Abstract
Background
WRINKLED1 (WRI1) encodes a transcription factor, belonging to the APETALA2 (AP2) family, and plays a key role in regulating plant oil biosynthesis. As a newly woody oil crop, tree peony (Paeonia rockii) was notable for the abundant unsaturated fatty acids in its seed oil. However, the role of WRI1 during the accumulation of P. rockii seeds oil remains largely unknown.
Results
In this study, a new member of the WRI1 family was isolated from P. rockii and was named PrWRI1. The ORF of PrWRI1 consisted of 1269 nucleotides, encoding a putative protein of 422 amino acids, and was highly expressed in immature seeds. Subcellular localization analysis in onion inner epidermal cells showed that PrWRI1 was located at the nucleolus. Ectopic overexpression of PrWRI1 could significantly increase the total fatty acid content in Nicotiana benthamiana leaf tissue and even PUFAs in transgenic Arabidopsis thaliana seeds. Furthermore, the transcript levels of most genes related to fatty acids (FA) synthesis and triacylglycerol (TAG) assembly were also up-regulated in transgenic Arabidopsis seeds.
Conclusions
Together, PrWRI1 could push carbon flow to FA biosynthesis and further enhance the TAG amount in seeds with a high proportion of PUFAs.
Similar content being viewed by others
Background
Fatty acids are important structural components for cells and provide the necessary energy for human beings. According to the presence or absence of double bonds in its long aliphatic chain, fatty acids could be classified into two major groups, including saturated and unsaturated fatty acids (UFAs). There is growing scientific evidence showing that the supply of UFAs, especially n-3 polyunsaturated fatty acids (PUFAs) could reduce the risk of coronary heart disease [1, 2]. The most common n-3 PUFAs in plant oil are α-linolenic acid (ALA). ALA is mainly found in canola (5%), soybean (2%), and walnut oils (1%) [3]. Recently, the tree peony seeds were identified as a novel source of edible oil with abundant UFAs (> 90%), and a high proportion of ALA (> 40%), which is much higher than many other plant oils [4,5,6]. Therefore, tree peony could be regarded as an excellent model for investigating the synthesis and production of ALA.
In plants, lipid accumulation mainly includes fatty acid (FA) synthesis and triglyceride (TAG) assembly. Particularly, sucrose is converted into pyruvate via glycolysis with pyruvate kinase (PK) catalyzing the final step, which provides precursors for fatty acid production [7]. Then, the acetyl-CoA is rapidly generated from pyruvate by the action of the plastidial pyruvate dehydrogenase complex (PDHC) to maintain the de novo fatty acid biosynthesis [8]. In the first step, the formation of malonyl-CoA from acetyl-CoA was catalyzed by the biotin carboxyl carrier protein (BCCP) [9]. Then, malonyl-CoA was converted to malonyl-ACP with the action of malonyl-CoA:ACP transacylase (MCAT) and reduced by enoyl-ACP reductase (ENR). After the series of subsequential condensation reactions driven by 3-ketoacyl-ACP synthases isoform (KAS), 16:0-ACP or 18:0-ACP were formed and hydrolyzed by thioesterases (FATA/FATB) for export from the plastid to the acyl-CoAs pool [10]. In the endoplasmic reticulum (ER), acyl-CoAs are used for the sequential acylation of glycerol-3-phosphate (G3P) backbone to produce TAGs either by Kennedy pathway, including G3P acyltransferase (GPAT), lysophosphatidic acid acyltransferase (LPAT), and diacylglycerol acyltransferase (DGAT) or by acyl exchange from phosphatidylcholine (PC) to diacylglycerol (DAG) by the phospholipid: diacylglycerol acyltransferase (PDAT) [11,12,13].
Transcription factors (TFs) such as Leafy cotyledon 1 (LEC1), Leafy cotyledon 2 (LEC2), Abscisic and insensitive 3 (ABI3), FUSCA 3 (FUS3), and Wrinkled 1 (WRI1) also were found played an important role in regulating the biosynthesis of Arabidopsis seed oil [14,15,16]. One of the most important TFs is WRI1, which is located in the downstream of lipid regulation network and was recently identified as a target of KIN10, the major SUCROSE NON-FERMENTATION1-RELATED KINASE1 involved in sugar/energy homeostasis [15, 17]. WRI1, belonging to APETALA2 (AP2) transcription factor family, was first identified in the Arabidopsis mutant line with wrinkled seed and 80% fewer TAGs compared to the wild type [18]. The genes regulated by WRI1 were involved in glycolysis, lipoic acid, and FA biosynthesis pathways [19]. And, it has been demonstrated that WRI1 is able to bind to the promoters of genes encoding key enzymes including biotin carboxyl carrier protein isoform 2 (BCCP2), acyl carrier protein 1 (ACP1), and keto-ACP synthase 1 (KAS1), which provide precursors (acyl chain and glycerol backbones) for various lipid biosynthetic pathways [20, 21]. And, the overexpression of AtWRI1or its orthologs from rapeseed (Brassica napus L.), corn (Zea mays L.), and oil palm (Elaeisguineensis Jacq), has led to varying degrees of increases in oil accumulation of seeds [22,23,24]. And, the recent findings showed that WRI1 is the key regulator of oil biosynthesis in P. ostii developing endosperm [25, 26]. However, the role of WRI1 during the accumulation of P. rockii seeds oil has not been uncovered.
In the present study, we investigated the role of WRI1 in the seeds of P. rockii with high UFAs. Its expression patterns in different tissues and seed development stages were also analyzed. Further, PrWRI1 was cloned and characterized by transient expression in the leaves of Nicotiana benthamiana and Arabidopsis using a stable transformation approach. Over-expression of PrWRI1 in Arabidopsis increased the total fatty acids, mainly UFAs in the seeds. Furthermore, the transcript level of genes involved in acyl editing and transfer pathways also increased in the transgenic Arabidopsis. Identification and characterization of the PrWRI1 would be meaningful for the genetic improvement of oil crops and could also lay foundations for the synthetic biology of FAs.
Results
Isolation and structural analysis of PrWRI1
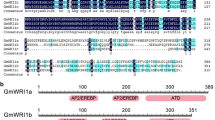
The full-length cDNA sequence of PrWRI1 was identified by sequence similarity search of the AtWRI1 encoding sequence against the P. rockii transcriptome assembly and named as PrWRI1. The ORF of PrWRI1 consisted of 1269 nucleotides, and it encodes a putative protein of 422 amino acids with a predicted molecular mass of 46.93 kDa and a pI of 5.52. The deduced amino acid sequence analysis showed that this protein had two typical AP2/ERF DNA-BD at 56–125 amino acid (aa) and 159–222 aa. Additionally, PrWRI1 has conserved YRG and RAYD residues in two AP2/ERF domains, which suggests that PrWRI1 might belong to the AP2 subfamily (Fig. 1).
There is a high identity in the conserved AP2 domains of PrWRI1, when compared with those of BnWRI1 (ADO16346.1), AtWRI1 (AAP80382.1), PtWRI1 (XP_002311921.2), and GmWRI1 (XP_006596986.1). But, the sequences at the C-terminal regions are diverged (Fig. 2A). Phylogenetic analysis indicated that the PrWRI1s are classified into the same group with those of Arabidopsis and B. napus among the various WRI1s that are currently identified from plants (Fig. 2B). And, PrWRI1 has a much closer relationship with GmWRI1 (soybean).
A Comparison of the deduced amino acid sequences for AP2/ERF-related proteins that have high sequence similarity with PrWRI1. Amino acids that are the all same among five different species are shown in red background. The conserved signature motifs are highlighted by blue boxes. The AP2/ERF domains were marked with black boxes. B Phylogenic comparison of the PrWRI1 protein and some AP2/ERF-related protein sequences based on the selected AP2/ERF domain amino acid sequences for those proteins
PrWRI1 expression in different tissues and developing seeds
To gain further insights to the organ-specific expression of PrWRI1, the expression patterns of PrWRI1 in different tissues of tree peony were determined using quantitative Real-time PCR (qRT-PCR). In non-seed tissues, PrWRI1 was higher expressed in root and pistil than in others (Fig. 3A). As shown in Fig. 3B, the transcript abundances of PrWRI1 in developing seeds were much higher than that of roots in P. rockii. The PrWRI1 was markedly activated, reached a maximum expression level at 40 and 60 DAP, and then gradually decreased throughout the seed maturation. Therefore, the transcription factor PrWRI1 might mainly function during tree peony seed development period.
Subcellular localization of the PrWRI1 protein
To further detect the subcellular localization of PrWRI1, the 35S::PrWRI1-GFP translational fusion protein was generated and transiently transformed with the marker pBV220-cherry into onion inner epidermal peels by Agrobacterium-mediated transformation method. The results showed that the red fluorescence of pBV220-mCherry was merged with the green fluorescence of 35S::PrWRI1-GFP in the nucleolus, indicating the subcellular localization of PrWRI1 to the nucleolus (Fig. 4).
Transient overexpression of PrWRI1
As an advantageous transient expression system, the N. benthamiana leaf tissue also has been successfully applied to characterize the functions of genes involved in FA biosynthesis. The PrWRI1 was over-expressed under the control of dual CaMV 35S promoters in N. benthamiana leaves. The Tomato bushy stunt virus (TBSV)-encoded p19 protein (P19) was also co-transformed as an inhibitor of ectopic gene silencing. Six days after infiltration, the lipid droplets (LDs) in leaves were observed under the confocal fluorescence microscope. According to the results, the number of LDs was significantly increased in PrWRI1-overexpressed leaves, when compared with that in mock- and P19-transformed control leaves (Fig. 5). And, when compared to the leaves that had just been transformed with PrWRI1, more LDs were found in leaves that had also been transformed with P19.
Generation of transgenic Arabidopsis and gene expression analysis
In order to investigate the role of PrWRI1 in the stable genetic transformation system, a plant over-expression vector harboring PrWRI1 driven by the 35S promoter was constructed and transformed into wild-type Arabidopsis (Columbia-0). In total of five independent T3 homozygous transgenic lines with high expression of PrWRI1 were identified by RT-PCR for further analysis.
The length and width of seeds from T3 transgenic lines and wild-type were measured. The seeds of transgenic Arabidopsis are plumper in shape, and larger in 100 seeds weight, when compared with wild-type ones (Fig. 6A, B and C). The average weight of 100 transgenic seeds was 1.72 mg, which was 37.6% higher than that of wild seeds. Furthermore, the total fatty acid content in transgenic lines (#1 and #4) was significantly higher than that in wild-type (Fig. 6D). And, the relative abundance of PUFAs were significantly increased, while the proportion of monounsaturated fatty acids were declined in #1 and #4 transgenic lines (Fig. 6E). Overall, these results suggested that PrWRI1 might play an important role in enhancing oil accumulation and changing FA composition.
Furthermore, the expression of various FA and TAG biosynthesis genes (including PKP-β1, GPDH, BCCP2, β-PDHC, KASI, MCAAT, EAR, FATA, FAD2, FAD3, GPAT, LPAAT, DGAT, PDAT) in transgenic Arabidopsis seeds were also examined by qRT-PCR. The expression levels of genes related to pyruvate synthesis in glycolysis (PKP-β1), FA de novo synthesis (BCCP2, β-PDHC, FATA), FA desaturation (FAD2, FAD3), and TAG assembly (DGAT, PDAT) were increased diversely in #1 and #4 transgenic line seeds (Fig. 7).
Discussion
WRI1 was the key transcription factor regulating fatty acid biosynthesis and was first identified in Arabidopsis [27]. And, its orthologs have been identified in many plants including Brassica napus [22], Glycine max [28], Ricinus connunis [29], and Zea mays [30]. In the present study, the PrWRI1 gene was identified and cloned from the seeds of P. rockii, which was characterized by high ALA content. The analysis of amino acid sequences showed that PrWRI1 had conserved YRG and RAYD residues, which were proposed to be functional in DNA binding, and might belong to the AP2 TF’s family [31]. And, comparison of WRI1 orthologs across many diverse plant species revealed a short protein sequence “VYL” also presented in the first AP2 domain of PrWRI1 (Fig. 2A). Studies have shown that the impairment of the function of WRI1 protein in Arabidopsis could be resulted by the mutation of a single amino acid in “VYL”, suggesting the essential role of “VYL” for AtWRI1 function [24]. However, recent studies have shown that the functional importance of "VYL" is in question because it is absent from AsWRI1c, RcWRI1-B, and OsWRI1-1 [29, 32, 33]. With the identification of AtWRI1 with its cognate double-stranded DNA, the mechanism by which WRI1 works will be further understood [34]. In addition, although the C-terminal regions of PrWRI1 are diverged, bioinformatics analysis showed that PrWRI1 has much closer relationship with GmWRI1 (soybean), whose downstream genes are responsible for fatty acid synthesis, elongation, and desaturation [28].
During the development of P. rockii seeds, the content of fatty acid is relatively low in the immature seeds, then there is a period of rapid oil accumulation, and finally, the FA content enters into a relatively stable period with a slight decrease [35]. The expression levels of PrWRI1 in immature seeds (40 and 60 DAP) were much higher than those in other periods during the development of P. rockii seeds (Fig. 3B). The results are in agreement with those in many other plants, such as coconut [36] and camelina [37]. That indicated PrWRI1 is actively transcribed during seed development, especially during the fore and middle periods. Besides, the expression level of PrWRI1 was much higher in roots than in other non-seed tissues in P. rockii. The relatively high expression level in P. rockii roots points to a root-specific function. In A. thaliana, WRI1 has been shown to have a role in root auxin homeostasis by controlling the expression of auxin carrier genes [38].
In the studies of transient overexpression of PrWRI1, the tomato bushy stunt virus (TBSV)-encoded p19 protein (P19) was also co-transformed as an inhibitor of ectopic gene silencing [39]. The P19 protein of TBSV is involved in various important activities, including the suppression of posttranscriptional gene silencing, virus movement, and symptom induction [40]. And, P19 was often applied to enhance heterologous gene expression with harmless to plants [41]. According to the results, the number of LDs was much more in PrWRI1 and P19 co-overexpressed N. benthamiana leaves than that in mock- and P19-transformed control leaves, which indicates that P19 was an effective tool in the investigation of genes associated with lipid accumulation. Ectopic expression of WRI1s from castor and oat were also found to enhance TAG levels in N. benthamiana fresh leaves relative to the control [29, 33]. In addition to this, the visualizations of LDs in N. benthamiana leaves will be greatly helpful for the investigation of TAG accumulations, which also have been used in our other study on PrASIL1 [42]. And, our results would provide a promising strategy to increase the production of vegetable leaves oils to meet the increasing demand for edible oil [43].
WRI1 could regulate the metabolic processes, particularly glycolysis, during seed development [44]. The overexpression of WRI1 alters the expression of target genes involved in glycolysis and fatty acid synthesis, and further enhancing the carbon flow from glycolysis to fatty acid synthesis in seeds and finally increasing the accumulation of seeds oil. The present study showed that the average weight of 100 PrWRI1-overexpressed transgenic Arabidopsis seeds was heavier than that of wild seeds. Similarly, ectopic expression of BnWRI1-1 and BnWRI1-2 from Brassica napus could significantly increase seed weights by around 40%, while the morphology of seeds was similar among transgenic lines and wild-type Arabidopsis [22]. This might have resulted from the increase in the sizes of cells and cotyledons in the seeds of transgenic lines. Besides, the content of total FA and PUFAs were also enhanced in transgenic lines (#1 and #4), when compared with that in wild-type. Similar results were also observed in the seeds of transgenic soybean lines overexpressing GmWRI1a gene [28]. The increased proportion of PUFAs, including C18:2 and C18:3, was also observed in transgenic Arabidopsis overexpressing PoWRI1 from Peasonia ostii [45]. In sum, WRI1 played an important role in enhancing oil accumulation and changing the FA composition of tree peony seeds oil.
In addition to phenotypic observation and FA measurement of transgenic Arabidopsis seeds, the expression levels of genes involved in glycolysis and fatty acid synthesis were also determined. According to the results, the transcript levels of genes related to FA de novo synthesis (BCCP2, β-PDHC, FATA) were increased, especially in transgenic lines (#1 and #4), which promotes the increase of raw materials for fatty acid synthesis and provides the material basis for fatty acid accumulation. As shown in Supplementary Table 3, a stronger correlation in the expression trends between WRI1 and genes relating with FA de novo was also observed in developing P. rockii seeds based on our previous transcriptome data [35]. Besides, the transcript levels of genes related to desaturation (FAD2, FAD3), and TAG assembly (DGAT, PDAT) were increased by varying degrees in transgenic Arabidopsis than those in wild-type ones. Taken together, these findings indicated that overexpression of PrWRI1 could enhance the transcript levels of genes involved in the FA biosynthesis pathway, and consequently promote the accumulation of TAG in transgenic seeds. Similar results were also observed in over-expressed CoWRI1 (Cocos nucifera) and PoWRI1 (Paeonia rockii) transgenic Arabidopsis [36, 45]. The results indicated that the overexpression of PrWRI1 not only promoted the flow of carbon source to FA metabolic pathway in transgenic Arabidopsis seeds, but also drove the expression of most genes related to FA biosynthesis.
Conclusions
This investigation aimed to characterize PrWRI1, which has been isolated from P. rockii with high UFAs. PrWRI1 had two typical AP2/ERF domains, and was similar to GmWRI1 in structure. Besides, the expression patterns of PrWRI1 in P. rockii suggested that PrWRI1 was highly expressed in immature seeds. And, our results showed that PrWRI1 over-expressed transient and stable system could increase total FAs and also change the FA compositions. Further, the expression of most genes related to FA synthesis and TAG assembly was increased in transgenic Arabidopsis seeds. Therefore, PrWRI1 could push carbon flow to FA biosynthesis and further enhance the TAG amount in seeds. The mechanisms on the increased PUFAs in PrWRI1-overexpressed transgenic Arabidopsis seeds also need further investigation. Our results would provide a better understanding of WRI1 transcriptional mechanisms in tree peony and could also be used in oil crops genetic research.
Materials and methods
Plant materials and growth conditions
Tree peony (Paeonia rockii) with the same genetic origin was grown in the wild tree peony germplasm repository at Yangling, Shaanxi Province, China. They were identified by Professor Li-xin Niu from Northwest A&F University. The voucher specimens of Paeonia rockii were deposited into the Herbarium of the National Oil Peony Engineering Technology Research Center, China. Different tissues including the root, stem, leaf, calyx, petal, stamen, pistil, and developing seeds (20, 40, 60, 80, and 100 days after flowering) were collected for transcript level analysis. All the samples were immediately frozen in liquid nitrogen and stored at -80 ℃ for further studies. Arabidopsis thaliana (ecotype Columbia-0) and Nicotiana benthamiana plants used for transformation were grown in growth chambers at 21/25 ℃ (day/night) with a 16 h light/8 h dark.
Gene identification and isolation
Total RNA was extracted from the seeds collected at 20 days after pollination (DAP) according to the protocol of TIANGEN RNA Prep Pure Plant Kit (Tiangen Biotech Co. Ltd., Beijing, China). A full-length cDNAs library was constructed by using the PrimeScript® RT Reagent Kit with gDNA Eraser (Takara, Japan). The specific primers (Supplementary Table 1) for identifying the open reading frame (ORF) of the PrWRI1 gene in tree peony were designed based on transcriptome data [35]. The PrWRI1 was amplified and ligated into the pMD19-T vector and then sequenced.
Protein sequence and phylogenetic tree analysis
The nucleotide and amino acid sequence analysis were performed using DNAMAN software. Homology search was conducted using the BLAST server in National Center for Biotechnology Information (NCBI). Dendrograms for phylogenetic analysis were performed on MEGA (version 5.1) software and multiple sequence alignment was conducted using CLUSTALW.
Quantitative RT-PCR (qRT-PCR) analysis
Total RNA from various tissues of Paeonia rockii and Arabidopsis seeds (15 days after pollination) were isolated for transcript expression. The extraction of total RNA and the synthesis of cDNA were performed according to the method described above. The qRT-PCR was performed using SYBR® Premix Ex TaqTM (Perfect Real Time) kit (Takara, Dalian, China) in StepOnePlus Real-time PCR System (Applied Biosystems). Primers used for qRT-PCR were listed in Supplementary Table 2. The PCR reaction and data analysis were conducted according to the methods described previously [46]. All qRT-PCR experiments were performed in triplicate for each gene.
Subcellular localization
The PrWRI1 ORF without the stop codon was inserted into vector P2300-GFP using BamHI and SalI sites, generating 35S::PrWRI1-GFP construct. Then, the constructed plasmid and the maker pBV220-mCherry were transformed into Agrobacterium tumefaciens strain EHA105 by electroporation, followed by the infection of onion inner epidermal peels using the agroinfiltration method (Horsch et al., 1984). The transformed cells were incubated for 24 h at 25℃ in the dark and the fluorescence was monitored using a laser scanning confocal microscope (Leica TCS SP8). The excitation wavelengths were 488 nm for GFP and 561 nm for markers.
Vector construction
In order to investigate the activity of PrWRI1 visually, the coding sequence of PrWRI1 was cloned into the SacII and BamHI restriction sites of pK34 entry vector, and then the recombinant pK34 vector with double CaMV 35S promoters and a terminator sequence was digested with AscI for entry into plant expression vector, pB110. The vector was then transiently expressed in Nicotiana benthamiana leaves by Agrobacterium-mediated transformation.
The ORF of PrWRI1 were inserted into the vector pCAMBIA1300 under the control of Arabidopsis seed-specific promoter 2S2 by the digestion of KpnI and BamHI. The generated constructs were transformed into Agrobacterium tumefaciens GV3101 using the freeze–thaw method, and then they were used for the transformation of wild-type Arabidopsis by the floral dip method.
Transient expression in tobacco leaves
Five/six-day-old tobacco (N. benthamiana) leaves were chosen for infiltration. The pB110-PrWRI1 construct was transiently transformed in N. benthamiana leaves individually or with the viral silencing suppressor protein P19 [47]. After infiltration, tobacco was transferred into the growth chamber and allowed to grow for 6/7 days to express the protein.
Then leaf discs were collected and placed into the Falcon tubes containing 4% formaldehyde in 1X phosphate buffered saline (PBS). The samples were washed three times with 1X PBS, after being shaken at 75 rpm for one hour. Finally, the leaf discs were stained with 4 μg/ml Nile Red in 1X PBS at room temperature in a rotational shaker at 100 rpm for 15 min in the dark. Then, each leaf disc was observed immediately under the confocal fluorescence microscope (Leica TCS SP8). The excitation wavelength for Nile Red is 488 nm and the emission wavelength is 560 to 620 nm.
The total number of lipid droplets (LDs) was counted by ImageJ software. Six biological replicates were conducted for each expression vector. Leaves infected with the empty vector or P19 vector were sampled as controls.
Generation of transgenic Arabidopsis
The harvested seeds from transformed Arabidopsis plants were selected on 1/2 MS plates containing 20 mg/L hygromycin (hyg). Hyg-resistant seedlings were then transplanted into the moistened potting soil as T0 transformants and followingly confirmed by PCR analysis. Seeds from homozygous T3 transgenic lines with 100% hyg resistance were collected for further studies.
Determination of seed weight and seed size
The seeds from wide-type and transgenic Arabidopsis lines were randomly counted and weighed using a microbalance. To determine the seeds’ sizes, they were examined and photographed using a Leica KL2 microscope (Leica Microsystems, Germany). The length and width of the seeds were measured using the program Image J (http://imagej.nih.gov.zzulib.vpn358.com/ij/) in accordance with the software's instructions.
FA analysis
FA extraction and methylation were conducted according to the procedures described previously (Li et al., 2015). Gas chromatograph-mass spectrometer (Thermo Scientific trace 1310 GC-ISQ) and TriPlus RSH robotic sampler (Thermo Scientific) were used to analyze FAs. The helium was used as carrier gas in the TG-WaxMS capillary column (30 m × 0.25 mm internal diameter, 0.25 μm film thickness; Thermo Fisher Scientific, USA). Qualitative FA analysis was achieved using tridecylic acid as an internal standard. The FAs content was expressed as milligrams per gram dry weight (DW) of a sample. All samples were analyzed in triplicate.
Statistical analysis
All experiments were performed in three biological replicates. The results were expressed as mean values ± standard deviations (SD). The significance of the difference between WRI1-overexpressed lines and wild-type was analyzed using One-way ANOVA. Statistical analysis was conducted using SPSS software (version 22.0 for Windows). All figures were generated using Origin 8.0 (Origin Software, Inc., OriginLab, USA).
Availability of data and materials
All relevant data are included in the manuscript and its supporting materials.
Abbreviations
- FA:
-
Fatty acid
- UFAs:
-
Unsaturated fatty acids
- PUFAs:
-
Polyunsaturated fatty acids
- ALA:
-
α-Linolenic acid TAG: Triglyceride
- PK:
-
Pyruvate kinase
- PDHC:
-
Pyruvate dehydrogenase complex
- BCCP:
-
Biotin carboxyl carrier protein
- MCAT:
-
Malonyl-CoA:ACP transacylase
- ENR:
-
Enoyl-ACP reductase
- KAS:
-
3-Ketoacyl-ACP synthases isoform
- FATA/FATB:
-
Thioesterases
- ER:
-
Endoplasmic reticulum
- G3P:
-
Glycerol-3-phosphate
- GPAT:
-
G3P acyltransferase
- LPAT:
-
Lysophosphatidic acid acyltransferase
- DGAT:
-
Diacylglycerol acyltransferase
- PC:
-
Phosphatidylcholine
- DAG:
-
Diacylglycerol
- PDAT:
-
Phospholipid: diacylglycerol acyltransferase
- TFs:
-
Transcription factors
- LEC1:
-
Leafy cotyledon 1
- LEC2:
-
Leafy cotyledon 2
- ABI3:
-
Abscisic and insensitive 3
- FUS3:
-
FUSCA 3
- WRI1:
-
Wrinkled 1
- AP2:
-
APETALA2
- aa:
-
Amino acid
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- TBSV:
-
Tomato bushy stunt virus
- LDs:
-
Lipid droplets
- DAP:
-
Days after pollination
- DW:
-
Dry weight
References
de Roos B, Mavrommatis Y, Brouwer IA. Long-chain n-3 polyunsaturated fatty acids: new insights into mechanisms relating to inflammation and coronary heart disease. Br J Pharmacol. 2009;158(2):413–28.
Demaison L, Moreau D. Dietary n-3 polyunsaturated fatty acids and coronary heart disease-related mortality: a possible mechanism of action. Cell Mol Life Sci. 2002;59(3):463–77.
Christopoulou E, Lazaraki M, Komaitis M, Kaselimis K. Effectiveness of determinations of fatty acids and triglycerides for the detection of adulteration of olive oils with vegetable oils. Food Chem. 2004;84(3):463–74.
Li S-S, Yuan R-Y, Chen L-G, Wang L-S, Hao X-H, Wang L-J, Zheng X-C, Du H. Systematic qualitative and quantitative assessment of fatty acids in the seeds of 60 tree peony (Paeonia section Moutan DC.) cultivars by GC-MS. Food Chem. 2015;173:133–40.
Orsavova J, Misurcova L, VavraAmbrozova J, Vicha R, Mlcek J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int J Mol Sci. 2015;16(6):12871–90.
Zheng J, Yang J, Yang X, Cao Z, Cai S, Wang B, Ye J, Fu M, Zhang W, Rao S, et al. Transcriptome and miRNA sequencing analyses reveal the regulatory mechanism of alpha-linolenic acid biosynthesis in Paeonia rockii. Food Res Int. 2022;155:111094.
Schwender J, Ohlrogge JB, Shachar-Hill Y. A flux model of glycolysis and the oxidative pentosephosphate pathway in developing Brassica napus embryos. J Biol Chem. 2003;278(32):29442–53.
Johnston ML, Luethy MH, Miernyk JA, Randall DD. Cloning and molecular analyses of the Arabidopsis thaliana plastid pyruvate dehydrogenase subunits. Plant Physiol. 1997;114(3):666–666.
Ohlrogge J, Browse J. Lipid biosynthesis. Plant Cell. 1995;7(7):957–70.
Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, Debono A, Durrett TP, et al. Acyl-lipid metabolism The arabidopsis book. 2013;11:e0161–e0161.
Napier JA. The production of unusual fatty acids in transgenic plants. Annu Rev Plant Biol. 2007;58:295–319.
Bates PD, Durrett TP, Ohlrogge JB, Pollard M. Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiol. 2009;150(1):55–72.
Zhang M, Fan J, Taylor DC, Ohlrogge JB. DGAT1 and PDAT1 acyltransferases have overlapping functions in arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell. 2009;21(12):3885–901.
North H, Baud S, Debeaujon I, Dubos C, Dubreucq B, Grappin P, Jullien M, Lepiniec L, Marion-Poll A, Miquel M, et al. Arabidopsis seed secrets unravelled after a decade of genetic and omics-driven research. Plant J. 2010;61(6):971–81.
Roscoe TT, Guilleminot J, Bessoule J-J, Berger F, Devic M. Complementation of seed maturation phenotypes by ectopic expression of abscisic acid insensitive3, FUSCA3 and leafy cotyledon2 in arabidopsis. Plant Cell Physiol. 2015;56(6):1215–28.
Devic M, Roscoe T. Seed maturation: Simplification of control networks in plants. Plant Sci. 2016;252:335–46.
Zhai Z, Liu H, Shanklin J. Phosphorylation of WRINKLED1 by KIN10 results in its proteasomal degradation, providing a link between energy homeostasis and lipid biosynthesis. Plant Cell. 2017;29(4):871–89.
Focks N, Benning C. wrinkled1: A novel, low-seed-oil mutant of arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol. 1998;118(1):91–101.
Weselake RJ, Taylor DC, Rahman MH, Shah S, Laroche A, McVetty PBE, Harwood JL. Increasing the flow of carbon into seed oil. Biotechnol Adv. 2009;27(6):866–78.
Baud S, Wuilleme S, To A, Rochat C, Lepiniec L. Role of WRINKLED1 in the transcriptional regulation of glycolytic and fatty acid biosynthetic genes in arabidopsis. Plant J. 2009;60(6):933–47.
Maeo K, Tokuda T, Ayame A, Mitsui N, Kawai T, Tsukagoshi H, Ishiguro S, Nakamura K. An AP2-type transcription factor, WRINKLED1, of Arabidopsis thaliana binds to the AW-box sequence conserved among proximal upstream regions of genes involved in fatty acid synthesis. Plant J. 2009;60(3):476–87.
Liu J, Hua W, Zhan G, Wei F, Wang X, Liu G, Wang H. Increasing seed mass and oil content in transgenic Arabidopsis by the overexpression of wri1-like gene from Brassica napus. Plant Physiol Biochem. 2010;48(1):9–15.
Shen B, Allen WB, Zheng P, Li C, Glassman K, Ranch J, Nubel D, Tarczynski MC. Expression of ZmLEC1 and ZmWRI1 increases seed oil production in maize. Plant Physiol. 2010;153(3):980–7.
Ma W, Kong Q, Arondel V, Kilaru A, Bates PD, Thrower NA, Benning C, Ohlrogge JB. WRINKLED1, A ubiquitous regulator in oil accumulating tissues from arabidopsis embryos to oil palm mesocarp. Plos One. 2013;8(7):e68887.
Xiu Y, Wu G, Tang W, Peng Z, Bu X, Chao L, Yin X, Xiong J, Zhang H, Zhao X, et al. Oil biosynthesis and transcriptome profiles in developing endosperm and oil characteristic analyses in Paeonia ostii var. lishizhenii. J Plant Physiol. 2018;228:121–33.
Sun J, Chen T, Liu M, Zhao D, Tao J. Analysis and functional verification of PoWRI1 gene associated with oil accumulation process in paeonia ostii. Int J Mol Sci. 2021;22(13):6996.
Cernac A, Benning C. WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in arabidopsis. Plant J. 2004;40(4):575–85.
Chen L, Zheng Y, Dong Z, Meng F, Sun X, Fan X, Zhang Y, Wang M, Wang S. Soybean (Glycine max) WRINKLED1 transcription factor, GmWRI1a, positively regulates seed oil accumulation. Mol Genet Genomics. 2018;293(2):401–15.
Ji X-J, Mao X, Hao Q-T, Liu B-L, Xue J-A, Li R-Z. Splice variants of the castor WRI1 gene upregulate fatty acid and oil biosynthesis when expressed in tobacco leaves. Int J Mol Sci. 2018;19(1):146.
Pouvreau B, Baud S, Vernoud V, Morin V, Py C, Gendrot G, Pichon J-P, Rouster J, Paul W, Rogowsky PM. Duplicate maize wrinkled1 transcription factors activate target genes involved in seed oil biosynthesis. Plant Physiol. 2011;156(2):674–86.
Okamuro JK, Caster B, Villarroel R, VanMontagu M, Jofuku KD. The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proc Natl Acad Sci USA. 1997;94(13):7076–81.
Mano F, Aoyanagi T, Kozaki A. Atypical splicing accompanied by skipping conserved micro-exons produces unique WRINKLED1, an AP2 domain transcription factor in rice plants. Plants (Basel). 2019;8(7):207.
Yang Z, Liu X, Li N, Du C, Wang K, Zhao C, Wang Z, Hu Y, Zhang M. WRINKLED1 homologs highly and functionally express in oil-rich endosperms of oat and castor. Plant Sci. 2019;287:110193.
Qiao Z, Kong Q, Tee WT, Lim ARQ, Teo MX, Olieric V, Low PM, Yang Y, Qian G, Ma W, et al. Molecular basis of the key regulator WRINKLED1 in plant oil biosynthesis. Sci Adv. 2022;8(34):1211.
Zhang Q, Yu R, Sun D, Rahman M, Xie L, Hu J, He L, Kilaru A, Niu L, Zhang Y. Comparative transcriptome analysis reveals an efficient mechanism for α-Linolenic acid synthesis in tree peony seeds. Int J Mol Sci. 2018;20(1):65.
Sun R, Ye R, Gao L, Zhang L, Wang R, Mao T, Zheng Y, Li D, Lin Y. Characterization and ectopic expression of CoWRI1, an AP2/EREBP domain-containing transcription factor from coconut ( Cocos nucifera L.) endosperm, changes the seeds oil Content in transgenic Arabidopsis thaliana and Rice ( Oryza sativa L.). Front Plant Sci. 2017;8:63.
An D, Kim H, Ju S, Go YS, Kim HU, Suh MC. Expression of camelina WRINKLED1 isoforms rescue the seed phenotype of the arabidopsis wri1 mutant and increase the triacylglycerol content in tobacco leaves. Front Plant Sci. 2017;8:34.
Kong Q, Ma W, Yang H, Ma G, Mantyla JJ, Benning C. The arabidopsis WRINKLED1 transcription factor affects auxin homeostasis in roots. J Exp Bot. 2017;68(16):4627–34.
Brioudes F, Jay F, Voinnet O. Suppression of both intra- and intercellular RNA silencing by the tombusviral P19 protein requires its small RNA binding property. New Phytol. 2022;235(3):824–9.
Uhrig JF, Canto T, Marshall D, MacFarlane SA. Relocalization of nuclear ALY proteins to the cytoplasm by the tomato bushy stunt virus P19 pathogenicity protein. Plant Physiol. 2004;135(4):2411–23.
Saxena P, Hsieh Y-C, Alvarado VY, Sainsbury F, Saunders K, Lomonossoff GP, Scholthof HB. Improved foreign gene expression in plants using a virus-encoded suppressor of RNA silencing modified to be developmentally harmless. Plant Biotechnol J. 2011;9(6):703–12.
Yang W, Hu J, Behera JR, Kilaru A, Yuan Y, Zhai Y, Xu Y, Xie L, Zhang Y, Zhang Q, et al. A tree peony trihelix transcription factor PrASIL1 represses seed oil accumulation. Front Plant Sci. 2021;12:796181.
Kim HU, Lee K-R, Jung S-J, Shin HA, Go YS, Suh M-C, Kim JB. Senescence-inducible LEC2 enhances triacylglycerol accumulation in leaves without negatively affecting plant growth. Plant Biotechnol J. 2015;13(9):1346–59.
Cernac A, Andre C, Hoffmann-Benning S, Benning C. WRI1 is required for seed germination and seedling establishment. Plant Physiol. 2006;141(2):745–57.
Sun J, Chen T, Liu M, Zhao D, Tao J. Analysis and functional verification of PoWRI1 gene associated with oil accumulation process in paeonia ostii. Int J Mol Sci. 2021;22(13):6996.
Xie L, Hu J, Zhang Q, Sun Q, Zhang Y, Niu L: Influence of pollen sources on the expression of FA and TAG biosynthetic pathway genes in seeds of Paeonia rockii during the rapid oil accumulation. entia Horticulturae 2019, 243:477–483.
Behera JR, Rahman MM, Bhatia S, Shockey J, Kilaru A. Functional and predictive structural characterization of WRINKLED2, a unique oil biosynthesis regulator in avocado. Front Plant Sc. 2021;12:648494.
Acknowledgements
We are grateful to Aruna Kilaru for assistance with providing the vector.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant No. 31901357), Postdoctoral Research Funding Project of Shaanxi Province (Grant No. K3380220028), General project of China Postdoctoral Science Foundation (Grant No. 2019M663845), and Natural Science Basic Research Project of Shaanxi Province (Grant No. 2021JQ-143).
Author information
Authors and Affiliations
Contributions
LX and JH carried out the experiments. LX analyzed the data. LX, QZ, and HG conceived and designed the experiments. LX wrote the manuscript. LX, SW and RX analyzed the data and revised the manuscript. WY and ZY assisted with doing the experiments. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was carried out in compliance with relevant institutional, national, and international guidelines and legislation for plant ethics.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table 1. Primers used for gene isolation and vector construction in the present study. Supplementary Table 2. Primers used for qRT-PCR analysis in the present study. Supplementary Table 3. The correlation analysis on the expression trends between WRI1 and other genes relating to fatty acid biosynthesis in developing P. rockii seeds based on the transcriptome data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xie, L., Hu, J., Yan, Z. et al. Tree peony transcription factor PrWRI1 enhances seed oil accumulation. BMC Plant Biol 23, 127 (2023). https://doi.org/10.1186/s12870-023-04127-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04127-9