Abstract
Background
The ratio of CO2 mesophyll conductance (gm) to Ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco) content has been suggested to positively affect photosynthetic nitrogen use efficiency (PNUE). The anatomical basis of gm has been quantified, but information on the relationship between cell-level anatomies and PNUE is less advanced. Here, hydroponic experiments were conducted in rice plants supplied with ammonium (NH4+) and nitrate (NO3−) under three N levels (low, 0.71 mM; intermediate, 2.86 mM; high, 7.14 mM) to investigate the gas exchange parameters, leaf anatomical structure and PNUE.
Results
The results showed a lower PNUE in plants supplied with high nitrogen and NH4+, which was positively correlated with the gm/Rubisco ratio. A one-dimensional within-leaf model revealed that the resistance to CO2 diffusion in the liquid phase (rliq) dominated the overall mesophyll resistance (rm), in which CO2 transfer resistance in the cell wall, cytoplasm and stroma were significantly affected by nitrogen supply. The chloroplast surface area exposed to intercellular space (Sc) per Rubisco rather than the gm/Sc ratio was positively correlated with PNUE and was thus considered a key component influencing PNUE.
Conclusion
In conclusion, our study emphasized that Sc was the most important anatomical trait in coordinating gm and PNUE with contrasting N supply.
Similar content being viewed by others
Background
Photosynthetic nitrogen use efficiency (PNUE), determined as the ratio of photosynthesis rate (Pn) to leaf organic nitrogen content [1], is a key component of nitrogen use efficiency (NUE) and an indicator of the relationship between leaf nitrogen (N) and Pn. Under the present atmosphere, the unsaturated CO2 concentration in C3 leaves influences the carboxylation of Ribulose-1,5-disphosphate (RuBP) and results in a finite Pn, which fails to match the increase in leaf N and induces a decrease in PNUE [2]. By using an “evolutionary” algorithm, the partitioning of photosynthetic enzymes was altered based on a fixed total amount of protein-nitrogen for maximizing Pn, and the result showed that an increase in Ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco) was required to maximize Pn [3]. It was also well documented that higher leaf N allocation into Rubisco was linked with an enhancement in PNUE [4].
Numerous studies have clarified that the enhancement in Rubisco activity is another favorable candidate for improving RuBP carboxylation efficiency and Pn because of its poor catalytic ability under ambient conditions due to the low CO2 concentration and the low affinity for CO2 [1, 5]. As the substrate of Rubisco, CO2 concentration in the chloroplast (Cc), which is determined by stomatal conductance (gs) and mesophyll conductance (gm), plays a dominant role in regulating Rubisco activity [6, 7]. It has been demonstrated that gm induces 40% of the total decrease in CO2 concentration between the atmosphere and the carboxylation sites of Rubisco [8]. In a previous study, Li et al. [5] argued that an increase in gm was not sufficient to meet the carboxylation demand of the increased Rubisco content and eventually resulted in a decreased PNUE. Therefore, it is speculated that factors affecting gm would influence Rubisco activity and the relationship between Pn and leaf N content.
Evidence is now mounting that gm is largely dependent on leaf anatomical characteristics, including leaf thickness, cell wall thickness and chloroplast morphology [9, 10]. Higher leaf density and thicker mesophyll cell walls contribute to a reduction in gm [9, 11,12,13,14], and mesophyll and/or chloroplast surface areas exposed to the intercellular space, Smes and Sc, respectively, are positively correlated with gm [15]. The overall importance of different anatomical traits in the restriction of gm varies [16]. For gymnosperms, the strongest sources of gm are cell wall and chloroplast thickness, variation in chloroplast shape and size, and Sc [9]. In lycophytes and bryophytes, the highest CO2 diffusive resistance is mainly driven by extremely high cell wall thickness and low Sc [17]. Even though the anatomical factors influencing gm have been widely studied, the role of these anatomical factors in influencing PNUE and their relative contribution in rice plants are still largely unknown.
Leaf anatomy is remarkably influenced by N nutrition; for example, decreasing leaf thickness and smaller chloroplasts with no starch granules have been detected in nitrogen-deficient leaves, while high-N leaves have more large chloroplasts with well-developed grana, stroma lamellae and starch granules per mesophyll cell [5, 18,19,20]. For different nitrogen forms, increased leaf thickness and a doubling of chloroplast volume with a larger internal membrane length have been found in NH4+-fed plants compared with NO3−-fed plants [21,22,23]. In this study, we examine the responses of leaf anatomical characteristics, including leaf thickness, mesophyll cell size, chloroplast length and thickness, chloroplast number per mesophyll cell under NH4+ and NO3− nutrition with different N levels; moreover, we discuss the implications for understanding leaf trait variation with changes in N nutrition along the PNUE. Our objectives of the present study were as follows: (1) to identify the response of PNUE and leaf anatomical traits upon NH4+ and NO3− nutrition at different N levels; (2) to clarify the role of leaf anatomical factors in coordinating the gm and PNUE under NH4+ and NO3− nutrition supply; and (3) to investigate the most limiting fraction of leaf anatomy in determining PNUE under different N supply.
Results
Effects of nitrogen supply on rice photosynthetic nitrogen use efficiency (PNUE)
Compared with those with low nitrogen supply (LAN and LNN), rice biomass and leaf area with intermediate and high nitrogen supply increased by 70–73% and 33–42% under NH4+ nutrition and by 30–48% and 40–41% under NO3− nutrition, respectively (Table 1). There were no significant differences in rice biomass and leaf area between the intermediate and high nitrogen supply conditions. Rice biomass was less affected by nitrogen forms at the same nitrogen level, while for intermediate nitrogen supply, the leaf area was decreased by 10% in NO3−-fed plants than in NH4+-fed plants (Table 1). The leaf N content (NL) was 54–62% and 66–80% higher under intermediate N supply and high N supply than under low N supply and was decreased by 9–11% under NO3− nutrition compared with that under NH4+ nutrition (Table 1). The Rubisco content was 26, 21 and 21% lower under low NO3− (LNN), intermediate NO3− (MNN) and high NO3− (HNN) than that under low NH4+ (LAN), intermediate NH4+ (MAN) and high NH4+ (HAN), respectively (Table 1). The stomatal conductance (gs) was less affected by N supply than mesophyll conductance (gm), which was increased by 72 and 24% in HAN and HNN, respectively, compared with that in LAN and LNN and decreased by 4–30% under NO3− compared to NH4+ nutrition (Table 1). Neither nitrogen supply levels nor nitrogen forms affected the chloroplast CO2 concentration (Cc). With increasing leaf N content, the light-saturated photosynthetic rate (Pn) increased, while the photosynthetic nitrogen use efficiency (PNUE) decreased (Table 1, Fig. 1a, b). The PNUE was 21, 17 and 14% higher in LNN, MNN and HNN than in LAN, MAN and HAN, respectively (Table 1). Positive correlations existed between PNUE and both the Cc/Rubisco ratio and the gm/Rubisco ratio (Fig. 1c, d).
The relationship between leaf N content (NL) and the photosynthetic rate (Pn) (a) and photosynthetic nitrogen use efficiency (PNUE) (b) and the relationship between PNUE and the ratio of chloroplast CO2 concentration to Rubisco (Cc/Rubisco) (c) and the ratio of mesophyll conductance to Rubisco (gm/Rubisco) (d). Each point represents one replicate (four replicates per treatment). The lines represent the following regression equations: a y = − 1.8756 × 2 + 11.3310x + 13.0290, R2 = 0.6228, P < 0.05; b y = − 0.0763x + 0.3565, R2 = 0.7886, P < 0.05; c y = 0.0017x + 0.0774, R2 = 0.8295, P < 0.01; d y = 1.5663x + 0.0733, R2 = 0.4215, P < 0.01
Effects of nitrogen supply on leaf anatomical properties
With increasing leaf N supply levels, leaf thickness (TL) and mesophyll cell thickness (Tm) increased in NH4+-fed plants but decreased in NO3−-fed plants (Supplementary Fig. S1, Fig. 2a, b). Leaf dry mass per area (MA), leaf density (DL) and mesophyll cell wall thickness (Tmc) were increased by high N supply either with NH4+ or NO3−, and were lower under NO3− nutrition than under NH4+ nutrition. Mesophyll surface area exposed to intercellular airspace (Smes) and the chloroplast surface area facing intercellular airspace (Sc) were upregulated significantly by increasing the nitrogen supply level (Fig. 2b). The Smes increased by 22–37 and 21% under intermediate and high N supply conditions in NH4+ and NO3− nutrition, respectively, and the corresponding Sc increased by 22–38% and 21–24%, both compared with their respective low N supply conditions. No obvious differences in Sc between NH4+ and NO3− under low N levels were observed, but the Sc decreased by 11 and 20% under MNN and HNN, respectively, compared to that under MAN and HAN (Fig. 2b).
Effects of different nitrogen supply levels on integral leaf variables (a), mesophyll cell (b) and chloroplast (c, d) anatomical characteristics. MA (g m− 2), leaf dry mass per area. TL (μm), leaf thickness. DL (g m− 2 μm− 1), leaf density. Tm (μm), mesophyll thickness. Tmc (μm), mesophyll cell wall thickness. Smes (μm2 μm− 2), mesophyll surface area exposed to intercellular airspace. Sc (μm2 μm− 2), chloroplast surface area exposed to intercellular airspace. Nc, chloroplast number per mesophyll cell. Lc (μm), chloroplast length. Tc (μm), chloroplast thickness. Secc (μm2), chloroplast section area. Volc (μm3), chloroplast volume. Surc (μm2), chloroplast surface area. The error bars indicate the standard deviation and at least 15 replicates were conducted for each parameter
We further analyzed the chloroplast number per mesophyll cell (Nc), chloroplast length (Lc), chloroplast thickness (Tc), chloroplast surface area (Surc), chloroplast volume (Volc) and chloroplast section area (Secc). Lc was lower in NO3−-fed plants than in NH4+-fed plants under intermediate and high N levels (Supplementary Fig. S1, Fig. 2c). Compared with that in LAN and LNN, the Tc was increased by 13–27% in MAN and HAN and 23–29% in MNN and HNN (Supplementary Fig. S1, Fig. 2c). Higher Surc and Volc were observed under high N supply. The chloroplast size was decreased under NO3− nutrition by 10–22%, 15–33%, and 10–40% in Surc, Volc and Secc under low N, intermediate N and high N supply, respectively (Supplementary Fig. S1, Fig. 2d).
Anatomical limitations of mesophyll conductance
The values of gm calculated according to the methods of Harley et al. [24] and Tomas et al. [16] were strongly positively linearly correlated (Supplementary Fig. S2, R2 = 0.936). Further quantitative analysis showed that both the resistance in the gas phase (rias) and proportion of gas-phase limitations (lias) of gm had little impact on the overall mesophyll resistance (Fig. 3), and that the liquid phase resistance (rliq) was responsible for the limited gm majority, among which stroma played a dominant role. High N supply significantly increased the resistance in the cell wall (rcw) and stroma (rst); compared with low N supply, gm limited by the stroma (lst) was increased by 9–10% under moderate N supply and by 9–13% under high N supply (Fig. 3b). Consistent with the absolute cytoplasm resistance, gm limited by the cytoplasm (lcyt) and cell wall (lcw) were downregulated under high N supply and NO3− nutrition, respectively (Fig. 3). Among all the components, rcw was the primary component affected by N forms and was 19, 23 and 16% higher under NH4+ nutrition than under NO3− nutrition in low N, intermediate N and high N supply, respectively (Fig. 3a).
Anatomical limitations of mesophyll conductance (gm) (a) and the share of the overall gm limitation (b) by cell wall (cw), plasma membrane (pl), chloroplast envelope (en), cytoplasm (cyt) and stroma (st) in rice leaves supplied with NH4+ (AN) or NO3− (NN) under 3 different amounts, low N (0.71 mM, LAN and LNN), intermediate N (2.86 mM, MAN and MNN), and high N (7.14 mM, HAN and HNN). The inset figure shows the anatomical limitations of gm and the share of the overall gm limitation by gas phase. The error bars indicate the standard deviation and at least 15 replicates were conducted for each parameter. Different letters indicate statistically significant differences (P < 0.05) between different treatments
Discussion
Effects of N supply on the gm/Rubisco ratio and photosynthetic nitrogen use efficiency (PNUE)
Decreased PNUE under high N supply has been reported in previous and present studies (Table 1, Fig. 1) [5, 25,26,27]; referring to N forms, higher PNUE under NO3− nutrition than NH4+ nutrition in the present study is consistent with results in barley (Hordeum vulgare L.) [28], pine [29], and cucumber [30]. Leaf nitrogen allocation is an important factor influencing PNUE. Onoda et al. [31] indicated that a higher fraction of photosynthetic nitrogen in electron transport and Rubisco would contribute to increased PNUE in leaves with lower dry mass per area (MA), while in leaves with higher MA, the over-investment of nitrogen in photosynthetic nitrogen and/or cell walls would reduce PNUE [1]. The effect of the proportion of Rubisco in leaf N content on PNUE can be expressed based on Eq. (6):
Our study detected that the Rubisco allocation ratio was increased under high nitrogen supply but decreased under NO3− nutrition compared with NH4+ nutrition; however, the portion of Rubisco in leaf N content was not associated with PNUE (Fig. 4a). These results implied that Rubisco activity, rather than its content, played a dominant role in regulating PNUE [1, 5, 32].
The relationship between photosynthetic nitrogen use efficiency (PNUE) and the ratio of Rubisco to leaf N content (Rubisco/NL) (a), the difference between intercellular CO2 concentration and chloroplast CO2 concentration (Ci-Cc) (b), the ratio of mesophyll conductance to chloroplast surface area exposed to intercellular airspace (gm/Sc) (c), and the ratio of chloroplast surface area exposed to intercellular airspace to Rubisco (Sc/Rubisco) (d). Data represent the mean of 4 replicates for PNUE, Rubisco, NL, Ci, Cc, gm and at least 15 replicates for Sc. The line in the figure represents the following regression equation: y = 0.0299x – 0.0019, R2 = 0.9700, P < 0.01
An increased Rubisco allocation ratio requires increased CO2 partial pressure at the carboxylation site (Cc) to meet carboxylation demands; however, the extent of the increase in Cc was less than that in Rubisco content, which resulted from the finite stomatal conductance (gs) and mesophyll conductance (gm). Li et al. [5] demonstrated that the smaller increases in gm relative to Rubisco content resulted in relatively lower CO2 levels in chloroplasts and PNUE (Fig. 1c, d), which implied that the gm/Rubisco ratio rather than the absolute value of gm was the key factor that regulates PNUE. We further compared the gap between estimated gm and Cc proposed by Harley et al. [24] (Eq. 5, 6) and theoretical Cc (Cc-Theoretical) and gm (gm-Theoretical), which were calculated as follows based on Ding et al. [27], to evaluate the equilibrium state of gm and Rubisco under different N nutrition conditions:
As shown in Fig. 5, both theoretical and estimated Cc and/or gm, as well as the differences between them, increased obviously with increasing leaf N content, and the gap between theoretical and estimated Cc and/or gm under NH4+ nutrition was larger than that under NO3− nutrition. These results confirmed that the balance between Rubisco content and Cc and/or gm was weaker when high N and NH4+ were supplied, and the relatively lower Cc failed to meet the carboxylation demands of the increased Rubisco content, resulting in decreased PNUE (Fig. 1c, d).
The relationships between the leaf-N content (NL) and the estimated and theoretical chloroplast CO2 concentration (Cc) (a, b) and the estimated and theoretical mesophyll conductance (gm) (c, d) under NH4+ (a, c) and NO3− (b, d) nutrition. Each point represents one replicate (four replicates per treatment). The dashed lines represent the theoretical chloroplast CO2 concentration and mesophyll conductance, which were calculated according to the ratio of Cc/Rubisco and gm/Rubisco constant at low-N levels, and the theoretical Cc and gm at intermediate and high N levels was Rubisco (intermediate or high N) × (Cc/Rubisco or gm/Rubisco (low N)). The lines represent the estimated Cc and gm. Rice leaves supplied with NH4+ (AN) or NO3− (NN) under 3 different amounts, low N (0.71 mM, LAN and LNN), intermediate N (2.86 mM, MAN and MNN), and high N (7.14 mM, HAN and HNN)
Overall importance of leaf anatomy in determining gm and PNUE
When leaf nitrogen content was expressed on a leaf dry mass basis, no significant differences in leaf N content between NH4+ and NO3− nutrition were obtained. Therefore, the discrepancies in PNUE between different N forms were primarily caused by the difference in MA (Fig. 2), which resulted from leaf anatomy characteristics such as leaf density (DL), leaf thickness (TL) and cell wall thickness (Tmc). In NO3−-fed plants, the lower MA was the ultimate result of lower DL and Tmc. However, a lower MA was not always related to a lower gm, as observed in the present study; Hassiotou et al. [8] found a negative relationship between MA and gm in the range of 100–500 g m− 2 of MA, while Hanba et al. [33] clarified a positive relationship in the same range of MA. These contrasting results were explained by different ways to enhance MA, as the increases in DL and TL were associated with higher gm, while the opposite conclusion would be obtained if the increase in MA was a result of a thickened cell wall [8]. Our positive correlation between MA and gm implied that the contributions of DL and/or TL compensated for the inhibitory effect of Tmc on gm.
To qualify the relative importance of each leaf anatomy trait in explaining gm, a one-dimensional within-leaf model was calculated to clarify the limitation of gm in each process [16]. The results showed that more than 90% of the total limitation of gm came from Lliq, which was a consequence of limitation in the cell wall (Lcw), plasma membrane (Lpl), envelope (Len), cytoplasm (Lcyt), and stroma (Lst) (Fig. 3) [34]. The decreased contribution of cytoplasmic resistance to gm under high N resulted from the decreased distance between adjacent chloroplasts, rather than the distance between the cell wall and chloroplasts [16], and the increase in chloroplast thickness (Tc) extended the transport path for CO2 from the chloroplast membrane to the carboxylation site in the interior of chloroplasts and resulted in an increasing in rst [35, 36]. Except for the resistance of each part, the chloroplast surface area exposed to intercellular airspace (Sc) was a paramount factor affecting CO2 liquid resistance (rliq). However, the decreased rcyt and increased Sc partially compensated for the increased rst under high N supply and resulted in an increased gm, although the effects were weak and did not match the increase in Rubisco content.
Considering the dominant role of Sc in determining gm, the gm/Rubisco in Eq. (1) was replaced by the product of gm/Sc and Sc/Rubisco to demonstrate the effect of leaf anatomies and gm on PNUE, which can be expressed as follows:
According to the formula above and Terashima et al. [37], a positive correlation would be summarized between PNUE and gm/Sc, which emphasized the potential role of leaf anatomical characteristics except for Sc, as well as the activity of carbonic anhydrase (CA) in contributing to PNUE [37]. However, the weak negative relationship between gm/Sc and PNUE and the significant positive correlation between the Sc/Rubisco ratio and PNUE suggested the dominant role of Sc in influencing PNUE. Due to the limited knowledge of the relationship between PNUE and the Sc/Rubisco ratio, Onoda et al. [14] did not take this component into consideration when they analyzed the physiological and structural tradeoffs underlying the leaf economics spectrum, and they argued that Sc per Rubisco may not correlate strongly with MA or PNUE. However, we detected that Sc per Rubisco was a critical parameter associated with PNUE in rice plants supplied with different N nutrition levels. Similar results were well documented in a review by Terashima et al. [15] and Terashima et al. [37], in which they speculated that, from the perspective of Rubisco and nitrogen use efficiency, thicker leaves with larger Sc were advantageous because the increased ratio of Sc to Rubisco would increase chloroplast CO2 concentration. The increased Sc/Rubisco ratio in NO3−– and high N–fed plants partially resulted from the lower leaf density, which allowed more chloroplast surface area to be exposed to intercellular airspace (Fig. 2a).
Conclusions
In conclusion, we demonstrated that PNUE is decreased in rice plants supplied with high N and ammonium nutrition, which results from unbalanced increases in gm and Rubisco content. Nitrogen-induced variation in gm is associated with leaf anatomical traits, especially chloroplast surface area exposed to intercellular airspace (Sc). We further concluded that the Sc/Rubisco ratio is directly related to the response of PNUE to N supply and that its increase is advantageous to the increase in PNUE.
Methods
Plant material and growth conditions
Rice seeds (Oryza sativa L., ssp. japonica inbred, cv. ‘Zhendao 11’) were purchased from Mingtian Seed Company (Nanjing, China), disinfected with 10% H2O2 for 30 min and germinated in 2.0 mM CaSO4 at 25 °C. The rice seedlings were transferred to 6 L rectangular containers (30 × 20 × 10 cm) when the seedlings developed 2.5 visible leaves, and one quarter-strength mixture of NH4+ and NO3− nutrient solution (for composition, see below) was supplied. Three days later, the seedlings were transferred to a one half-strength nutrient solution. After 6 days, the seedlings were supplied with full-strength nutrient solution for 1 week, after which the seedlings were supplied with either (NH4)2SO4 (AN) or Ca (NO3)2 (NN) at three different N levels: low N (0.71 mM), intermediate N (2.86 mM), and high N (7.14 mM). Thus, six treatments were applied: LAN (low NH4+), MAN (intermediate NH4+), HAN (high NH4+), LNN (low NO3−), MNN (intermediate NO3−), and HNN (high NO3−). In addition, the macronutrients in the solution were as follows (mM): 0.32 P as KH2PO4, 1.02 K as K2SO4 and KH2PO4 and 1.65 Mg as MgSO4. The micronutrients were (μM) as follows: 35.8 Fe as Fe-EDTA, 9.10 Mn as MnCl2·4H2O, 0.52 Mo as (NH4)6Mo7O24·4H2O, 18.5 B as H3BO3, 0.15 Zn as ZnSO4·7H2O, 0.16 Cu as CuSO4·5H2O and 100 Si as Na2SiO3·9H2O. CaCl2 was added to the AN, LNN, and MNN solutions to adjust the Ca level to that of the HNN treatment. The nitrification inhibitor dicyandiamide (DCD) was added to each nutrient solution to prevent the oxidation of NH4+. The nutrient solutions were changed every 3 days, and the pH was adjusted to 5.50 ± 0.05 each day with 0.1 M HCl or NaOH. All the treatments were replicated 5 times with a completely randomized design. The temperature in the greenhouse was maintained at 30 °C during the day and 18 °C at night. Light was supplied by SON-TAGRO 400 W bulbs, and the distance between the light and the rice plants was approximately 60 cm. The light intensity was maintained at a minimum of 1000 μmol photons m− 2 s− 1 at the leaf level using a 14-h photoperiod.
Measurement of biomass and leaf N content
After all the measurements were completed, plant dry weight was determined after oven-drying at 105 °C for 30 min and then at 70 °C to a constant weight. Pictures of the leaves used for the measurement of Pn were taken with a camera along with a benchmark to calibrate, and the leaf area was obtained by ImageJ Pro Plus, after which the leaves were dried and digested with H2SO4-H2O2 at 260–270 °C. The leaf N concentration was determined using a digital colorimeter (AutoAnalyzer 3; Bran+Luebbe).
Gas exchange measurements
Twenty days after treatments, a Li–Cor 6400 infrared gas analyzer was used for the simultaneous measurement of light–saturated photosynthesis (Pn) and chlorophyll fluorescence on the newly expanded leaves from 9:00 to 15:00. Leaf temperatures were 25 °C, the relative humidity was 45%, and photosynthetic photon flux density (PPFD) was 1500 μmol m− 2 s− 1 for all measurements. After equilibration to a steady state, Pn was recorded and the photosynthetic nitrogen use efficiency (PNUE) was calculated as the ratio of Pn to the leaf nitrogen content per leaf area. The fluorescence (Fs) was also measured simultaneously, and a 0.8 s saturating pulse of light (approx. 8000 μmol m− 2 s− 1) was applied to measure the maximum fluorescence (Fm′). The efficiency of photosystem II (ΦPSII) was calculated as ΦPSII = 1- Fs/Fm′. The total electron transport rate (JT) was calculated as JT = ΦPSII × PPFD×αleaf × β, where αleaf and β were leaf absorption and the proportion of quanta absorbed by photosystem II, respectively. In this study, αleaf was also assumed to be 0.85, and β was assumed to be 0.5 [38].
The following equations proposed by Harley et al. [24] were used to calculate the CO2 mesophyll conductance (gm) and chloroplast CO2 concentration (Cc):
where Ci is the intercellular CO2 concentration, Γ* is the CO2 compensation point and Rd is the mitochondrial respiration rate in the light. In the present experiment, Γ* and Rd were measured on newly expanded leaves according to the method of Li et al. [39]. PPFDs in the cuvette were controlled as the series of 150, 300, and 600 μmol m− 2 s− 1. At each PPFD, the ambient CO2 concentration in the cuvette was adjusted as the series of 25, 50, 75 and 100 μmol CO2 mol− 1. Thirty minutes prior to initiating measurements, leaves were placed in the cuvette with a PPFD of 600 μmol photons m− 2 s− 1 and a Ca of 100 μmol CO2 mol− 1.
Anatomical analysis
For the anatomical analysis, approximately 1–2 mm2 leaf sections were cut and fixed in FAA (95% ethanol: glacial acetic acid: formalin: distilled water = 10:1:2:7), dehydrated in ethanol series, and embedded in paraffin. After cutting into 6 μm transverse sections with a microtome and mounting on glass, the glass was stained with Safranin O and fast green and then mounted in DPX mounting medium. Images of each section were obtained with a light microscope (BX 53, Olympus) with a CCD camera (eXcope X3, DIX, Korea). Leaf thickness (TL), mesophyll thickness (Tm), leaf density (DL), and the volume fraction of intercellular air space (fias) were measured and/or calculated from at least 5 sections from four different leaves, and at least 5 different fields of view were observed for a given section of images. DL and fias were calculated as:
where MA is the specific leaf weight (g m− 2), ΣSm is the total sectional area of mesophyll cells, and W is the width of the section.
For the transmission electron microscope (TEM) analysis, approximately 1–2 mm2 leaf sections were cut from the middle of newly expanded leaves using two razor blades, fixed in 2.5% glutaraldehyde (0.1 mol L− 1 phosphate buffer, pH 7.0) and postfixed with 2% osmium tetroxide. Specimens were dehydrated in a graded acetone series and embedded in Epon 812. Ultrathin cross-sections of 90 nm for transmission electron microscopy (TEM) were cut with a Power Tome-XL ultramicrotome, stained with 2% uranyl acetate, and examined with an H-7650 transmission electron microscope. For each sample, 15 cross-sections were chosen to measure mesophyll cell wall thickness (Tmc) and total length of the mesophyll cells (Lmes) and chloroplasts (Lch) facing the intercellular air space. At least 40 chloroplasts from TEM were observed to measure the chloroplast traits, including chloroplast length (Lc), chloroplast thickness (Tc), chloroplast section area (Secc), distance between two neighbor chloroplasts (ΔLchl), and chloroplast distance from the cell wall (ΔLcyt). The surface area of mesophyll cells to the intercellular air-spaces (Smes), the surface area of chloroplasts exposed to intercellular airspace (Sc), the chloroplast surface area (Surc) and volume (Volc) were calculated by using the following formula:
where W is the width of the measured section, and F is the curvature correction factor and taken as 1.55 [40].
where a = Lc/2, and b = Tc/2.
The chloroplast number per mesophyll cell (Nc) was determined according to the method of Pyke [41]. Briefly, the leaves were cut into 1–5 mm widths with a scalpel or razor blade, submerged in 3.5% (v/v) glutaraldehyde in a tube and kept in the dark at room temperature for 1 h. The glutaraldehyde solution was then replaced with 0.1 M Na-EDTA (pH 9), and the leaf discs were heat-blocked at 60 °C for 12 h and incubated overnight in the dark at 4 °C. To view chloroplasts in individual cells, a piece of tissue was removed from the tube with fine forceps and placed on a microscope slide in a drop of water. A scalpel handle was used to tap and macerate the tissue fairly vigorously, and a Leica DM2700 M microscope with DIC/Nomarski optics was used to image and count chloroplast numbers with changing focus to avoid duplicate and uncounted chloroplasts (Fig. S3).
The qualification of the anatomical limitations of mesophyll conductance
The one-dimensional gas diffusion model of Tomas et al. [16] was applied in our present study to determine the anatomical limitations of mesophyll conductance, which was given as:
where gias and gliq are the gas phase conductance and liquid phase conductance, respectively. R is the gas constant (8.31 Pa m3 K− 1 mol − 1), Tk is the absolute temperature, and H is Henry’s law constant (2943.3 Pa m3 K− 1 mol − 1 for CO2). The gias, was calculated as:
where rias is the resistance of the gas phase to CO2, Da is the diffusion coefficient for CO2 in the gas phase and is set to 1.51 × 10− 5 m2 s− 1 at 25 °C, fias is the volume fraction of intercellular air space, ΔLias was taken as half of the mesophyll thickness, and ς is the diffusion path tortuosity (1.57 m m− 1).
The gliq was determined by different components in the cell, including the conductance in the cell wall (gcw), plasma membrane (gpl), cytosol (gcyt), chloroplast envelope (gen), and stroma (gst). Eventually, gliq was calculated as:
where rcw, rpl, rcyt, ren, rst are the reciprocal terms of gcw, gpl, gcyt, gen and gst, respectively. We used an estimate of 0.0035 m s− 1 for the gpl and gen as Tomas et al. [16] suggested. In addition, gcw, gcyt, and gst were calculated as:
where i stands for cell wall, cytosol, or stroma conductance. rf,i accounted for the reduction in the aqueous phase diffusion coefficient for CO2 (Dw, 1.79 × 10− 9 m2 s− 1 at 25 °C) and was taken as 1.0 for cell walls and 0.3 for cytosol and stroma, respectively. pi was the effective porosity (m3 m− 3) and was taken as 1.0 for the cytosol and stroma and 0.28 for the cell walls. ΔLi (m) is the diffusion path length in the corresponding component of the diffusion pathway.
The proportion of gm determined by limited gas-phase conductance (lias) was calculated as:
The share of gm by different components of the cellular phase conductance (li) was determined as:
Statistical analysis
One-way ANOVA was applied to assess the differences in each parameter among the treatments with the SPSS 16.0 statistical software package. Significant differences (P < 0.05) among treatments are indicated by different letters using the least significant difference test.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- C C :
-
Chloroplast CO2 concentration
- C i :
-
Intercellular CO2 concentration
- D L :
-
Leaf density
- g m :
-
Mesophyll conductance
- g s :
-
Stomatal conductance
- HAN:
-
High NH4+
- HNN:
-
High NO3−
- LAN:
-
Low NH4+
- L c :
-
Chloroplast length
- LNN:
-
Low NO3−
- M A :
-
Specific leaf weight
- MAN:
-
Intermediate NH4+
- MNN:
-
Intermediate NO3−
- N c :
-
Chloroplasts number per mesophyll cell
- N L :
-
Leaf nitrogen content
- P n :
-
Net photosynthetic rate
- PNUE:
-
Photosynthetic nitrogen use efficiency
- S c :
-
Chloroplast surface area facing intercellular air spaces
- Sec c :
-
Chloroplasts section area
- S mes :
-
The surface area of mesophyll cells to the intercellular air-spaces
- Sur c :
-
Chloroplast surface area
- Vol c :
-
Chloroplast volume
- T c :
-
Chloroplast thickness
- T L :
-
Leaf thickness
- T m :
-
Mesophyll thickness
- T mc :
-
Mesophyll cell wall thickness
References
Poorter H, Evans JR. Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia. 1998;116:26–37.
Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol. 2002;130:1992–8.
Zhu XG, De SE, Long SP. Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: a numerical simulation using an evolutionary algorithm. Plant Physiol. 2007;145:513–26.
Ripullone F, Grassi G, Lauteri M, Borghetti M. Photosynthesis–nitrogen relationships: interpretation of different patterns between Pseudotsuga menziesii and Populus × euroamericana in a mini-stand experiment. Tree Physiol. 2003;23:137.
Li Y, Ren BB, Ding L, Shen QR, Peng SB, Guo SW. Does chloroplast size influence photosynthetic nitrogen use efficiency? PLoS One. 2013;8:e62036.
Flexas J, Barbour MM, Brendel O, Cabrera HM, Carriqui M, Diaz-Espejo A, Douthe C, Dreyer E, Ferrio JP, Gago J, et al. Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci. 2012;193–194:70–84.
Lorimer GH. The carboxylation and oxygenation of Ribulose-1,5-Bisphosphate: the primary events in photosynthesis and photorespiration. Annu Rev Plant Physiol. 2003;32:349–82.
Hassiotou F, Ludwig M, Renton M, Veneklaas EJ, Evans JR. Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls. J Exp Bot. 2009;60:2303–14.
Veromann-Jürgenson L-L, Tosens T, Laanisto L, Niinemets Ü. Extremely thick cell walls and low mesophyll conductance: welcome to the world of ancient living! J Exp Bot. 2017;68:1639–53.
Veromann-Jürgenson L-L, Brodribb TJ, Niinemets Ü, Tosens T. Pivotal role of mesophyll conductance in shaping photosynthetic performance across 67 structurally diverse gymnosperm species. Int J Plant Sci. 2020;181:116–28.
Hassiotou F, Renton M, Ludwig M, Evans JR, Veneklaas EJ. Photosynthesis at an extreme end of the leaf trait spectrum: how does it relate to high leaf dry mass per area and associated structural parameters? J Exp Bot. 2010;61:3015–28.
Niinemets U, Wright IJ, Evans JR. Leaf mesophyll diffusion conductance in 35 Australian sclerophylls covering a broad range of foliage structural and physiological variation. J Exp Bot. 2009;60:2433–49.
Evans JR, Kaldenhoff R, Genty B, Terashima I. Resistances along the CO2 diffusion pathway inside leaves. J Exp Bot. 2009;60:2235–48.
Onoda Y, Wright IJ, Evans JR, Hikosaka K, Kitajima K, Niinemets Ü, Poorter H, Tosens T, Westoby M. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 2017;214:1447–63.
Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S. Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J Exp Bot. 2006;57:343–54.
Tomas M, Flexas J, Copolovici L, Galmes J, Hallik L, Medrano H, Ribas-Carbo M, Tosens T, Vislap V, Niinemets U. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. J Exp Bot. 2013;64:2269–81.
Carriquí M, Roig-Oliver M, Brodribb TJ, Coopman R, Gill W, Mark K, Niinemets Ü, Perera-Castro AV, Ribas-Carbó M, Sack L, et al. Anatomical constraints to nonstomatal diffusion conductance and photosynthesis in lycophytes and bryophytes. New Phytol. 2019;222:1256–70.
Bondada BR, Syvertsen JP. Leaf chlorophyll, net gas exchange and chloroplast ultrastructure in citrus leaves of different nitrogen status. Tree Physiol. 2003;23:553–9.
Bondada BR, Syvertsen JP. Concurrent changes in net CO2 assimilation and chloroplast ultrastructure in nitrogen deficient citrus leaves. Environ Exp Bot. 2005;54:41–8.
Poorter H, Niinemets U, Poorter L, Wright IJ, Villar R. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol. 2009;182:565–88.
Golvano M, Felipe M, Cintas A. Influence of nitrogen sources on chloroplast development in wheat seedlings. Physiol Plant. 1982;56:353–60.
Raab TK, Terry N. Nitrogen source regulation of growth and photosynthesis in Beta vulgaris L. Plant Physiol. 1994;105:1159–66.
Antal T, Mattila H, Hakala-Yatkin M, Tyystjarvi T, Tyystjarvi E. Acclimation of photosynthesis to nitrogen deficiency in Phaseolus vulgaris. Planta. 2010;232:887–98.
Harley PC, Loreto F, Dimarco G, Sharkey TD. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol. 1992;98:1429–36.
Sage RF, Pearcy RW. The nitrogen use efficiency of C3 and C4 plants. Plant Physiol. 1987;84:954–8.
Makino A, Nakano H, Mae T. Responses of Ribulose-1,5-bisphosphate carboxylase, cytochrome-F, and sucrose synthesis enzymes in rice leaves to leaf nitrogen and their relationships to photosynthesis. Plant Physiol. 1994;105:173–9.
Ding L, Gao LM, Liu W, Wang M, Gu M, Ren BB, Xu GH, Shen QR, Guo SW. Aquaporin plays an important role in mediating chloroplastic CO2 concentration under high-N supply in rice (Oryza sativa L) plants. Physiol Plant. 2015;156:215–26.
Lopes MS, Nogues S, Araus JL. Nitrogen source and water regime effects on barley photosynthesis and isotope signature. Funct Plant Biol. 2004;31:995–1003.
Yao BQ, Cao J, Zhao CM, Rengel Z. Influence of ammonium and nitrate supply on growth, nitrate reductase activity and N-use efficiency in a natural hybrid pine and its parents. J Plant Ecol. 2011;4:275–82.
Valentine AJ, Osborne BA, Mitchell DT. Form of inorganic nitrogen influences mycorrhizal colonisation and photosynthesis of cucumber. Sci Hortic. 2002;92:229–39.
Onoda Y, Hikosaka K, Hirose T. Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct Ecol. 2004;18:419–25.
Cheng LL, Fuchigami LH. Rubisco activation state decreases with increasing nitrogen content in apple leaves. J Exp Bot. 2000;51:1687–94.
Hanba YT, Miyazawa SI, Terashima I. The influence of leaf thickness on the CO2 transfer conductance and leaf stable carbon isotope ratio for some evergreen tree species in Japanese warm-temperate forests. Funct Ecol. 1999;13:632–9.
Lu ZF, Lu JW, Pan YH, Lu PP, Li XK, Cong RH, Ren T. Anatomical variation of mesophyll conductance under potassium deficiency has a vital role in determining leaf photosynthesis. Plant Cell Environ. 2016;39:2428–39.
Terashima I, Miyazawa S-I, Hanba YT. Why are sun leaves thicker than shade leaves? — consideration based on analyses of CO2 diffusion in the leaf. J Plant Res. 2001;114:93–105.
Muller O, Oguchi R, Hirose T, Werger MJ, Hikosaka K. The leaf anatomy of a broad-leaved evergreen allows an increase in leaf nitrogen content in winter. Physiol Plant. 2009;136:299–309.
Terashima I, Hanba YT, Tholen D, Niinemets Ü. Leaf functional anatomy in relation to photosynthesis. Plant Physiol. 2011;155:108–16.
Manter DK, Kerrigan J. A/Ci curve analysis across a range of woody plant species: influence of regression analysis parameters and mesophyll conductance. J Exp Bot. 2004;55:2581–8.
Li Y, Ren BB, Yang XX, Xu GH, Shen QR, Guo SW. Chloroplast downsizing under nitrate nutrition restrained mesophyll conductance and photosynthesis in rice (Oryza sativa L.) under drought conditions. Plant Cell Physiol. 2012;53:892–900.
Scafaro AP, Von Caemmerer S, Evans JR, Atwell BJ. Temperature response of mesophyll conductance in cultivated and wild Oryza species with contrasting mesophyll cell wall thickness. Plant Cell Environ. 2011;34:1999–2008.
Pyke K. Analysis of plastid number, size, and distribution in Arabidopsis plants by light and fluorescence microscopy. Methods Mol Biol. 2011;774:19–32.
Acknowledgements
Not applicable.
Funding
This work was financially supported by the National Key Research and Development Program of China (2017YFD0200206) and the Special Fund for Agriculture Profession of China (20150312205). The funders have no role in the study design, data analysis and interpretation, and manuscript writing, but just provide the financial support.
Author information
Authors and Affiliations
Contributions
S.W.G. and L.M.G. conceived and designed the experiments; L.M.G. and K.L.X. performed the experiments; L.M.G. and Z.F.L. analyzed the data and wrote the paper; L.M.G., Z.F.L. and L.D. helped in analysis of the results and manuscript writing; all authors discussed the results and wrote the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
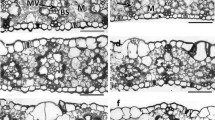
Figure S1. Representative light micrographs (A ~ F; scale bar = 200 μm) and transmission electron micrographs (G ~ L; scale bar = 5 μm; M ~ R, scale bar = 1 μm) of rice leaves supplied with NH4+ (AN) or NO3− (NN) under 3 different amounts, low N (0.71 mM, LAN and LNN), intermediate N (2.86 mM, MAN and MNN), and high N (7.14 mM, HAN and HNN). UEP, upper epidermis; LEP, lower epidermis; V, vascular bundle; CP, chloroplast; CW, cell wall; SG, starch grain; OG, osmiophilic globule. Figure S2. The relationship between mesophyll diffusion conductance (gm) measured with the Harley et al. method and gm modeled with anatomical parameters (Eq. 13–16). Values are means ± SD of four replicates. The data were fitted by linear regression. Broken lines correspond to the 1:1 relationship. Figure S3. Differential interference contrast image of chloroplasts in mesophyll cells separated from leaves. Leaves were cut into small pieces and fixed with 3.5% glutaraldehyde, and the mesophyll cells were individually dispersed on the glass plate and observed by microscopy. The red circles in the figure indicate individual mesophyll cells, and the chloroplast numbers therein were counted; the arrows indicate that the mesophyll cells did not separate efficiently. Bars = 20 μm.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gao, L., Lu, Z., Ding, L. et al. Anatomically induced changes in rice leaf mesophyll conductance explain the variation in photosynthetic nitrogen use efficiency under contrasting nitrogen supply. BMC Plant Biol 20, 527 (2020). https://doi.org/10.1186/s12870-020-02731-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-020-02731-7