salinity stress on plant growth is complex and depends on several factors, including the level of salinity, type of salt, and the specific plant species involved [4].
Traditional breeding programs have been extensively utilized to enhance crop salinity tolerance. However, achieving commercial success has proven difficult, primarily due to the complexity of this phenomenon. The genetic and physiological traits associated with tolerance to environmental stresses, including salinity, pose significant obstacles in conventional breeding approaches [5]. To address these challenges, gene transfer methods are currently employed to enhance tolerance to salt, although achieving tolerance to multiple stresses through gene transfer is difficult. Recently, grafting onto tolerant rootstocks has emerged as a promising and environmentally friendly technique for enhancing crop yield, offering benefits such as resistant to pests, diseases, and environmental stress [6, 7, 8, 9, 10, 11 and 12].
One of the environmentally friendly ways to mitigate yield reduction and increase resistance to soil diseases (especially damping off) and environmental stresses in the Solanaceae and Cucurbitaceae genotypes is to graft them onto resistant rootstocks [8, 13]. This technique allows plant breeders to harness the advantageous traits of both the rootstock and scion. Rootstock can significantly affect plant growth, yield, and fruit quality [7, 14, 15 and 16]. Numerous reports emphasize the pivot role of rootstock selection in conferring tolerance to environmental stresses, pathogens, and suboptimal soil growth conditions. Successful grafting ensures the scion’s ability to yield high and provide products of superior quality, while the rootstock increases stress tolerance related to the soil conditions. The interaction between rootstock and scion plays a crucial role in determining scion’s tolerance to environmental stresses [17]. In grafted plants, tolerance to salinity stress can be attributed to the accumulation of proline and total soluble sugars [18], enhanced antioxidant capacity [19], and reduced of sodium and chlorine accumulation in the scion [20].
Grafting is a reciprocal process, in which both the rootstock and the scion affect plant’s tolerance to salinity stress [21]. Studies in potato (Solanum tuberosum L.) [22], melon (Cucumis melo L.) [23], tomato (Solanum lycopersicum L.) [24], watermelon (Citrullus lanatus L.) [25], and cucumber (Cucumis sativus L.) [26, 27] plants highlighted the importance of rootstock in conferring salinity stress tolerance in grafted plants. In tomato, however, both the rootstock and scion contribute to salinity stress tolerance [28].
Cucumber is low-calorie vegetable rich in minerals and phenolic compounds. As a glycophyte plant, cucumber is extremely sensitive to soil salinity. Salinity stress adversely affects cucumber growth as a result of osmotic stress, which is followed by ion toxicity. The osmotic stress leads to nutrient imbalances, reactive oxygen species (ROS) production, and membrane damage thereby reducing yield and product quality [29]. Research indicates that high salinity tolerance in grafted cucumber plants is linked to increased leaf potassium concentration [20]. Grafting cucumber onto fig leaf gourd (Cucurbita ficifolia Bouche L.) has shown to increase yield and tolerance to salinity [29, 30]. However, cucumber fruit quality and taste can be negatively affected necessitating a careful rootstock selection to increase tolerance both under abiotic and biotic stresses, while improving the yield and quality of grafted cucumber fruit. Luffa (Luffa aegyptiaca L.) has been introduced as a promising rootstock for cucumber, demonstrating increased salt-resistant and cucumber growth. According to Guo et al. [31], this growth increase can be attributed to increased plant height, leaf number, photosynthesis, antioxidant activity, total soluble sugars, and potassium accumulation in aerial plant parts.
Selenium is an essential micronutrient crucial for animal and human health as well as plant growth and development [32]. To improve the quality of agricultural products and mitigate Se deficiency problems in society, the biological addition of Se, widely known as Se biofortification, has gained attention [33]. The World Health Organization (WHO) has recommended a daily intake of approximately 55 µg Se for adults. Selenium deficiency directly affects human health since more than 40 types of diseases, such as Keshan 2 disease, cancer, cardiovascular diseases, liver diseases, and cataracts, have been linked to its inadequate levels in the human body. Plants play a crucial role in transferring Se from the soil to the human food chain [34].
In plants, Se has emerged as a beneficial element that can mitigate the adverse effects of heat stress [35], heavy metals [36], ultraviolet radiation [37], drought [38], and salinity [39]. According to Lan et al. [40], Se can help alleviate the oxidative damaged induced by stress due to the increased activity of antioxidant enzymes (peroxidase, catalase, etc.) and number of antioxidant compounds in the body (anthocyanins, flavonoids, phenolic compounds, etc.). Selenium is one of the essential components of the antioxidant enzyme system aiding in the scavenging of free radicals produced by salinity stress conditions [40]. This leads to improved photosynthesis, ion homeostasis and increased plant growth and yield [41]. Moreover, appropriate levels of Se have been shown to reduce the negative effects of salinity stress by enhancing the plant’s defense mechanism and regulating sodium carriers [42]. As shown by Regni et al. [43], Se increased tolerance to salinity stress in olive (Olea europaea L.) plants, resulting in increased leaf dry weight, RWC, proline content, and photosynthesis [43]. Similarly, in bean (Phaseolus vulgaris L.), Se biofortification resulted in enhanced shoot and root fresh weight, chlorophyll, carotenoid, RWC, proline, total soluble sugars, peroxidase, and catalase enzymes when plants were exposed to a concentration of 50 mM NaCl [44]. Additionally, foliar Se application improved photosynthesis and water use efficiency (WUE) in tomato plants under salinity stress conditions, leading to increased tomato plant growth and a reduction in oxidative stress-induced damage [45].
Because of the increasing demand for food and the widespread occurrence of salinity-affected soils, research on plant responses to salinity stress has rapidly expanded in recent decades. Sustainable cultivation practices such as grafting plants onto resistant rootstocks and biofortification have been proposed as promising alternative. Despite the extensive research conducted on grafting of cucumber, little is known on the interactive effects of grafting and Se biofortification. Given the relative new subject of vegetable grafting in Iran, a study was designed with the aim to investigate the effect of Se grafted cucumber performance under salinity stress conditions.
Materials and methods
Plant materials and experimental treatments
In this study, the experimental treatments were implemented in a factorial design, based on completely randomized design with three replications. The research was conducted in a greenhouse at Razi University of Agriculture and Natural Resources. The first factor consisted of different salinity levels namely 0, 50, and 100 mM of sodium chloride; the second factor was the foliar application of Se at three levels of 0, 5, and 10 mg L-1 of sodium selenate, and the third factor included both grafted and non-grafted plants. Selenium foliar application was performed using sodium selenate salt and was applied simultaneously with the induction of salinity stress and Se foliar spraying was done once. Sodium selenate was purchased from Sigma Company.
Cucumber seeds of the Nagene variety and pumpkin seeds were obtained from the Pakan Bazr Isfahan Company. The seeds were sown in 5 cm diameter plastic containers with an equal mixture of soil, sand, and rotten manure. Pumpkin seeds were planted three to four days prior to cucumber seeds to ensure compatibility in the stem diameter between the rootstock and the scion to ensure success in grafting. After 25 days of seed cultivation, the Hole insertion grafting was performed as follows: the rootstock, which had cotyledon and true leaves, was prepared by carefully removing the true leaf and the rootstock terminal (apical) bud. A hole of 1-1.5 cm is made in the stem center using a toothpick. The scion plant, consisting only of cotyledons, was cut approximately 2 cm below the cotyledons. Finally, the scion was inserted into the hole created in the rootstock. The Steps to perform the grafting are shown in Fig. 1. Grafted plants were then transferred to 10 L plastic pots. Subsequently, the grafted seedlings were placed in grafting chambers with a relative humidity of 95%, complete darkness, and a temperature of 27–29 ºC. After three days, the relative humidity was steadily reduced until it reached the appropriate levels for optimum growth for the upcoming 14 days. Once the grafted plants had acclimatized and developed three true leaves, salinity stress was applied by adding NaCl to the irrigation water up to the end of the growth period. The salinity level increased gradually to reach the desired stress level. Selenium foliar spraying was done once and simultaneously with the application of salt stress. Pest and disease control, plant pruning and support for vertical growth, irrigation, and temperature and humidity regulation were all provided for grafted and non-grafted seedlings. The environmental conditions of the greenhouse during the cucumber growth period included a day temperature of 22–26 °C and the night temperatures of 18–20 °C, light intensity 6000–10,000 lx and relative humidity between 50 and 70%.
Morphometric parameters
The morphological features studied were plant height, fresh weight of root, shoot and fruit, number of leaves and fruits, single plant yield, and total yield. Fruits were harvested three times per week between May 4 and June 20. At each harvest, the total number of fruits and weight of fruits was recorded separately. Mean fruit weight and total fruit yield were calculated.
Physiologic parameters
Photosynthetic pigments
The amount of leaf chlorophyll was measured according to the method of Lichtenthaler [46]. Briefly, 0.1 g of fresh tissue of cucumber leaf was homogenized with 10 ml of 80% acetone inside the mortar. The obtained homogeneous mixture was centrifuged for 10 min at 3000 rpm, and finally, the upper part of the extract was separated. The absorbance of the samples was read at 645, 663 and 470 nm using the spectrophotometric method (Kerry 100 model, Varian, America), and the amount of chlorophyll and carotenoid was calculated in mg FW-1 using the following formulas:
RWC
For RWC assay, the fresh leaf discs (with 1.5 cm diameter) were weighted (FW), placed in a petri dish containing 30 ml cool distilled water for 24 h and then their turgid weight (TW) measured. To measure dry weight (DW), leaf discs were dried in an oven (35 L smart model, Shimaz Company, Iran) for 48 h at 72 ºC and then weighed. The leaf RWC was calculated as following formula [47]:
Electrolyte leakage (EL)
The method of Ben Hamed et al. (2007) was used to measure the leakage of electrolytes [48]. Firstly, the cucumber leaf segments (discs with 1.5 cm diameter) were immersed in 40 ml deionized water and the tubes shaken (120 rpm) immediately for 12 h under room temperature, and the electrical conductivity of the samples (EC1) was measured with an EC meter (Jenway model, England company). Then, they were autoclaved at 121 °C for 20 min, and after reaching 25 °C, the electrical conductivity of the samples (EC2) was measured again, and the ion leakage percentage was calculated from the following equation.
Proline and soluble carbohydrates
The first 0.5 g of leaf tissue was ground with liquid nitrogen in a mortar. Then, 5 ml of 95% ethanol was immediately added to it and shaken vigorously. The upper part of the resulting solution was separated, and its sediments were washed twice with 5 ml of 70% ethanol, and their upper phase was added to the previously collected supernatant. The obtained solution was centrifuged at 3500 rpm for 10 min. After separating the liquid and solid phases, the liquid part was kept inside the refrigerator at a temperature of 4 ºC.
To determine the amount of proline, 1 ml of the above-mentioned alcoholic extract was diluted with 10 ml of distilled water, and 5 ml of Ninhydrin reagent was added to it. The composition of the Ninhydrin reagent for each sample included 0.125 g of Ninhydrin + 2 ml of 6 M Phosphoric acid and 3 ml of Glacial acetic acid. After adding the Ninhydrin reagent, 5 ml of Glacial acid was added, and the resulting mixture was placed in a boiling water bath at 100 ºC for 45 min. After removing the samples from the boiling water bath and cooling them, 10 ml of benzene was added to each sample and shaken vigorously until proline entered the benzene phase. The samples were then left to stand still for 30 min. Standard solutions of proline were prepared with concentrations of 0 to 0.1 µmol ml-1. Finally, the light absorption of standard solutions and samples was measured at a wavelength of 515 nm with a spectrophotometer (Kerry 100 model, Varian, America) [49].
To calculate the soluble sugars, 0.1 ml of the alcoholic extract was added to 3 ml of freshly prepared anthrone (150 mg of anthrone + 100 ml of 72% sulfuric acid). It was placed in a boiling water bath for 10 min. At this time, a colored substance was formed. Glucose standards were prepared from 0 to 0.1 µmol ml-1. Finally, the light absorption of standard solutions and samples was read with a spectrophotometer (Kerry 100 model, Varian, America) at a wavelength of 625 nm [50].
Total phenol and flavonoid content
To prepare methanolic extract, 0.5 g the fresh tissue of the leaf was crushed well in a mortar in the presence of 3 ml of 85% methanol and then smoothed. This methanolic extract was used to measure total phenol and flavonoids.
The amount of total phenol was determined using Folin–Ciocalteu reagent [51]. In this method, 300 µl of methanolic extract was mixed with 1500 µl of diluted folin solution (10:1 ratio with distilled water). After keeping it for 8 min at 25 °C, 1200 µl of 7% sodium bicarbonate solution were added. After 90 min of shaking on a shaker at a speed of 120 rpm at room temperature and in the dark, the absorbance of the samples was measured with a spectrophotometer at a wavelength of 765 nm (model Kerry 100, Varian, America). Using the standard curve of gallic acid, total phenol was calculated as mg of gallic acid g-1 FW.
The amount of total flavonoid was measured by the aluminum chloride calorimetric method [52]. Fifty µl of methanol extract was mixed with 10 µl of aluminum chloride (10%), 10 µl of potassium acetate (1 M), and 280 µl of deionized water. After vortexing, the samples were kept at room temperature for 40 min. The absorbance of the samples was read at a wavelength of 415 nm with a spectrophotometer. Total flavonoids were calculated in mg of quercetin g-1 FW using the quercetin standard curve.
Total protein content
The Bradford method [53] was employed to determine the amount of soluble proteins. For this purpose, 0.5 g of fresh leaves was mixed with 6.25 ml of extraction buffer solution and kept for 24 h. To prepare 1 L of extraction buffer solution, 121.4 g of Tris was dissolved in 1 L of distilled water, and the acidity of the solution was changed to 6.8 by normal hydrochloric acid until the desired buffer solution was obtained. After the mentioned period, the leaves were wholly ground in a mortar and then centrifuged at 6000 rpm for 20 min. Then, the sampler took 0.1 ml of the centrifuged upper solution, and 5 ml of Bio-Rad reagent was added to it. 100 mg of Coomassie Brilliant Blue − 250 was mixed with 50 ml of pure ethanol to prepare the reagent, and then it was brought to a volume of approximately 800 ml with distilled water and filtered. Finally, the volume of the filtered solution was increased to 1000 ml with 100 ml of pure phosphoric acid and distilled water. The resulting solution was placed in a spectrophotometer (Kerry 100 model, Varian, America) along with the extraction buffer solution, and its absorbance was read at a wavelength of 595 nm. To prepare the standard solution, 100 mg of bovine albumin was dissolved in 1 ml of extraction buffer and then made up to 1000 ml with distilled water. Then, a standard of 10 to 90 ppm was prepared from the solution, and its absorbance was read with a spectrophotometer at the mentioned wavelength.
Antioxidant enzyme activities
Enzyme extract was first prepared to measure the activity of antioxidant enzymes. Briefly, the frozen leaf tissue was first ground in a mortar in the presence of liquid nitrogen, and 0.1 g of it was added to a plastic tube containing 1 ml of extraction buffer and mixed. The sample was passed through a strainer, and the prepared extract was centrifuged for 15 min at a speed of 10,000 rpm at a temperature of 4 ºC and the clear supernatant solution was slowly separated; the resulting solution was used to measure the activity of each of the antioxidant enzymes as described below.
To determine the activity of the catalase enzyme, first, 50 µl of plant extract was mixed with 3 ml of extraction buffer containing 50 mM sodium phosphate (pH 7.8) and 2 mM ethylenediaminetetraacetic acid (EDTA). The reaction of catalase enzyme was started by adding 5 µl of 30% hydrogen peroxide to this mixture. The changes in optical absorption of the samples were recorded at a wavelength of 240 nm for 10 min. Each unit of catalase enzyme activity was considered the amount of enzyme that reduces 1 µl of hydrogen peroxide per minute. The amount of enzyme activity was expressed as units per mg of leaf protein. Each unit of CAT activity was considered as the 1.0 ml enzyme that reduces 1.0 µmol H2O2 min-1 [54].
Peroxidase enzyme activity was measured by spectrophotometry [55]. The first, 3 ml of extraction buffer (50 mM sodium phosphate (pH 7.8) and 2 mM ethylenediaminetetraacetic acid (EDTA) was poured into both control and sample cuvettes to start the peroxidase enzyme reaction. Five µl of 30% hydrogen peroxide and 5 µl of glycol were added to them. These two cuvettes were placed in the spectrophotometer, and the number read became 0. Then, 50 µl of plant extract were added to the sample cuvette, and the changes in light absorption of the samples at 465 nm wavelength, which indicates the degree of degradation and decrease in H2O2 concentration, were recorded every 10 s for 120 s. Each unit of peroxidase enzyme activity was considered as the amount of enzyme that reduces 1 µl of H2O2 ml-1 min-1.
Sodium and potassium concentration
After washing, the leaf samples were dried, placed inside the envelope, and placed in an oven at 72 ºC for 48 h. After drying, the samples were milled, then 0.2 g of the milled samples were poured into the test tube, and 2 ml of concentrated nitric acid was added to them and placed in a water bath at 60 ºC for 60 min. After 60 min, the temperature was increased to reach 100 ºC and kept at this temperature for 90 min. After cooling down the test tubes containing the samples to the laboratory temperature, 0.2 ml of 37% hydrogen peroxide was added to the samples, and the samples were left for 30 min to complete the reaction. After 30 min, the samples were filtered and their final volume was diluted to 25 ml by distilled water. This extract was used to measure sodium and potassium elements by the flame photometer (450G electronic flame photometer) [56].
Statistical analyses
The experimental treatments were implemented factorial, based on completely randomized design with three replications containing two vines per each replicate. Data were analyzed with SAS (9.1) statistical software. Mean comparisons were performed with Duncan’s multiple range test at the 5% level of significance. All data were presented as mean ± standard deviation.
Results
Morphometric parameters
Salinity, Se, and grafting significantly affected the growth characteristics of cucumber. The highest plant height (345.100 cm), fresh plant weight (135.833 g), fresh root weight (47.933 g), number of nodes (61.777), number of fruit (59.700), fresh fruit weight (72.733 g), plant yield (4342.2 g) and total yield (614.60 g m-2) were observed in grafted cucumbers with a concentration of 10 mg L-1 of sodium selenate and 0 mM of NaCl (Table 1). Salinity, Se and grafting had no significant effect on plant and root dry weight. According to the obtained results, Se has improved the effects of salinity in transplanted plants so that in all three levels of salinity stress, the growth characteristics and yield of cucumber increased significantly with the increase of Se.
Physiological characteristics
Physiological traits
The results showed that salinity and Se significantly affected the amount of photosynthetic pigments in cucumber leaf. Unlike Se, salinity harms the amount of photosynthetic pigments in cucumber leaf. The highest amount of chlorophyll a (4.966 mg g-1 FW), chlorophyll b (1.936 mg g-1 FW), total chlorophyll (6.933 mg g-1 FW), and carotenoid (0.596 mg g-1 FW) was observed in the treatment of 0 mM of NaCl along with 10 mg L-1 of sodium selenate. With increasing Se concentration, the amount of photosynthetic pigments in cucumber leaf increased in all three levels of salinity stress compared to the control. At the same time, with the increase of Se concentration in non-salinity conditions, the number of photosynthetic pigments increased (Table 2).
Proline
According to the results (Table 3), with increasing levels of salinity stress and Se, the proline content of cucumber leaf increased compared to the control. The highest content of proline (32.667 mg g-1 FW) was found in grafted cucumber and 100 mM of NaCl along with 10 mg L-1 of sodium selenate.
Soluble carbohydrates
The amount of soluble carbohydrates increases as salinity and Se levels increased. Only in the highest salinity stress and Se level did the results (Table 3) show a significant difference between grafted and non-grafted cucumber plants. The highest amount of soluble carbohydrates (13.666 mg g-1 FW) was observed in grafted cucumber and 100 mM of NaCl along with 10 mg L-1 of sodium selenate.
Total protein
In grafted and non-grafted cucumber, with the increase of Se and salinity level, the amount of total protein increased, which was significantly different from the control treatment. Thus, the highest amount of total protein (18.666 mg g-1 FW) was observed in grafted cucumber with 100 mM of NaCl along with 10 mg L-1 of sodium selenate.
Total phenol and flavonoid
With increasing Se concentration, the total phenol content of cucumber leaf increased under salinity stress condition. The highest amount of total phenol (1.090 mg g-1 FW) and total flavonoid (3.966 mg g-1 FW) were in the treatment of 100 mM of NaCl and 10 mg L-1 of sodium selenate of grafted plants, significantly different from the control treatment (Table 3).
Antioxidant enzymes
With increasing Se concentration and salinity stress, catalase and peroxidase enzymes activity increased (Table 3). The results show no significant difference between grafted and non-grafted plants at the lowest and highest concentrations of Se and salinity stress. Still, grafted cucumbers had higher catalase and peroxidase enzyme activity under salinity stress conditions than non-grafted plants (Table 3).
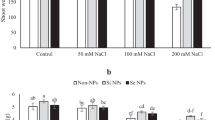
RWC
According to the results of mean comparison of effect different levels of salinity and Se on RWC cucumber leaf (Fig. 2), unlike salinity, Se increased the amount of RWC. The highest amount of RWC (78.77%) was in the treatment of 0 mM of NaCl and 10 mg L-1 of sodium selenate (Fig. 2). According to the obtained results, in the condition of salt stress, with the increase of Se level, the amount of RWC increased.
EL
The results mean comparison of effect different levels of salinity and Se on EL cucumber leaf show that, contrary to Se, salinity has increased the amount of EL in cucumber leaf. The maximum amount of EL of cucumber leaf (92.37%) was in treating 100 mM of NaCl and 0 mg L-1 of sodium selenate (Fig. 3).
Sodium and potassium concentration
The amount of potassium decreased as salinity stress increased, but the amount of sodium increased. Selenium reduced the quantity of sodium while increasing the amount of potassium in cucumber leaf and root (Table 4). The highest amount of potassium in leaf (20.566 mg g-1 DW) and root (32.523 mg g-1 DW) of cucumber was in treating 0 mM NaCl with 10 mg L-1 sodium selenate. In contrast, the highest amount of sodium was observed in treating 100 mM of NaCl and 0 mg L-1 of sodium selenate (Table 4).
Discussion
Salinity seriously threatens agricultural and horticultural crops, leading to reduced growth and yield. Growth is directly related to plant productivity and yield. As a result, it has been widely recognized as a critical indicator in most physiological research. Salinity decreases turgor pressure and DNA synthesis by inducing osmotic stress, ion imbalance, and ion toxicity [45]. All salinity-induced changes in plant metabolism, which include physiological and biochemical processes such as photosynthesis, ion homeostasis, and antioxidant activity, lead to reduced growth [57].
Salt stress has reduced the growth characteristics of grafted and non-grafted cucumbers. In this study, the height and fresh weight of cucumber plant and fruit decreased under salt stress compared to the control (Table 1). The weight loss of air parts under salinity stress conditions can be due to the accumulation of harmful ions such as chlorine and sodium, which are detrimental or cause disturbances in the absorption of water and other minerals. Also, salinity increases the amount of energy required to maintain the cell’s standard conditions, and as a result, less energy is left for growth needs [58]. Under salinity stress conditions, the absorption and transfer of water and minerals from the roots to the leaves decrease. The plant reduces its photosynthetic level by reducing the number and surface of the leaves, which also reduces the plant’s photosynthetic capacity [59]. According to the results of this research, salinity stress, by lowering RWC leaf and causing ion toxicity, led to a decrease in the growth characteristics, yield and yield components of cucumber, which is consistent with the results of Semida et al. (2021) in onion (Allium cepa) and Karaca et al. (2023) in tomato [60, 61].
Similar to the outcomes shown in cucumber [62] and garlic (Allium sativum L.) [32], under salinity stress conditions, foliar application of Se (10 mg L-1 sodium selenate) improved vegetative traits compared to the control treatment. Transferring them to the roots through water and nutrient elements absorption, the osmotic balance is essential in plant tolerance to salinity stress conditions, which is associated with increasing plant growth characteristics [63]. Under salinity stress conditions, Se causes an increase in the RWC, which leads to more water retention in the tissues. Under salinity stress, Se can be said to increase the absorption of macronutrients like magnesium, potassium, phosphorus, and nitrogen while decreasing the absorption of sodium. It also increases proline levels, RWC, and the activity of antioxidant enzymes [64], thereby resulting in enhanced cucumber yield (Table 1).
Cucumber is sensitive to salinity; thus, identifying salinity-tolerant rootstock appears to be critical. Pumpkin has shown more tolerance to environmental stress than cucumber [65]. According to the results (Table 1), no significant difference was observed between grafted and non-grafted cucumbers in the treatment without salinity stress. Still, at high levels of salinity stress, the growth characteristics studied in grafted plants were higher than non-grafted plants; this shows the positive effect of grafting and using pumpkin rootstock under salinity stress conditions and follows the results obtained in the pepper (Capsicum annuum L.) [66]. According to the conducted studies, grafting can affect water absorption and nutrient elements [67, 68]. In the present study, grafting and foliar application of Se improved the growth characteristics of cucumber under salinity stress conditions. In salinity stress conditions, grafting tomato on eggplant (Solanum melongena L.) has improved tomato fruit’s physiological condition and yield. Eggplant as a rootstock increases the amount of proline and antioxidant enzymes, including catalase, and decreases the amount of sodium in grafted plants [69], according to what was observed in our research. Grafting tomato on potato under salinity stress conditions increased the yield of tomato fruit. The interaction between the rootstock and scion can increase the scion’s growth and biomass and also play a role in the distribution of assimilates between the source (leaf, stem, and root) and the sink (fruit) [18]. Rootstock will play an important role in fruit yield and growth, and there are many reports on increasing tolerance to environmental stress in grafted fruit trees. At the same time, pumpkin rootstock and Se were tested for the first time for cucumber salinity tolerance increase (Table 1). Pumpkin rootstock and Se significantly increased plant height, number of leaves, and fruit yield of grafted cucumber, which can be attributed to the increase of compatible osmolytes (proline, total soluble sugars), antioxidant enzymes, and reduction of sodium absorption and transport under salinity stress conditions. Some studies employed foliar Se application and grafting to increase tomato fruit growth, yield, and quality. According to the results, grafting with 2 and 4 µg L-1 of Se resulted in increased cherry tomato yield and nutritional compounds [70].
In salinity stress conditions, Se has increased the amount of photosynthetic pigments, consistent with the results of Hawrylak-Nowak (2009) in cucumber plants [71]. In garlic, by applying 8 mg L-1 of sodium selenate under salinity stress conditions the amount of chlorophyll and carotenoid was increased [32]. The reduction of photosynthetic pigments under salinity stress conditions has been reported in various plants [72], which can be due to the accumulation of sodium ions in chloroplasts, the deterioration of chloroplast and thylakoid membranes, the reduction of enzymes responsible for the synthesis of photosynthetic pigments, the reduction of the stability of pigment-protein complexes due to the presence of ions, the prevention of new chlorophyll biosynthesis due to the synthesis of more proline, the lack of magnesium and potassium ions - as the main elements in the synthesis of chlorophyll, the reduction of the ratio of potassium to sodium, the attack of ROS caused by oxidative stress and peroxidation, the decomposition of chlorophyll and the activation of the chlorophyllase enzyme, and finally the reduction of the content of chlorophyll [73]. The foliar application of Se can increase the content of chlorophyll and carotenoids in the leaves of plants subjected to salinity stress conditions by reducing oxidative tension and preventing the destruction of chlorophyll molecules. Carotenoids have a protective role against oxidative stress and are also effective in detoxifying chlorophyll and reducing the toxic effects of free radicals [74].
Carbohydrates, under stress conditions, in addition to playing a role in osmoregulation, also have a protective role against oxidative stress through free electrons in their structural rings [75]. It appears that using Se to increase soluble carbohydrates production is a viable step in plant protection against oxidative stress. Basil (Ocimum basilicum L.) has also shown a rise in soluble carbohydrates under salinity stress conditions [76].
The findings showed that grafting positively affected the amount of proline in cucumber leaves under salinity stress conditions so that the highest amount of proline was in the grafted cucumber and the treatment of 0 mM of NaCl with 10 mg L-1 of sodium selenate. These findings were consistent with those obtained in tomato [77] and pepper [66]. Proline is one of the compatible substances most plants produce under stress conditions and helps maintain osmotic balance. In fact, the increase of proline in plants under salinity stress is the plant’s reaction to reducing water potential in the root environment [78]. By lowering the osmotic potential of root cells, proline creates water and nutrient absorption conditions. At the same time, proline induces the transcription of salinity stress-resistant proteins so that the plant tolerates salinity stress conditions [79]. Raising the proline level with foliar Se application may help plants resist salinity stress by enhancing the antioxidant defense system. Under salinity stress conditions, Se leads to increased accumulation of some compatible osmolytes, including proline and total soluble sugars [80]. According to our results, Se increased the amount of chlorophyll a and b, proline, and catalase enzyme activity in salinity stress in garlic plant [32].
According to the findings (Table 3), salinity and Se positively affected total soluble protein in grafted cucumber leaf compared to control plants. The highest amount of cucumber leaf protein was observed in the treatment of grafted plants with 100 mM of NaCl and 10 mg L-1 of sodium selenate (Table 3). Selenium enhances the amount of proteins under salinity stress conditions by protecting proteins with sulfhydryl groups and stimulating the nitrate reductase enzyme gene transcription [81]. Under salinity stress conditions, Se enhanced the amount of protein and soluble carbohydrates in wheat (Triticum aestivum L.), according to the studies [41].
According to the results of comparing the averages (Table 3), in high levels of salinity stress and Se, no significant difference in the flavonoid content of cucumber leaf was seen between grafted and non-grafted plants. While with increasing Se concentration and salinity stress, the flavonoid content of cucumber leaf increased (Table 3). Phenolic compounds are essential in inhibiting lipid peroxidation and scavenging free radicals. In salinity stress conditions, the increase of phenolic compounds is directly related to the production of free radicals. The rise in phenolic compounds under salinity stress conditions is a plant stress-resistance mechanism [82]. At the same time, the increase of phenolic compounds under salinity stress conditions is associated with an increase in the production of lignins, which helps to increase the plant’s resistance to stresses [83]. Under salinity stress conditions, Se can play a protective role by activating the production of the phenylalanine ammonia-lyase enzyme, which is necessary for producing phenolic compounds, and lead to an increase in phenolic compounds [84]. According to the results, Se has increased proline, total phenol, flavonoid, and antioxidant enzymes in stevia (Stevia rebaudiana Bertoni) under salinity stress [85]. Flavonoids are essential because of their role in non-enzymatic defense systems. The amount of flavonoids is significantly affected by environmental conditions. When a plant detects stress, its defensive mechanism, which includes flavonoids, is activated and strengthened to deal with the stress [78].
Selenium and salinity stress positively affected peroxidase enzyme activity (Table 3). The maximum amount of peroxidase enzyme activity was detected in the grafted plants with the highest Se concentration and salinity stress. That exhibited a significant difference from the control treatment. The antioxidant system helps protect cells against free radicals. In this study, the activity of antioxidant enzymes such as catalase and peroxidase increased under salinity stress conditions and foliar application of Se (Table 3), following the results obtained in the Stachys byzantina (Stachys byzantine L.) [86]. Environmental stresses are associated with oxidative stress and increased production of ROS, which destroy membranes and peroxidation of lipids. ultimately causing the leakage of materials the cell and cell death. Plants can clear these free radicals against oxidative stress using their antioxidant system, which includes ascorbate peroxidase, catalase, superoxide dismutase, peroxidase, and non-enzymatic antioxidants like ascorbate, glutathione, and alphatocopherol, according to the findings of this study [78]. By increasing the activity of antioxidant enzymes, Se causes the removal of active oxygen and, as a result, reduces the oxidation of lipids in membranes and the surface of malondialdehyde [87]. Selenium suppresses the enzymes glutathione peroxidase and hydrogen peroxide, then the enzymes ascorbate peroxidase, catalase, and glutathione reductase clean up the hydrogen peroxide residues [88].
The highest RWC of cucumber leaf was seen in the highest concentration of Se (10 mg L-1 sodium selenate) in all three degrees of salinity stress. Selenium increased the RWC of cucumber leaf under salinity stress conditions (Fig. 2). Measuring the RWC is one indicator that estimates the plant’s resistance to salinity stress. Salinity lowers the cultivation bed’s water potential, affects the volume of water the cucumber roots can absorb, and ultimately lowers the RWC. Selenium helps to maintain the osmotic pressure of cucumber in salinity stress by producing compatible osmolytes. The production of compatible osmolytes reduces the osmotic pressure inside the cell, which helps keep the water inside and prevents the cell from drying out. By helping to absorb water from the soil solution, it increases the water pressure and the relative amount of RWC [89].
In all three concentrations of salinity stress, with increasing Se concentration, the amount of electrolyte leakage of cucumber leaf has decreased (Fig. 3). In salinity stress, electrolyte leakage decreases as antioxidant activity increases, and Se reduces electrolyte leakage by protecting cell membranes [90]. Because the cell membrane is a primary target in many environmental stresses, including salinity, membrane stability under stress conditions is one of the indicators of tolerance [91]. Therefore, measuring the amount of electrolyte leakage is one of the good indicators for measuring the amount of oxidative damage to the membrane. Due to the cytoplasmic membrane’s vulnerability, the cell’s contents leak out, and the amount of this damage is determined by measuring electrolyte leakage [92].
The fundamental strategy for regulating solute accumulation in the plant is reducing sodium transport from the root to the air organ and absorbing more potassium than sodium. Salinity lowers potassium levels in cucumber plant root (Table 4); indeed, one of the harmful impacts of salinity is the disruption of potassium absorption. The reduction of potassium absorption due to the presence of the competing sodium ion is due to the similarity of the size of the hydrated radius of these two ions [62]. As a result, their transfer proteins are misdiagnosed. Because of the presence of high levels of sodium in the surrounding environment of the roots during salinity stress, in addition to disrupting potassium absorption and causing damage to the root membranes, the selective selection of these membranes also changes [93]. Potassium is one of the most abundant elements in plants, and it is required to form proteins, enzymes, and photosynthesis. It plays a role in regulating osmotic potential, and with increasing pH and sodium, its availability for plants decreases [91]. Under condition of salinity stress, Se increases potassium. It decreases the amount of sodium in the seedling index by binding sodium to the root cell wall, reducing salinity stress damage. Sodium ion blocks potassium ion transport channels on the membrane surface, while Se can affect gene expression of sodium transporters and hydrogen pumps. The appropriate Se concentration can increase the expression of tonoplasty H+ ATPase and Na+/H+ antiport in the root membrane, reducing sodium ion transport to the restricted aerial parts and toxicity [87]. According to the findings (Table 4), the root contains higher sodium than the cucumber leaf that, according to the observations of Gou et al. [64]., in grafted cucumber on rootstock pumpkin lowers sodium transfers from root to leaf (Table 4).
Conclusions
Considering that for the first time the effect of selenium and transplantation on salinity tolerance of greenhouse cucumber was studied, the results showed that pumpkin has more tolerance to salt stress than cucumber, which is accompanied by increase in growth characteristics, compatible osmolytes (proline, total soluble sugars), compounds (total phenol, flavonoid), antioxidant enzymes (catalase) and accumulation potassium of cucumber leaf. Selenium, along with grafting, had an effective role in increasing salt tolerance in cucumber. As a result, it can be said that the use of pumpkin rootstock and 10 mg L-1 of sodium selenate is a good strategy to tolerate salinity and improve the quality and yield of grafting cucumber plants.
Data availability
All data are available in the manuscript file.
References
Feng D, Gao Q, Liu J, Tang J, Hua Z, Sun X. Categories of exogenous substances and their effect on alleviation of plant salt stress. Eur J Agron. 2023;142:126656. https://doi.org/10.1016/j.eja.2022.126656.
Hassani A, Azapagic A, Shokr N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat Commun. 2021;12(1):6663. https://doi.org/10.1038/s41467-021-26907-3.
Okur B, Örçen N. Soil salinization and climate change. In: Prasad MNV, Pietrzykowski M, editors. Climate Change and Soil interactions. Amsterdam, The Netherlands: Elsevier; 2020. pp. 331–50.
Zhao S, Zhang Q, Liu M, Zhou H, Ma C, Wang P. Regulation of plant responses to salt stress. Int J Mol Sci. 2021;22:1–16. https://doi.org/10.3390/ijms22094609.
Abdelaal Kh. Cucumber grafting onto pumpkin can represent an interesting tool to minimize salinity stress. Physiological and anatomical studies. Middle East J Agric Res. 2017;6:953–75. https://www.researchgate.net/publication/323883911.
Savvas D, Papastavrou D, Ntatsi G, Ropokis A, Olympios C, Hartmann H, Schwarz D. Interactive effects of Grafting and Manganese Supply on Growth, Yield, and nutrient uptake by Tomato. HortScience Horts. 2009;44(7):1978–82. https://doi.org/10.21273/HORTSCI.44.7.1978.
Ntatsi G, Savvas D, Ntatsi G, Kläring H, Schwarz D. Growth, yield, and metabolic responses of temperature-stressed Tomato to Grafting onto rootstocks differing in Cold Tolerance. J Am Soc Hortic Sci. 2014;139(2):230–43. https://doi.org/10.21273/JASHS.139.2.230.
Ntatsi G, Savvas D, Papasotiropoulos V, Katsileros A, Zrenner RM, Hincha DK, Zuther E, Schwarz D. Rootstock Sub-optimal Temperature Tolerance determines transcriptomic responses after Long-Term Root cooling in Rootstocks and scions of grafted tomato plants. Front Plant Sci. 2017;8:911. https://doi.org/10.3389/fpls.2017.00911.
Savvas D, Öztekin GB, Tepecik M, Ropokis A, Tüzel Y, Ntatsi G, Schwarz D. Impact of grafting and rootstock on nutrient-to-water uptake ratios during the first month after planting of hydroponically grown tomato. J Hortic Sci Biotechnol. 2017;92(3):294–302. https://doi.org/10.1080/14620316.2016.1265903.
Fu X, Feng YQ, Zhang XW, Zhang YY, Bi HG, Ai XZ. Salicylic acid is involved in rootstock–Scion communication in improving the Chilling Tolerance of Grafted Cucumber. Front. Plant Sci. 2021;12:1–16. https://doi.org/10.3389/fpls.2021.693344.
Jang Y, Moon JH, Kim SG, Kim T, Lee OJ, Lee HJ, Wi SH. Effect of low-temperature tolerant rootstocks on the growth and Fruit Quality of Watermelon in Semi-forcing and Retarding Culture. Agronomy. 2023;13:1–17. https://doi.org/10.3390/agronomy13010067.
Yetisir H, Uygur V. Responses of grafted watermelon onto different gourd species to salinity stress. J Plant Nutr. 2010;33:315–27. https://doi.org/10.1016/j.hpj.2018.08.003.
Huang Y, Bie Z, He S, Hua B, Zhen A, Liu Z. Improving cucumber tolerance to major nutrients induced salinity by grafting onto Cucurbita ficifolia. Environ Ex Bot. 2011;69:32–8. https://doi.org/10.1016/j.envexpbot.2010.02.002.
Rouphael Y, Edelstein M, Savvas D, Colla G, Ntatsi G, Kumar P, Schwarz D. Grafting as a Tool for Tolerance of Abiotic stress. Vegetable Grafting: Principles and Practices. 2017;171–215. https://doi.org/10.1079/9781780648972.0171.
Ropokis A, Ntatsi G, Kittas C, Katsoulas N, Savvas D. Impact of Cultivar and Grafting on Nutrient and Water Uptake by Sweet Pepper (Capsicum annuum L.) grown hydroponically under Mediterranean climatic conditions. Front. Plant Sci. 2018;9:1244. https://doi.org/10.3389/fpls.2018.01244.
Consentino BB, Rouphael Y, Ntatsi G, Pasquale CD, Iapichino G, D’Anna F, Bella SL, Sabatino L. Agronomic performance and fruit quality in greenhouse grown eggplant are interactively modulated by iodine dosage and grafting. Sci Hortic. 2022;295:110891. https://doi.org/10.1016/j.scienta.2022.110891.
Parthasarathi TE, Ephrath J, Lazarovitch N. Grafting of tomato (Solanum lycopersicum L.) onto potato (Solanum tuberosum L.) to improve salinity tolerance. Sci Hortic. 2021;282:1–9. https://doi.org/10.1016/j.scienta.2021.110050.
Lu K, Sun J, Li Q, Li X, Jin S. Effect of cold stress on growth, physiological characteristics, and Calvin-Cycle-related gene expression of grafted Watermelon seedlings of different Gourd rootstocks. Horticulturae. 2021;7:1–13. https://doi.org/10.3390/horticulturae7100391.
Lόpez-Gόmez E, San Juan MA, Diaz-Vivancos P, Mataix beneyto J, Garcia-Legaz MF, Hernández JA. Effect of rootstocks grafting and boron on the antioxidant system and salinity tolerance on Ioqut plants (Eriobotyra Japonica Lidl). Environ Exp Bot. 2007;60:151–8. https://doi.org/10.1016/j.envexpbot.2006.10.007.
Zhu J, Bie Z, Huang Y, Han X. Effect of grafting on the growth and ion concentrations of cucumber seedlings under NaCl stress. J Soil Sci Plant Nutr. 2008;54:895–902. https://doi.org/10.1111/j.1747-0765.2008. 00306.x.
Etehadnia M, Waterer D, De Jong H, Tanino KK. Scion and Rootstock effects on ABA-mediated plant growth regulation and salt tolerance of acclimated and unacclimated potato genotypes. J Plant Growth Regul. 2008;27:125–40. https://doi.org/10.1007/s00344-008-9039-6.
Shaterian J, Georges F, Hussain A, Tanin KK. Root to shoot communication and abscisic acid in calreticulin (CR) gene expression and salt stress tolerance in grafted diploid potato (Slanum sp.) clones. Environ Ex Bot. 2005;53:323–32. https://doi.org/10.1016/j.envexpbot.2004.04.008.
Ulas F, Alim Aydın AU, Halit Y. Grafting for sustainable growth performance of melon (Cucumis melo) under salt stressed hydroponic condition. EJSD. 2019;8:201–210. https://doi.org/10.14207/ejsd.2019.v8n1p201.
Singh H, Kumar P, Kumar A, Kyriacou MC, Colla G, Rouphael Y. Grafting tomato as a tool to improve salt tolerance. Agronomy. 2020;10:1–22. https://doi.org/10.3390/agronomy10020263.
Yanyan Y, Shuoshuo W, Min W, Biao G, Qinghua SHI. Effect of different rootstocks on the salt stress tolerance in watermelon seedlings. Hortic Plant J. 2018;4:239–49. https://doi.org/10.1016/j.hpj.2018.08.003.
Colla G, Rouphae Y, Reac E, Cardarelli M. Grafting cucumber plants enhance tolerance to sodium chloride and sulfate salinization. Sci Hortic. 2012;135:177–85. https://doi.org/10.1016/j.scienta.2011.11.023.
Elsheery NI, Helaly MN, Omar SO, John SVS, Zabochnicka-Swiątek M, Kalaji HM, Rastogi A. Physiological and molecular mechanisms of salinity tolerance in grafted cucumber. S Afr J Bot. 2020;130:90–102. https://doi.org/10.1016/j.sajb.2019.12.014.
Santa-Cruz A, Martinez-Rodriguez MM, Perez-Alfocea F, Romero-Aranda R, Bolarin MC. The rootstock effect on the tomato salinity response depends on the shoot genotype. Plant Sci. 2002;162:825–31. https://doi.org/10.1016/S0168-9452(02)00030-4.
Bayoumi Y, Abd-Alkarim E, El-Ramady H, El-Aidy F, Hamed ES, Taha N, Prohens J, Rakha M. Grafting improves Fruit Yield of Cucumber plants grown under combined heat and soil salinity stresses. Horticulturae. 2021;7:1–14. https://doi.org/10.3390/horticulturae7030061.
Huang Y, Tang R, Cao Q, Bie Z. Improving the fruit yield and quality of cucumber by grafting onto the salt tolerant rootstock under NaCl stress. Sci Hortic. 2009;122:26–31. https://doi.org/10.1016/j.scienta.2009.04.004.
Guo Z, Qin Y, Lv J, Wang X, Dong H, Dong X, Zhang T, Du N, Piao F. Luffa rootstock enhances salt tolerance and improves yield and quality of grafted cucumber plants by reducing sodium transport to the shoot. Environ Pollut. 2023;316:1–16. https://doi.org/10.1016/j.envpol.2022.120521.
Khademi Astaneh R, Bolandnazar S, Zaare Nahandi F, Oustan S. Effects of selenium on enzymatic changes and productivity of garlic under salinity stress. S Afr J Bot. 2019;121:447–55. https://doi.org/10.1016/j.sajb.2018.10.037.
Motesharezadeh B, Ghorbani S, Alikhani HA, Fatemi R, Ma Q. Investigation of different selenium sources and supplying methods for Selenium Enrichment of Basil vegetable (a Case Study under Calcareous and Non-calcareous Soil systems). Recent Pat Food Nutr Agric. 2020;12:73–82. https://doi.org/10.2174/2212798411666200611101032.
Genchi G, Lauria G, Catalano A, Sinicropi MS, Carocci A. Biological Activity of Selenium and its impact on Human Health. Int J Mol Sci. 2023;24(3):1–19. https://doi.org/10.3390/ijms24032633.
Saleem MF, Kamal MA, Shahid M, Saleem A, Shakeel A, Anjum Sh. A. Exogenous Selenium-Instigated Physiochemical transformations Impart Terminal Heat Tolerance in BT Cotton. J Soil Sci Plant Nutr. 2020;20:274–83. https://doi.org/10.1007/s42729-019-00139-3.
Wu C, Dun Y, Zhang Z, Li M, Wu G. Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil. Ecotoxicol Environ Saf. 2020;190:110091. https://doi.org/10.1016/j.ecoenv.2019.110091.
Golob A, Novak T, Marsic NK, Sircelj H, Stibij V, Jersa A, Kroflic A, Germ M. Biofortification with selenium and iodine changes morphological properties of Brassica oleracea L. var. Gongylodes and increases their contents in tubers. Plant Physiol Biochem. 2020;150:234–43. https://doi.org/10.1016/j.plaphy.2020.02.044.
Ali J, Jan IU, Ullah H. Selenium supplementation affects vegetative and yield attributes to escalate drought tolerance in okra. Sarhad J Agric. 2020;36:120–9. https://doi.org/10.17582/journal.sja/2020/36.1.120.129.
Elkelisha AA, Soliman MH, Alhaithlould HA, El-Esawie MA. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol Biochem. 2019;137:144–53. https://doi.org/10.1016/j.plaphy.2019.02.004.
Lan CY, Lin KH, Huang WD, Chen CC. Protective effects of selenium on wheat seedlings under salt stress. Agronomy. 2019;9:1–14. https://doi.org/10.3390/agronomy9060272.
Desoky E-SM, Merwad A-RMA, Abo El-Maati MF, Mansour E, Arnaout SMAI, Awad MF, Ramadan MF, Ibrahim SA. Physiological and biochemical mechanisms of exogenously Applied Selenium for Alleviating Destructive impacts Induced by salinity stress in Bread Wheat. Agronomy. 2021;11:1–18. https://doi.org/10.3390/agronomy11050926.
Rasool A, Shah WH, Mushtaq NU, Saleem S, Hakeem KR, ul Rehman R. Amelioration of salinity induced damage in plants by selenium application: a review. S Afr J Bot. 2022;147:98–105. https://doi.org/10.1016/j.sajb.2021.12.029.
Regni L, Palmerini CA, Del Pino AM, Businelli D, D’Amato R, Mairech H, Marmottini F, Micheli M, Pacheco PH, Proietti P. Effects of selenium supplementation on olive under salt stress conditions. Sci Hortic. 2021;278:109866. https://doi.org/10.1016/j.scienta.2020.109866.
Farag HAS, Ibrahim MFM, El-Yazied AA, El-Beltagi HS, El-Gawad HGA, Alqurashi M, Shalaby TA, Mansour AT, Alkhateeb AA, Farag R. Applied Selenium as a powerful antioxidant to mitigate the Harmful effects of salinity stress in snap Bean seedlings. Agronomy. 2022;12:1–19. https://doi.org/10.3390/agronomy12123215.
Wu H, Fan S, Gong H, Guo J. Roles of salicylic acid in selenium-enhanced salt tolerance in tomato plants. Plant Soil. 2022;484:569–88. https://doi.org/10.21203/rs.3.rs-1857198/v1.
Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–82. https://doi.org/10.1016/0076-6879(87)48036-1.
Schonfeld MA, Johnson RC, Carver BF, Mornhinweg DW. Water relations in winter wheat as drought resistance indicators. Crop Sci. 1988;28(3):526–31. https://doi.org/10.2135/cropsci1988.0011183X002800030021x.
Ben Hamed K, Castagna A, Salem EA, Ranieri A, Abdelly C. Sea fennel (Crithmum Maritimum L.) under salinity conditions: a comparison of leaf and root antioxidant responses. Plant Growth Regul. 2007;53:185–94. https://doi.org/10.1007/s10725-007-9217-8.
Irigoyen JJ, Einerich DW, Sanchez-Diaz M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa L.) plants. Physiol Plant. 1992;84:55–60. https://doi.org/10.1111/j.1399-3054.1992.tb08764.x.
Paquin R, Lechasseur P. Observation sur une method de dosage de la proline libre dans les extrats deplants. Canad J Bot. 1979;75:1851–4. https://doi.org/10.1139/b79-233.
Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–53. https://doi.org/10.5344/ajev.1965.16.3.144.
Bor JY, Chen HY, Yen G. Ch. Evaluation of antioxidant activity and inhibitory effect on nitric oxide production of some common vegetables. J Agric Food Chem. 2006;54:1680–6. https://doi.org/10.1021/jf0527448.
Bradford MM. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. https://doi.org/10.1016/0003-2697(76)90527-3.
Bergmeyer HU. Methods of enzymatic analysis. Berlin, Germany: Akademie Verlag; 1970. pp. 636–47.
Herzog V, Fahimi HD. A new sensitive colorimetric assay for peroxidase using 3,3’-diaminobenzidine as hydrogen donor. Anal Biochem. 1973;55:554–62. https://doi.org/10.1016/0003-2697(73)90144-9.
AOAC. Official method of analysis. Association of official analytical. Chemists, washington, DC: USA; 1990.
Hasanuzzaman M, Anwar Hossain M, Fujita M. Selenium in higher plants: physiological role, antioxidant metabolism and abiotic stress tolerance. J. Plant Sci. 2010;5:354–75. https://doi.org/10.3923/jps.2010.354.375.
Paris P, Matteo GD, Tarchi M, Tosi L, Spaccino L, Lauteri M. Precision subsurface drip irrigation increases yield while sustaining water use efficiency in Mediterranean poplar bioenergy plantations. For Ecol Manag. 2018;409:749–56. https://doi.org/10.1016/j.foreco.2017.12.013.
Yaldiz G, Camlica M. Selenium and salt interactions in sage (Salvia officinalis L.): growth and yield, chemical content, ion uptake. Ind Crops Prod. 2021;171:113855. https://doi.org/10.1016/j.indcrop.2021.113855.
Alinejadian Bidabadi A, Hasani M, Maleki A. The effect of amount and salinity of water on soil salinity and growth and nutrients concentration of spinach in a pot experiment. Iran J Soil Water Res. 2018;49:641–51. https://doi.org/10.22059/ijswr.2017.236843.667714.
Semida WM, Abd El-Mageed TA, Abdelkhalik A, Hemida KA, Abdurrahman HA, Howladar SM, Leilah AAA, Rady MOA. Selenium modulates antioxidant activity, osmoprotectants, and photosynthetic efficiency of onion under saline soil conditions. Agronomy. 2021;11:1–18. https://doi.org/10.3390/agronomy11050855.
Karaca C, Aslan GE, Buyuktas D, Kurunc A, Bastug R, Navarro A. Effects of salinity stress on drip-irrigated tomatoes grown under Mediterranean-Type Greenhouse conditions. Agronomy. 2023;13:1–18. https://doi.org/10.3390/agronomy13010036.
Shalaby TA, Abd-Alkarim E, El-Aidy F, Hamed ES, Sharaf-Eldin M, Taha N, El-Ramady H, Bayoumi Y, Dos Reis AR. Nano-selenium, silicon and H2O2 boost growth and productivity of cucumber under combined salinity and heat stress. Ecotoxicol Environ Saf. 2021;212:1–9. https://doi.org/10.1016/j.ecoenv.2021.111962.
Admasie MA, Kurunc A, Cengiz MF. Effects of exogenous selenium application for enhancing salinity stress tolerance in dry bean. Sci Hortic. 2023;320:112238. https://doi.org/10.1016/j.scienta.2023.112238.
Gou T, Yang L, Hu W, Chen X, Zhu Y, Guo J, Gong H. Silicon improves the growth of cucumber under excess nitrate stress by enhancing nitrogen assimilation and chlorophyll synthesis. Plant Physiol Biochem. 2020;152:53–61. https://doi.org/10.1016/j.plaphy.2020.04.031.
Farajimanesh A, Haghighi M, Mobli M. The Effect of different endemic cucurbita rootstocks on water relation and physiological changes of grafted cucumber under salinity stress. Int J Hortic Sci Technol. 2016;17:351–68. http://journal-irshs.ir/article-1-210-en.html.
Kacjan Maršić N, Štolfa P, Vodnik D, Košmelj K, Mikulič-Petkovšek M, Kump B, Vidrih R, Kokalj D, Piskernik S, Ferjančič B, Dragutinović M, Veberič R, Hudina M, Šircelj H. Physiological and biochemical responses of ungrafted and Grafted Bell Pepper Plants (Capsicum annuum L. var. Grossum (L.) Sendtn.) Grown under moderate salt stress. Plants. 2021;10(2):314. https://doi.org/10.3390/plants10020314.
Penella C, Nebauer SG, Quinones A, San Bautista A, Lopez-Galarza S, Calatayud A. Some rootstocks improve pepper tolerance to mild salinity through ionic regulation. Plant Sci. 2015;230:12–22. https://doi.org/10.1016/j.plantsci.2014.10.007.
Ulas F. Effects of grafting on growth, root morphology and leaf physiology of pepino (Solanum muricatum Ait.) As affected by salt stress under hydroponic conditions. int J Agric Environs food sci. 2021;5:203–12. https://doi.org/10.31015/jaefs.2021.2.10.
Sanwal SK, Man A, Kumar A, Kesh H, Kaur G, Rai AK, Kumar R, Sharma PC, Kumar A, Bahadur A, Singh B, Kumar P. Salt Tolerant Eggplant rootstocks modulate sodium partitioning in Tomato Scion and improve performance under saline conditions. Agriculture. 2022;12:1–15. https://doi.org/10.3390/agriculture12020183.
Sabatino L, La Bella S, Ntatsi G, Iapichino G, D’Anna F, De Pasquale C, Consentino BB, Rouphael Y. Selenium biofortification and grafting modulate plant performance and functional features of cherry tomato grown in a soilless system. Sci Hortic. 2021;285:110095. https://doi.org/10.1016/j.scienta.2021.110095.
Hawrylak-Nowak B. Beneficial effects of Exogenous Selenium in Cucumber seedlings subjected to salt stress. Biol Trace Elem Res. 2009;132:259–69. https://doi.org/10.1007/s12011-009-8402-1.
Afkari A, Farajpour P. Evaluation of the effect of vermicompost and salinity stress on the pigments content and some biochemical characteristics of borage (Borago Officinalis L.). JPEP. 2019;14(54):90. https://ecophysiologi.gorgan.iau.ir/article_668078.html?lang=en.
Shah SH, Houborg R, McCabe MF. Response of chlorophyll, carotenoid and SPAD-502 measurement to salinity and nutrient stress in wheat (Triticum aestivum L). Agronomy. 2017;7:1–20. https://doi.org/10.3390/agronomy7030061.
Hawrylak-Nowak B. Selenite is more efficient than selenate in alleviation of salt stress in lettuce plants. Acta Biol Crac Ser Bot. 2015;57:49–54. https://doi.org/10.1515/abcsb-2015-0023.
Labanowska M, Filek M, Kurdziel M, Bidzińska E, Miszalski Z, Hartikainen H. EPR spectroscopy as a tool for investigation of differences in radical status in wheat plants of various tolerances to osmotic stress induced by NaCl and PEGtreatment. J. Plant Physiol. 2013;170:136–45. https://doi.org/10.1016/j.jplph.2012.09.013.
de Oliveira Sousa VF, Dias TJ, Henschel JM, Júnior SDOM, Batista DS, Linné JA, Targino VA, da Silva RF. Castor bean cake increases osmoprotection and oil production in basil (Ocimum basilicum) under saline stress. Sci Hortic. 2023;309:111687. https://doi.org/10.1016/j.scienta.2022.111687.
Koleška I, Hasanagi ́c D, Oljaˇca R, Murti ́c S, Bosanˇci ́c B. Todorovi ́c V. influence of grafting on the copper concentration in tomato fruits under elevated soil salinity. AГРОЗНAЊЕ. 2019;20:37–44. https://doi.org/10.7251/AGREN1901037K.
Karimi R, Ebrahimi M, Amerian M. Abscisic acid mitigates NaCl toxicity in grapevine by influencing phytochemical compounds and mineral nutrients in leaves. Sci Hortic. 2021;288:1–10. https://doi.org/10.1016/j.scienta.2021.110336.
Khedr AHA, Abbas MA, Wahid AAA, Quick WP, Abogadallah GM. Proline induces the expression of salt-stress-responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt stress. J Exp Bot. 2003;54:2553–62. https://doi.org/10.1093/jxb/erg277.
Sattar A, Cheema MA, Abbas T, Sher A, Ijaz Wasaya A, Yasir TA, Abbas T, Hussain M. Foliar applied silicon improves water relations, stay green and enzymatic antioxidants activity in late sown wheat. Silicon. 2020;12:223–30. https://doi.org/10.1007/s12633-019-00115-7.
Nowak J, Kaklewski K, Ligocki M. Influence of selenium on oxidoreductive enzymes activity in soil and in plants. Soil Biol Biochem. 2004;36:1553–8. https://doi.org/10.1016/j.soilbio.2004.07.002.
Pietrak A, Salachna P, Łopusiewicz Ł. Changes in growth, ionic status, metabolites content and antioxidant activity of two FernsExposed to Shade, full sunlight, andSalinity. Int J Mol Sci. 2023;24:1–15. https://doi.org/10.3390/ijms24010296.
Shekari F, Abbasi A, Mustafavi SH. Effect of silicon and selenium on enzymatic changes and productivity of dill in saline condition. J Saudi Soc Agric Sci. 2017;16:367–74. https://doi.org/10.1016/j.jssas.2015.11.006.
Walaa AE, Shatlah MA, Atteia MH, Sror HAM. Selenium induces antioxidant defensive enzymes and promotes tolerance against salinity stress in cucumber seedlings (Cucumis sativus). Arab Univ J Agric Sci. 2010;18:65–76. https://doi.org/10.21608/ajs.2010.14917.
Sheikhalipour M, Esmaielpour B, Gohari G, Haghighi M, Jafari H, Farhadi H, Kulak M, Kalisz A. Salt stress mitigation via the Foliar application of Chitosan-Functionalized Selenium and Anatase Titanium Dioxide nanoparticles in Stevia (Stevia rebaudiana Bertoni). Molecules. 2021;26:1–20. https://doi.org/10.3390/molecules26134090.
Sharifi P, Amirnia R, Torkian M, Shirani Bidabadi S. Protective role of exogenous selenium on salinity-stressed Stachys byzantine plants. J Soil Sci Plant Nutr. 2021;21:2660–72. https://doi.org/10.1007/s42729-021-00554-5.
Xu S, Zhao N, Qin D, Liu S, Jiang S, Xu L, Sun Z, Yan D, Hu A. The synergistic effects of silicon and selenium on enhancing salt tolerance of maize plants. Environ Exp Bot. 2021;187:104482. https://doi.org/10.1016/j.envexpbot.2021.104482.
Rahman M, Rahman K, Sathi KS, Alam MM, Nahar K, Fujita M, Hasanuzzaman M. Supplemental Selenium and Boron Mitigate Salt-Induced oxidative damages in Glycine Max L. Plants. 2021;10:1–16. https://doi.org/10.3390/plants10102224.
Levent Tuna A, Kaya C, Dikilitas M, Yokas IB, Burun B, Altunlu H. Comparative effects of various salicylic acid derivatives on key growth parameters and some enzyme activities in salinity stressed maize (Zea mays L.) plants. Pak. J. Bot. 2007;39:787–798. https://hdl.handle.net/20.500.12809/5077.
Khademi Astaneh R, Bolandnazar S, Zaare Nahandi F, Oustan S. Effects of selenium on enzymatic changes and productivity of garlic under salinity stress. S Afr J Bot. 2021;121:447–55. https://doi.org/10.1016/j.sajb.2018.10.037.
Liu H, Xiao C, Qiu T, Deng J, Cheng H, Cong X, Cheng S, Rao S, Zhang Y. Selenium regulates antioxidant, photosynthesis, and cell permeability in plants under various Abiotic stresses: a review. Plants. 2023;12:1–17. https://doi.org/10.3390/plants12010044.
Jouyban Z. The effect of salt stress on plant growth. J Eng Appl Sci. 2012;2:7–10. https://api.semanticscholar.org/CorpusID:39278033.
Acknowledgements
The authors of the article are grateful to Razi University and Agricultural University of Athens.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
M.A. supervised the experiments, wrote and critically revised the manuscript. A.P. performed the experiments. G.G. analyzed the data. G.N. con-tributed to project administration, investigation and revision of the manuscript. All authors have read and agreed to the published version of the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Amerian, M., Palangi, A., Gohari, G. et al. Enhancing salinity tolerance in cucumber through Selenium biofortification and grafting. BMC Plant Biol 24, 24 (2024). https://doi.org/10.1186/s12870-023-04711-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04711-z