Abstract
Background
Contamination−free culture is a prerequisite for the success of in vitro − based plant biotechnology. Aseptic initiation is an extremely strenuous stride, particularly in woody species. Meanwhile, over−sterilization is potentially detrimental to plant tissue. The recent rise of machine learning algorithms in plant tissue culture proposes an advanced interpretive tool for the combinational effect of influential factors for such in vitro − based steps.
Results
A multilayer perceptron (MLP) model of artificial neural network (ANN) was implemented with four inputs, three sterilizing chemicals at various concentrations and the immersion time, and two outputs, disinfection efficiency (DE) and negative disinfection effect (NDE), intending to assess twenty−seven disinfection procedures of Pistacia vera L. seeds. Mercury chloride (HgCl2; 0.05–0.2%; 5–15 min) appears the most effective with 100% DE, then hydrogen peroxide (H2O2; 5.25–12.25%; 10–30 min) with 66–100% DE, followed by 27–77% DE for sodium hypochlorite (NaOCl; 0.54–1.26% w/v; 10–30 min). Concurrently, NDE was detected, including chlorosis, hard embryo germination, embryo deformation, and browning tissue, namely, a low repercussion with NaOCl (0–14%), a moderate impact with H2O2 (6–46%), and pronounced damage with HgCl2 (22–100%). Developed ANN showed R values of 0.9658, 0.9653, 0.8937, and 0.9454 for training, validation, testing, and all sets, respectively, which revealed the uprightness of the model. Subsequently, the model was linked to multi−objective genetic algorithm (MOGA) which proposed an optimized combination of 0.56% NaOCl, 12.23% H2O2, and 0.068% HgCl2 for 5.022 min. The validation assay reflects the high utility and accuracy of the model with maximum DE (100%) and lower phytotoxicity (7.1%).
Conclusion
In one more case, machine learning algorithms emphasized their ability to resolve commonly encountered problems. The current successful implementation of MLP–MOGA inspires its application for more complicated plant tissue culture processes.
Similar content being viewed by others
Background
Plant tissue culture is a well−established strategy for the plant genetic breeding, the production of biologically active compounds, and the conservation and mass propagation of various species [1, 2]. It involves oversensitive, precise, and multi−variables processes considering the extended assortment of in vitro based−plant biotechnologies, for instance, organogenesis, somatic embryogenesis, somatic hybridization, haplodiploidization, hairy root culture, in vitro secondary metabolites production, Agrobacterium − mediated gene transformation, and recently as a crucial step in genome editing technologies [3,4,5,6]. In vitro cell fate is the outcome of the dynamic interaction mainly of three levels, environmental signal inputs and physical stimuli that act as initial triggers of regeneration, epigenetic and transcriptional cellular responses to those triggers leading to cellular reprogramming, and molecules that manage the development of the new stem cell niche [7]. As in vitro culture environment offers fertile sustenance for a wide range of microorganisms, an unavoidable step, successful culture initiation requires the eradication of all the contamination including filamentous fungi, yeasts, bacteria, viruses, viroids, and micro−arthropods (mites and thrips) which can alter the subsequent growth and development of the explant [8, 9]. Biological contamination is a serious problem in plant tissue culture [10,11,12]. Contaminants can release metabolites and proteins that affect the plant tissues and alter the composition and/or pH of the culture medium [13]. With a faster growth rate and in conjunction with the nutrient’s availability, contamination colonizes the medium and the explant eventually dies in a matter of days. This obstacle exacerbates financial losses in the commercial sector as well as limits experiment progress in research laboratories. The severity of this issue was corroborated by the adoption of microbiological quality assurance systems (e.g. Hazard Analysis Critical Control Point; HACCP procedures) to succor the requirements of commercial plant tissue culture laboratories [8]. Disinfection efficiency depends on the contamination type (epiphytic or endophytic and expressed or latent), the explant (type, age, size, choice of explant, sampling time, physiological state of the donor plant, and culture condition) as well on the disinfection procedure [14]. Accordingly, various compounds were applied to establish axenic culture e.g. hydrogen peroxide (H2O2), mercuric chloride (HgCl2), silver nitrate (AgNO3), silver nanoparticles, calcium hypochlorite (Ca(OCl)2), antibiotics, etc. Though, sodium hypochlorite (NaOCl) represents the more considered option for chemical disinfection with a broad antimicrobial spectrum, rapid bactericidal action, solubility in water, and relative stability [15]. There is no standard decontamination protocol. Occasionally, a research laboratory should adjust its own procedure that is well adapted to studied biological material namely, should not affect the viability and the regeneration capacity. Explant health is the main feature determining regeneration potential. Seed germination, seedling growth, and shoot regeneration were negatively affected by the increasing concentration and temperature of NaOCl solution in flax (Linum usitatissimum) [16]. A disinfecting agent can alter seed metabolism, trace amounts of NaOCl that remain on the surface of Lycopersicon esculentum Mill. seeds after sterilization interfere with subsequent uptake and incorporation of leucine into protein [17]. As well, it can influence the number and size of stomata and cells and the total chlorophyll content [18]. Even further, the disinfection procedure can affect the future development pathway of the explant [19]. Still, the conventional one factor at a time (OFAT) “optimization” way, i.e. by assessing some defined levels of the involved factors, e.g., sterilization agent, concentration, immersion time, temperature, shaking, etc., may give rise to limited results since, presumably, even untested miniature variation could be influential in the corresponding response.
The implication of artificial intelligence−optimization algorithms, a highly potent technology combination, in plant tissue culture has recently emerged with limited application due to the complex definition terms and computational algorithms. Notwithstanding, it was considered to achieve different purposes such as modeling the effects of light and sucrose, optimization of medium culture formulation, prediction and optimization of cell growth, shoot organogenesis, in vitro rooting, and somatic embryogenesis [20,21,22,23]. The adoption of modeling and 3D printing technologies for the design and development of a functional plant tissue culture vessel revealed a successful application to prototype novel culture vessels with independently controlled variable fluence rate/spectra LED lighting [24]. Light (quantity and particularly quality) is the main factor that affects photomorphogenesis and with high effect on protocol reproducibility among laboratories [25, 26]. Dissimilar to the classical linear regression−based analysis, the robustness of machine learning methods is that it makes it possible to take into account the overall interaction effect of the different involved variables in a particular event. This is primordial since the plant tissue culture approach encompasses dynamic, non − linear, and non − deterministic processes, therefore, able to interpret highly complex relationships between dependent and independent variables. Artificial neural networks (ANNs) are the most applied for modeling and optimization in plant tissue culture such as radial basis function (RBF), generalized regression neural network (GRNN), probabilistic neural network (PNN), neurofuzzy logic, support vector machine (SVM) with multilayer perceptron (MLP) is the most popular ANN [27]. The superiority of ANNs tool, compared with others modeling technologies such as response surface methodology (RSM), was extensively proved by various reports [28, 29]. ANNs were usually linked to various types of algorithms for optimization purposes. Regarding multi–objectives problems, several multi–objective evolutionary algorithms were developed including multi–objective genetic algorithm (MOGA) [30], nondominated sorting genetic algorithm (NSGA) [31], and fast nondominated sorting genetic algorithm (NSGA–II) [32]. Multi−objective genetic algorithm was suggested as an effective way to aggregate all objectives simultaneously and proposes a reasonable solution to a multi–objective problem following the investigation of a set of solutions, each of which satisfies the objectives at an acceptable level without being dominated by any other solution.
Pistachio (Pistacia vera L.), a luxury and high economically important crop from Anacardiaceae family, is cultured mainly in hot and dry climates including Western Asia, Asia Minor, Northern Africa, Southern Europe, and California [33]. A treasure species distinguished by great adaptability to marginal climatic and edaphic conditions such as drought, cold, calcareous, and rocky soils [34]. Though, its culture has been largely restricted, partially, by inefficient methods of vegetative propagation. Various attempts have been reported regarding the adoption of in vitro culture technology that revealed, most often, various physiological disorders (browning, shoot apical necrosis, phenolic compounds exudation, vitrification …) [35, 36]. To those, the hurdle of the culture aseptic initiation was frequently reported which is due to the surface colonizers as well as endogenous contamination [37,38,39]. Consequently, this species is still underexploited in arid and semi–arid regions.
Some works reported successful decontamination in Pistacia vera explants [38, 39]. Nonetheless, the protocol should be adjusted to take precautions against phytotoxicity and ensure high explants’ health which could be accomplished using computer−based tools. Thus, the modeling of surface disinfection protocol of field−derived explants was considered by comparing the effect of three sterilizing agents, their concentration, and the time exposure. Afterward, an optimized combination was defined using multi−objective genetic algorithm aimed to define the lowest effective chemical concentration for the shortest application time.
Materials and methods
Plant material, treatments, and in vitro culture conditions
Mature nuts of pistachio (Pistacia vera L.) elite Tunisian variety ‘Mateur’ were collected from 35 − year−old trees cultured in Medenine, Southeast of Tunisia. After removing the hull and the shell, the kernels were surface−sterilized under a laminar flow hood (ZHJH−C1214C Vertical Airflow; HEPA Filter) sterilized using UV radiation for 15 min and 75% ethanol (C2H5OH) immediately before the manipulation. Sterile gloves, continuously sprayed with 75% ethanol, were used. Three sterilizing agents were evaluated, sodium hypochlorite (NaOCl; 3.61% active chlorine) at 0.54, 0.90, and 1.26% (w/v), hydrogen peroxide (H2O2, 35%) at 5.25, 8.75, and 12.25%, both for 10, 20, and 30 min, and mercury (II) chloride (HgCl2; HiMedia, India) at 0.05, 0.1 and 0.2% for 5, 10 and 15 min (Table 1). The chemicals were added to the sterile distilled water (SDW) after cooling to room temperature under the laminar flow hood. Heating NaOCl solutions may cause unpredictable changes to the concentration of available Cl− [40]. The ranges of parameters were designated based on our preliminary experiments as well as considering the literature. Glass jars (300 ml) were used with 150 ml of disinfection solution. All the materials, including the glass jars, forceps, scalpel handle, and the filter paper (to eliminate the excess SDW from explants), were sterilized using an autoclave at 121 °C for 20 min. Disposable sterile surgical blades were used. Soaking of forceps and scalpel in 100% ethanol and flame sterilization are continuously carried out during the manipulation. Likewise, sterile filter paper layers were changed regularly between treatments. Stainless steel infuser was used to facilitate the recuperation of explants. New sterilizing and SDW solutions were reserved for each treatment to avoid any concentration of the chemical product. Explants were washed four times in SDW and inoculated in full strength Murashige and Skoog (MS) medium [41] devoid of plant growth regulators, supplemented with 3% (w/v) sucrose (HiMedia, India), 0.75% (w/v) agar (Biolife, Italy), 100 mg L− 1 ascorbic acid and distributed in glass tubes (200 mm × 25 mm) hermetically sealed using parafilm. The two cotyledons of each kernel were incubated separately (with one of them including the embryonic axis). pH of the medium was adjusted to 5.8 ± 0.1 prior to autoclaving at 121 °C for 20 min. A total of 522 cultures, including the control and the validation assay, were devoted to the current study with eighteen explants for each treatment and one explant/tube to avoid the risk of spreading contamination. Explants treated with SDW for 20 min served as the control. Cultures were incubated in a growth incubator at a controlled temperature of 25 ± 1 °C with a 16 h photoperiod (40 μmol m− 2 s− 1) for 20 days. Routinely monitoring of contamination was carried out during incubation, usually, detectable by the ‘halo’ effect around the contaminated explant, turbidity of the medium, cell destruction, etc. Ultimately, disinfection efficiency and potential negative disinfection impact were recorded by visual examination. The negative disinfection effect was evaluated considering only the contamination−free cultures.
Artificial neural network model
The ANN was developed based on four inputs, three sterilizing agents at different concentrations and the immersion time, and two outputs, disinfection efficiency (DE) and negative disinfection effect (NDE). A multilayer perceptron (MLP) model was implemented with hyperbolic tangent sigmoid transfer function in the hidden layer and linear transfer function in the output layer. The network was trained with Levenberg–Marquartd back−propagation algorithm. Twenty–eight treatments, including the control, were applied (Table 1), divided randomly into 3 datasets, with 70% (20 samples) for training, 15% (4 samples) for validation, and 15% (4 samples) for testing dataset. All the data were normalized between − 1 and 1 using Eq. (1) to attain dimensional consistency of the parameters and to ensure compatibility with the adopted transfer function [42]:
Where Mi is the normalized value, Mmax and Mmin are the maximum and the minimum values of the scaling range, Ni is the actual data to be normalized, Nmax and Nmin are the maximum and minimum values of the actual data.
Afterward, the developed model was transformed into a mathematical equation, through the weights and biases in conjunction with the transfer functions:
Where; x is the input variables, N is the number of neurons, J is the number of input variables, w1 is weight of hidden layer, w2 is weight of the output layer, b1 is bias of the hidden layer, and b2 is bias of the output layer.
The prediction performance of the developed model was statistically evaluated in terms of the root mean squared error (RMSE) [43] and the coefficient of determination (R2) [44] values as follows:
Where, xi is predicted value; xik is the experimental or actual value; xz is the mean of experimental value, and n is the number of observations.
Optimization of disinfection process
To achieve the main goal of the current work, multi−objective genetic algorithm (MOGA) solver was applied to balance the two considered aspects simultaneously i.e. maximize the disinfection efficiency and minimize the negative disinfection effect. As there are two outputs, two different objectives functions were defined by the developed ANN, both fit Eq. (2), and represent an appropriate approximation of the functional relationship between the inputs and the outputs variables. These two equations were introduced as a fitness function for the optimization step. The population type was a double vector with the creation, selection, mutation, and crossover functions were feasible population, tournament, adaptive feasible, and intermediate, respectively, completed with the following parameters: population size: 50, crossover fraction: 0.8, migration fraction: 0.2, stopping criteria: generations: 400, and stall generations: 100.
Statistical analysis
Matlab®8.3 (R2014a, The Mathworks Inc., Natick, USA) was used for the modeling and the optimization. Data were subjected to multivariate ANOVA analysis and the means were separated by Duncan test (P < 0.05) using IBM SPSS Statistics v.25.0 for Windows.
Results and discussion
Disinfection process
Contamination hazard is among the more destructive limitations in plant tissue culture, commonly encountered in worldwide involved laboratories, regardless of its source (biological material, inappropriate manipulation, …). A solid understanding of both the type and the potential sources of contaminants is required to prevent culture failure. Rigorous instructions should be respected in this technology. Otherwise, the culture will be discarded. Handled by an experienced manipulator in a specialized laboratory, contamination could be effectively managed to be overcome.
In the matter of surface disinfection, a total of twenty−seven treatments were evaluated for pistachio seeds disinfection in the current experiment. The studies concerned seed–derived material are with high–priority as the genetic variation, the main characteristic of the fertilization process, may generate a biological material with new interesting characters. Equally, P. vera is commonly used as a rootstock in this species during field or micrografting [45]. Furthermore, any advancement recorded in seed studies could be, high presumed, extended to tree–derived explant.
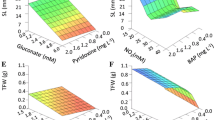
Multivariate ANOVA analysis reflected a high significant (p < 0.001) effect for the three implicated parameters viz. sterilizing agent, its concentration, and the processing time, as well as all their interactions (Table 2). A 100% contamination was registered in the control culture (treated with SDW). In respect of disinfection efficiency, HgCl2 appears the most effective (100%), then H2O2 (66–100%), followed by NaOCl (27–77%) (Table 1, Fig. 1). Synchronously, the direct contact with disinfectants affected negatively the explants’ health at different levels during some treatments. The undesirable effects (NDE) included chlorosis (or yellowing), hard embryo germination, embryo deformation, and browning tissue. The same order of chemical agent aforementioned was recorded i.e. a low NDE with NaOCl (0–14%), a moderate effect with H2O2 (6–46%), and pronounced damage with HgCl2 (22–100%). Thus, the best result, for both evaluated aspects, was obtained with H2O2 at 5.25 and 12.25% for 10 min (T10) and 20 min (T17), respectively. No NDE was obtained with T10, giving 86.6% of healthy sterile explants. Equally, a very close value was recorded with T17 (88.9%) following the ignoring of a small fraction of NDE. With stem segments, explants of P. vera were subjected to (i) 0.1% HgCl2 and 0.3% CaCl2 both for 10 min and (ii) 2.6% (w/v) NaOCl for 10 min with a few drops of a wetting agent. Both of them gave about 96% contamination−free culture [39]. Increasing NaOCl concentration from 5 to 20% (v/v) for 20 min, in apical tips treatment from adult male pistachio, showed better decontamination, meantime, the proportion of survived explants was negatively affected [38]. In Melia azedarach L. culture, the lowest contamination and browning response with the highest percentage of callus induction and growth were obtained with benomyl (a systemic fungicide) pretreatment (2 g L− 1) for 2 h and 7% H2O2 for 10 min and 2% (w/v) NaOCl for 12 min [46]. Hence, generally, variable but convergent levels of chemicals are recommended for effective decontamination.
Disinfection efficiency and negative disinfection impact of P. vera explants using three chemicals at different concentrations and immersion time, NaOCl (0.54, 0.9, 1.26% w/v; 10, 20, 30 min), H2O2 (5.25, 8.75, 12.25%; 10, 20, 30 min), and HgCl2 (0.05, 0.1, 0.2%; 5, 10, 15 min) after 20 days of culture. Different letters indicate a significant difference using Duncan’s test at P < 0.05
Theoretically, regardless of the potential induced damage, a more concentrated disinfectant, escorted or not with increased exposure time, will ensure more microorganisms eradication. This is well noticed, for instance, during in vitro sterilization of chrysanthemum including different concentrations of six sterilizing agents and the immersion time [47]. Yet, this notion was refuted here as more accentuated treatment does not necessarily show better asepsis. As Fig. 1 displays, there are fluctuations in the disinfection response even with the same chemical agent concentration noticed mainly with NaOCl and H2O2. Some effects of appraised disinfection procedures are presented in Fig. 2.
Effect of some disinfection procedures on P. vera explants after 20 days of culture: a Explants at day 0 of incubation; b germination of healthy zygotic embryos (T3:0.54% NaOCl, 30 min); c cotyledon explant with dark green color (T5: 0.9% NaOCl, 20 min); d deformed embryo germination (T27: 0.2% HgCl2, 15 min); e explant with chlorosis and browning symptoms (T26: 0.2% HgCl2, 10 min)
During disinfection processes, the biological activity of cells should not be affected and only contaminants should be eliminated. Although it seems a simple step, decontamination in some species is extremely difficult. In many cases, anti−microbial treatments only inhibit contaminants and low levels of these latter persist. Detection of latent contamination, namely, without visible symptoms on explant and/or visible growth on medium culture, may involve screening using general and semi−selective microbial growth media or serological and PCR − based molecular techniques for specific pathogens. Nonetheless, it is often difficult to detect low numbers of latent bacterial contaminants [8]. The latent aspect could be correlated to the absence of some growth factors/vitamins in the plant culture medium required for bacterial growth [48]. Moreover, the acidity of the medium and the release of antibacterial exudates by the plant cells/tissues can also suppress bacterial growth [49]. This kind of contamination could proliferate following the transfer from one stage to another which involved usually adjustment in the nutrient regimes.
Disinfection treatment can highly affect the subsequent response of the tissue, alongside a multitude of factors including even the explant orientation (vertical, inverted vertical, horizontal, and abaxial or adaxial face), which substantiates its importance. The use of hot water and plant preservative mixture (PPM), a broad–spectrum biocide/fungicide for plant tissue culture, improves the rate of bud germination and differentiation from 4 to 50% in ginger (Zingiber officinale) explants [19]. By testing two sterilization procedures for stem tips culture in P. vera, it was described that NaOCl was more conducive to rosette development (75%) but did not significantly differ from HgCl2 (66.7%) [39].
As above–stated, a wide range of surface disinfectants with varying degrees of effectiveness have been used in plant tissue culture, often with a few drops of a wetting agent (e.g. Tween 20). Still, NaOCl is the most widely applied compound [50, 51]. Based on its concentration, the germicidal effect of NaOCl was attributed predominantly to hypochlorous acid (HOCl) in diluted solution [52] and to its high pH (12.5–13.5) and hypochlorite ion (ClO−) oxidizing agent in concentrated form [53]. ClO− has a poor germicidal activity due to its inability to diffuse through the microbial plasma membrane and it exerts an oxidizing action only from outside of the cell. HOCl can penetrate across the cell wall and the lipid bilayer in the plasma membrane by passive diffusion due to its electrical neutrality, thus, can attack the microbial cell both from the outside and inside the cell, which is responsible for the potent germicidal activity of this agent. Both substances give rise to the inhibition of enzyme activity essential for the growth, damage to the membrane and DNA, and possibly an injury to membrane transport [15]. The pH adjusting (to pH = 7 and 10) reduced significantly the microbial contamination in Melia azedarach L. culture, but, adversely influenced the explant viability and callus induction and growth [46]. A positive effect was accorded to this disinfection agent during micropropagation of Kalanchoe tubiflora (Harvey) Hamet which has been assumed to correlate with the positive influence of stress (associated with explants disinfection) [54]. Chemical sterilization, using NaOCl, was proved to be useful as a replacement for thermal sterilization of nutrition media [55]. Chlorine dioxide was also used to sterilize the medium for tissue culture of potato (Solanum tuberosum L.) [56]. NaOCl can alleviate significantly the inhibitive effect of salt stress during the germination phase. Added to the germination solution, Allium cepa L. seed treated with a 0.1% NaOCl + 0.225 M NaCl for 7 d showed an improvement in the germination percentage, the radical length and number, and the fresh weight compared with the salt treatment [57]. In another aspect, NaOCl and H2O2 were described as effective for releasing dormancy of imbibed wild oat (Avena fatua L.) seeds via a modification of the properties of the hull and seed coat membranes and in the provision of additional oxygen to the seeds [58]. This fact was evidenced in this experiment. Pistachio zygotic embryos were able to respond to external stimulants and germination was noticed in all explants considering that the cultures were induced with dormant seeds. Besides its effect as a disinfectant agent, it was reported that NaOCl can be used as a dormancy−breaking agent by decomposing germination inhibitors [59], scarification of the seed coat [16], and increasing α–amylase activity [60].
Mercuric chloride is a highly toxic chemical reagent for humans and plant tissues [61,62,63]. Heavy metals, such as mercury, are known for their immunotoxic and neurotoxic properties and are environmental pollutants [64]. Nevertheless, this compound was used in various works for disinfection purposes in in vitro plant culture. Its toxic potential has been confirmed, in the present experiment, especially with the application of a high concentration for a long immersion time (Figs. 1 and 2). Whereas, H2O2 is a non–phytotoxic compound due to the activity of plant peroxidases and catalases allowing its transformation into water and oxygen [65]. In agreement, a relatively moderate negative impact was noticed here with this chemical.
As aforementioned, several disinfection protocols were proposed. The one factor at a time (OFAT) approach was commonly considered for the “optimization” of the process. For instance, for the decontamination of shoots or apical tips from male Pistacia vera L. cv. ‘Atlı’, NaOCl was applied at four concentrations 5, 10, 15, and 20% (v/v) for 10 min as the first step. Then, 10% NaOCl was selected for combination with five immersion times 5, 10, 20, 30, and 40 min. Subsequently, the presterilized explants (10% NaOCl for 30 min) were subjected to 10% H2O2 for 5 and 10 min [38, 66]. This approach is conventionally adopted, although, it does not allow highlighting the interactive effect between the different factors.
Artificial neural network modeling
To simulate the connection between the three sterilizing chemicals, their concentrations, and the immersion time, on one side, and the disinfection efficiency and the negative disinfection effect, on the other side, a multilayer perceptron (MLP) model was developed. The ANN consisted of three layers, the input layer with 4 neurons, the hidden layer with 10 neurons, and the output layer with 2 neurons (Fig. 3). The number of neurons in the hidden layer was decided using the trial−and − error method by changing the number of neurons until a maximum regression R and minimum mean squared error (MSE) were obtained. Therefore, the ANN topology was 4–10 − 2. Determining the construction of the MLP has the main function in its performance [67]. Each neuron in the hidden and the output layer has a bias (b) value and is linked to the previous layer’s neurons with an interconnection having a certain weight (w) and, altogether, the generated structure creates the network. The sizes for the weights matrices are 10 × 4 and 10 × 1 for both evaluated parameters, for joining the input layer to hidden layer and the hidden layer to output layer, respectively, while the size of the biases matrices are 10 × 1 and 1 for the neurons of the hidden layer and the output layer, respectively. The ANN dimensions were given in Eqs. (5, 6, 7, 8).
Details of developed feed–forward back–propagation neural network (Topology: 4–10 − 2): input layer with 4 neurons, one hidden layer with 10 neurons (only neuron N°1 was presented), and output layer with 2 neurons. W1: weight of hidden layer, W2: weight of output layer, b1: bias of hidden layer, b2: bias of output layer, x: inputs (x1: NaOCl (%), x2: H2O2 (%), x3: HgCl2 (%), x4: immersion time (min)), Y: outputs (Y1: disinfection efficiency (%), Y2: negative disinfection effect (%))
Post−training analysis showed R values: 0.9658, 0.9653, 0.8937, and 0.9454 for training, validation, testing, and all, respectively, revealed a good correlation between the predicted (output) and the actual (target) data (Fig. 4a). The performance evaluation, in terms of MSE, showed that the network manifests improvement and the best validation performance was obtained at 8 epochs with an MSE value of 6.65E− 02 (Fig. 4b). Below 8 epochs, MSE is high. Whereas, with a higher epoch number, the MSE of training data decreased, reflecting a network overfitting. By considering R for the training dataset (0.9658), thus, the best performance is automatically selected and the model is assumed to be adequate for data prediction. The predictive capacity of the model can be assessed also considering calculated parameters with 11.09 and 13.5 for RMSE and 0.804 and 0.839 for R2 values for DE and NDE, respectively (Table 3). Figure 4c shows that the data fitting errors, for training, validation, and testing are distributed within a reasonably good range and are very close to zero.
Optimization using multi–objective genetic algorithm
Dissimilar to single–objective problems, in multi–objective situations, it is not easy to find the optimal solution since the objective functions are usually in conflict with each other, that is means improving one will negatively affect the other. Thus, a balanced solution must be established. The presented conflict in the current experiment is to define the optimal combination of inputs to ensure the maximum value for the objective function of decontamination percentage output and, simultaneously, the minimum value for the objective function of negative disinfection effects. Following multi−objective genetic algorithm running, the proposed combination included the three sterilizing agents at different levels: 0.563%, 12.232%, and 0.068% for NaOCl, H2O2, and HgCl2, respectively, with an immersion time of 5.022 min. Subsequently, the validation assay has proved the accuracy of this tool with a high coincidence between the expected and the obtained values with 100% contamination−free culture and 7.1% only of negative disinfection effects. In the same framework, optimizing of in vitro sterilization of chrysanthemum was proposed using multilayer perceptron non–dominated sorting genetic algorithm–II (MLP–NSGAII) encompasses seven inputs, viz. six sterilizing agents and the immersion time, and two outputs including contamination frequency and explants viability [47]. They suggested MLP–NSGAII as an efficient method in different areas of in vitro culture.
Conclusion
Machine learning models and optimization algorithms, revolutionary computational technologies, have opened wide perspectives in the field of plant tissue culture, especially for recalcitrant species. This work represents a case study concerning the effectiveness of these tools and the successful implementation of multi–objective genetic algorithm that can be extended to any nonlinear multivariate processes. An optimized decontamination treatment consisting of 0.56% NaOCl, 12.23% H2O2, and 0.068% HgCl2 for 5.022 min was designed for in vitro P. vera seeds culture. The established protocol will be assessed for other types of explants in subsequent manipulations. It could be either efficient or needs some adjustment considering that the adjoining features can diverge among explants. Meanwhile, underpinned by suitable laboratory practices, the present investigation spotlighted the ingenuity of artificial intelligence technology to manage such steps regardless of the concerned explant. Similarly, optimization algorithms will have an unprecedented impact in further intricate tasks, for instance, in plant growth regulators management, the main decisive molecules for in vitro cell fate acquisition, an extremely precise aspect in plant tissue culture.
Availability of data and materials
All data concerning the current work were included in the manuscript. Other materials and any clarification that support the findings of this study are available from the corresponding author on reasonable request.
References
Hussain A, Qarshi IA, Nazir H, Ullah I. Plant tissue culture: current status and opportunities. In: Leva A, Rinaldi MR, editors. Recent advances in plant in vitro culture. Croatia: InTech; 2012. p. 1–28.
Loyola-Vargas VM, Ochoa-Alejo N. An introduction to plant tissue culture: advances and perspectives. In: Loyola-Vargas VM, Ochoa-Alejo N, editors. Plant cell culture protocols. New York: Springer; 2018. p. 3–13.
Peiró R, Gammoudi N, Yuste A, Olmos A, Gisbert C. Mature seeds for in vitro sanitation of the grapevine leafroll associated virus (GLRaV-1 and GLRaV-3) from grape (Vitis vinifera L.). span. J Agric Res. 2015;13:e1005.
San Pedro T, Gammoudi N, Peiró R, Olmos A, Gisbert C. Somatic embryogenesis from seeds in a broad range of Vitis vinifera L. varieties: rescue of true-to-type virus-free plants. BMC Plant Biol 2017;17(1):226.
Gammoudi N, San Pedro T, Ferchichi A, Gisbert C. Improvement of regeneration in pepper: a recalcitrant species. In Vitro Cell Dev Biol Plant. 2018;54:145–53.
Gammoudi N, Zerria K, Nagaz K, Ferchichi A. Enhancement of capsaicinoids in vitro production by abiotic elicitors in placenta-derived callus of Capsicum annuum L. Tunisian var. ‘Baklouti Medenine’. Biologia. 2019;74:725–32.
Sugimoto K, Temman H, Kadokura S, Matsunaga S. To regenerate or not to regenerate: factors that drive plant regeneration. Curr Opin Plant Biol. 2019;47:138–50.
Leifert C, Cassells AC. Microbial hazards in plant tissue and cell cultures. In Vitro Cell Dev Biol Plant. 2001;37:133–8.
Da Silva JAT, Kulus D, Zhang X, Zeng S, Ma G, Piqueras A. Disinfection of explants for saffron (Crocus sativus) tissue culture. Environ Exp Biol. 2016;14:183–98.
Cassells AC. Problems in tissue culture: culture contamination. In: Debergh PC, Zimmerman RH, editors. Micropropagation: technology and application. Dordrecht: Kluwer Academic Publishers; 1991. p. 31–44.
Niedz RP, Bausher MG. Control of in vitro contamination of explants from greenhouse-and field-grown trees. In Vitro Cell Dev Biol–Pl. 2002;38:468–71.
Sugii NC. The establishment of axenic seed and embryo cultures of endangered Hawaiian plant species: special review of disinfestation protocols. In Vitro Cell Dev Biol–Pl. 2011;47:157–69.
Cassells A. Pathogen and biological contamination management in plant tissue culture: Phytopathogens, vitro pathogens, and vitro pests. In Plant Cell Culture Protocols—Methods in Molecular Biology (Methods and Protocols); Loyola-Vargas, V., Ochoa-Alejo, N., Eds.; Humana Press: Totowa, NJ, USA. 2012; 877: 57–80.
Da Silva TJA, Winarto B, Dobranszki J, Zeng S. Disinfection procedures for in vitro propagation of Anthurium. Folia Hortic. 2015;27:3–14.
Fukuzaki S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006;11:147–57.
Yildiz M, Er C. The effect of sodium hypochlorite solutions on in vitro seedling growth and shoot regeneration of flax (Linum usitatissimum). Naturwissenschaften. 2002;89:259–61.
Abdul-Baki AA. Hypochlorite and tissue sterilization. Planta (Berl). 1974;115:373–6.
Telci C, Yıldız M, Pelit S, Onol B, Erkılıc EG, Kendir H. The effect of surface disinfection process on dormancy-breaking seed germination, and seedling growth of Lathyrus chrysanthus Boiss. Under in vitro conditions. Prog Ornam Plants. 2011;11:10–6.
Ma X, Gang DR. Metabolic profiling of in vitro micropropagated and conventionally greenhouse grown ginger (Zingiber officinale). Phytochemistry. 2006;67(20):2239–55.
Gago J, Martínez-Núñez L, Landín M, Flexas J, Gallego PP. Modeling the effects of light and sucrose on in vitro propagated plants: a multiscale system analysis using artificial intelligence technology. PLoS One. 2014;9:85989.
Arab MM, Yadollahi A, Eftekhari M, Ahmadi H, Akbari M, Khorami SS. Modeling and optimizing a new culture medium for in vitro rooting of G × N15 prunus rootstock using artificial neural network-genetic algorithm. Sci Rep. 2018;8(1):e9977.
Hesami M, Naderi R, Tohidfar M, Yoosefzadeh-Najafabadi M. Application of adaptive neuro-fuzzy inference system-non-dominated sorting genetic algorithm-II (ANFIS-NSGAII) for modeling and optimizing somatic embryogenesis of Chrysanthemum. Front Plant Sci. 2019;10:869.
García-Pérez P, Lozano-Milo E, Landín M, Gallego PP. Machine learning technology reveals the concealed interactions of phytohormones on medicinal plant in vitro organogenesis. Biomolecules. 2020;10(5):746.
Shukla MR, Singh AS, Piunno K, Saxena PK, Jones AMP. Application of 3D printing to prototype and develop novel plant tissue culture systems. Plant Methods. 2017;13:6–15.
Kendrick RE, Kronenberg GH, editors. Photomorphogenesis in plants. Dordrecht: Springer Science & Business Media Kluwer; 2012. p. 333–6.
Batista DS, Felipe SHS, Silva TD, Castro KM, Mamedes-Rodrigues TC, Miranda NA, et al. Light quality in plant tissue culture: does it matter? In Vitro Cell Dev Biol Plant. 2018;54:195–215.
Hesami M, Jones AMP. Application of artificial intelligence models and optimization algorithms in plant cell and tissue culture. Appl Microbiol Biotechnol. 2020;104:1–37.
Pilkington JL, Preston C, Gomes RL. Comparison of response surface methodology (RSM) and artificialneural networks (ANN) towards efficient extraction of artemisinin from Artemisia annua. Ind Crop Prod. 2014;58:15–24.
Gammoudi N, Mabrouk M, Bouhemda T, Nagaz K, Ferchichi A. Modeling and optimization of capsaicin extraction from Capsicum annuum L. using response surface methodology (RSM), artificial neural network (ANN), and Simulink simulation. Ind crop. Prod. 2021;171:113869.
Fonseca CM, Fleming PJ. Multiobjective genetic algorithms. In: IEE colloquium on ‘Genetic Algorithms for Control Systems Engineering’ (Digest No. 1993/130), 28 May 1993. London: IEE; 1993.
Srinivas N, Deb K. Multiobjective optimization using nondominated sorting in genetic algorithms. J Evol Comput. 1994;2(3):221–48.
Deb K, Pratap A, Agarwal S, Meyarivan T. A fast and elitist multiobjective genetic algorithm: NSGA-II. IEEE Trans Evol Comput. 2002;6(2):182–97.
Buffo RA, Han JH. Edible films and coatings from plant origin proteins. Innovations in Food Packaging. 2005:277–300.
Ghorbel A, Ben Salem-Fnayou A, Chatibi A, Twey M. Genetic resources of Pistacia. In: Tunisia SP, Hadj-Hassan A, editors. Project on underutilized Mediterranean species. Pistacia: towards a comprehensive documentation of distribution and use of its genetic diversity in central and West Asia, North Africa and Mediterranean Europe. Irbid: Report of the IPGRI workshop; 2001. p. 62–72.
Gannoun S, Lionakis SM, Gerasopoulos D. Aspects of in vitro culture of Pistacia terebinthus and Pistacia vera. Acta Hort (ISHS). 1995;419:201–6.
Benmahioul B. Amélioration de la micropropagation in vitro du pistachier (Pistacia vera L.) en vue de l’extension des vergers en Algérie. [Ph.D. Thesis.], vol. 129. Oran: University of Sciences and Technology of Oran Mohamed Boudiaf; 2009.
Onay A. Micropropagation of pistachio. In: Jain MS, Ishii K, editors. Micropropagation of Woody trees and fruits, 75. Dordrecht: Kluwer Acad. Press; 2003. p. 565–88.
Tilkat E, Süzerer V, Akdemir H, Ayaz Tilkat E, Ozden Çiftçi Y, Onay A. A rapid and effective protocol for surface sterilization and in vitro culture initiation of adult male pistachio (Pistacia vera L. cv. “Atlı”). Academia. J Sci Res. 2013;1:134–41.
Benmahioul B, Kaid-Harche M, Daguin F. In vitro regeneration of Pistacia vera L. from nodal explants. J For Sci. 2016;62:198–203.
Frais S, Ng YL, Gulabivala K. Some factors affecting the concentration of available chlorine in commercial sources of sodium hypochlorite. Int Endodontic J. 2001;34:206–15.
Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–97.
Lawal AI. An artificial neural network-based mathematical model for the prediction of blast-induced ground vibration in granite quarries in Ibadan, Oyo state, Nigeria. Scientific African. 2020;8:1–10.
Olalere OA, Abdurahman NH, bin Mohd Yunus R, Alara OR. Multi-response optimization and neural network modeling for parameter precision in heat reflux extraction of spice oleoresins from two pepper cultivars (Piper nigrum). J King Saud University-Science. 2019;31:789–97.
Ray A, Halder T, Jena S, Sahoo A, Ghosh B, Mohanty S, et al. Application of artificial neural network (ANN) model for prediction and optimization of coronarin D content in Hedychium coronarium. Ind Crop Prod. 2020;146:112186.
Onay A, Pirinç V, Yildirim H, Başaran D. In vitro micrografting of pistachio (Pistacia vera L. cv. Siirt). Plant Cell Tissue Organ Cult. 2004;77:215–9.
Ahmadpoor F, Zare N, Asghari R, Sheikhzadeh P. Sterilization protocols and the effect of plant growth regulators on callus induction and secondary metabolites production in in vitro cultures Melia azedarach L. AMB Exp. 2022;12(1).
Hesami M, Naderi R, Tohidfar M. Modeling and optimizing in vitro sterilization of chrysanthemum via multilayer perceptron-non-dominated sorting genetic algorithm-II (MLP-NSGAII). Front Plant Sci. 2019;10:282.
Leifert C, Waites WM. Bacterial growth in plant tissue cultures. J Appl Bacteriol. 1992;72:460–6.
Leifert C, Berger F, Steward GSAB, Waites WM. Plasmid profiles of lactobacillus plantarum found as contaminants in Hemerocallis plant tissue cultures. Let Appl Microbiol. 1994;19:377–9.
Lazo-Javalera MF, Troncoso-Rojas R, Tiznado-Hernández ME, Martrtínez-Tellez MA, Varg as-Arispuro I, Islas-Osuna MA, Rivera-Domínguez M. Surface disinfection procedure and in vitro regeneration of grapevine (Vitis vinifera L.) axillary buds. Springerplus. 2016.
Tung HT, Bao HG, Cuong DM, Ngan HTM, Hien VT, Luan VQ, et al. Silver nanoparticles as the sterilant in largescale micropropagation of chrysanthemum. In Vitro Cell Dev Biol – Plant. 2021;57:897–906.
Dantec CL, Duguet JP, Montiel A, Dumoutier N, Dubrou S, Vincent V. Chlorine disinfection of atypical mycobacteria isolated from a water distribution system. Appl Environ Microbiol. 2002;68:1025–32.
Estrela C, Estrela CRA, Barbin EL, Spano JC, Marchesan MA, Pecora JD. Mechanism of action of sodium hypochlorite. Braz Dent J. 2002;13:113–7.
Kulus D. Micropropagation of Kalanchoe tubiflora (Harvey) Hamet. Sci Nat Technol. 2015;9(1):14.
Pais AK, Silva APda, Souza JCde, Teixeira SL, Ribeiro JM, Peixoto AR, Paz CD. Sodium hypochlorite sterilization of culture medium in micropropagation of Gerbera hybrida cv. Essandre Afr J Biotechnology. 2016;15(36):1995–8.
Duan Y, Zhang H, Sun M, Zhao F, Xue T, Xue J. Use of chlorine dioxide to sterilize medium for tissue culture of potato. Sci Rep. 2019;9:10232.
Çavuşoğlu K, Doğu F, Çavuşoğlu D. Effects of sodium hypochlorite on some physiological and cytogenetical parameters in Allium cepa L. exposed to salt stress. Bangladesh J Bot. 2019;48:223–9.
Hsiao AI, Quick WA. Actions of sodium and hydrogen peroxide on seed dormancy and germination of wild oats, Avena fatua L. Weed Res. 1984;24:411–9.
Ogawa K, Iwabuchi M. A mechanism for promoting the germination of Zinnia elegans by hydrogen peroxide. Plant & Cell Physiol. 2001;42:286–91.
Kanecko Y, Morohashi Y. The effect of sodium hypochlorite treatment on the development of α-amylase activity in mung bean cotyledons. Plant Sci. 2003;164:287–92.
Patra M, Sharma A. Mercury toxicity in plants. Bot Rev. 2000;66:379–422.
Vaidya VS, Mehendale HM. Mercuric chloride (HgCl2). Encyclopedia of. Toxicology. 2014:203–6.
Yang J, Li G, Bishopp A, Heenatigala PPM, Hu S, Chen Y, et al. A comparison of growth on mercuric chloride for three Lemnaceae species reveals differences in growth dynamics that effect their suitability for use in either monitoring or remediating ecosystems contaminated with mercury. Front Chem. 2018;6:112.
Marinescu MV, Teodorescu A, Şuţan NA. Preliminary results on the in vitro propagation by leaf explants and axillary buds of Iris aphylla L. J Hortic For Biotechnol. 2013;17:279–82.
Arora A, Sairam RK, Srivastava GC. Oxidative stress and antioxidative system in plants. Curr Sci. 2002;82:1227–38.
Tilkat E, Onay A, Yildirim H, Ayaz E. Direct plant regeneration from mature leaf explants of pistachio. Pistacia vera L Sci Hortic. 2009;121:361–5.
Domingues GF, Soares VP, Leite HG, Ferraz AS, Ribeiro CAAS, Lorenzon AS, et al. Artificial neural networks on integrated multispectral and SAR data for high-performance prediction of eucalyptus biomass. Comput Electron Agric. 2020;168:105089.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not–for–profit sectors.
Author information
Authors and Affiliations
Contributions
NG: conceived, designed, performed the experiments, software, analyzed the data, wrote, and revised the paper. KN, AF: provision laboratory resources. The author(s) read and approved the final manuscript.
Authors’ information
Arid and Oases Cropping Laboratory, Arid Lands Institute (IRA), 4119 Medenine, Tunisia.
Najet Gammoudi, Kamel Nagaz.
National Institute of Agronomy of Tunis, 43 Charles Nicolle, 1082 Tunis, Tunisia.
Ali Ferchichi.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experiments did not utilize transgenic technology. All plant materials used in this study were obtained from cultivated plants and did not involve any endangered or protected species. Collection of plant material complied with institutional, national, and international guidelines and was conducted in accordance with local legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gammoudi, N., Nagaz, K. & Ferchichi, A. Establishment of optimized in vitro disinfection protocol of Pistacia vera L. explants mediated a computational approach: multilayer perceptron–multi−objective genetic algorithm. BMC Plant Biol 22, 324 (2022). https://doi.org/10.1186/s12870-022-03674-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03674-x