Abstract
Background
Autism Spectrum Disorder (ASD) is a neurodevelopmental condition that typically emerges early in childhood. This study aimed to explore the potential link between serum levels of vitamin B12 and homocysteine (Hcy) and the severity of ASD symptoms in children.
Methods
In this study, 50 children diagnosed with ASD comprised the observation group, while 50 healthy children constituted the control group. Serum levels of IL-17 A, Hcy, folate, and vitamin B12 were compared between the study group and control group, as well as among children with different degrees of ASD severity. The correlation between the Childhood Autism Rating Scale (CARS) score and serum levels of IL-17 A, Hcy, folate, and vitamin B12 was examined. Additionally, the relationship between serum IL-17 A and Hcy levels and their association with the severity ASD were explored.
Results
Compared to the control group, the observation group demonstrated elevated serum Hcy and IL-17 A levels alongside decreased folate and vitamin B12 levels. Individuals with severe ASD exhibited higher Hcy and IL-17 A levels but lower folate and vitamin B12 levels compared to those with mild to moderate ASD. The CARS score showed negative correlations with serum folate and vitamin B12 levels and positive correlations with serum IL-17 A and Hcy levels in ASD patients. Additionally, serum Hcy and IL-17 A levels were correlated with ASD severity.
Conclusion
Children diagnosed with ASD presented with reduced serum vitamin B12 levels and increased levels of Hcy, potentially contributing to the onset and severity of ASD.
Similar content being viewed by others
Introduction
Autism, also known as Autism Spectrum Disorder (ASD), is the most representative pervasive developmental disorder characterized by impairments in social interaction, communication, and the presence of restricted, stereotyped, and repetitive behaviors. According to estimates from the World Health Organization [1], Approximately 1 in 100 children is affected by autism. ASD exhibits a higher prevalence in males compared to females, with a male-to-female ratio of approximately 4:1 [2]. The etiology of autism is intricately complex, involving various factors such as genetics, environment, and neurodevelopment. Additionally, immune dysregulation, characterized by abnormal immune system activity, plays a crucial role in the pathogenesis of autism. Evidence of immune dysfunction is frequently observed in individuals with ASD, often associated with behavioral deterioration [3]. Folate and vitamin B12 play critical roles in one-carbon metabolism, and perturbations in their metabolism have been observed in numerous individuals with Autism Spectrum Disorder (ASD), implying a potential involvement of the folate-tryptophan cycle in autism pathogenesis [4].. Disruption of biochemical pathways associated with folate and vitamin B12 is linked to the onset and severity of autism. These pathways, including methylation and transsulfuration, are involved in neurodevelopmental processes. Dysregulation of these pathways may result in imbalances that could potentially impact the symptoms of autism [5, 6].. Genetic factors play a substantial role in autism, as evidenced by elevated concordance rates of ASD in monozygotic twins, as well as an increased risk in families with affected individuals. Autism involves numerous genes, including those associated with synaptic function, neuronal signaling, and chromatin remodeling [7]. However, the genetic architecture of autism is highly heterogeneous, with different combinations of genetic variants contributing to individual cases. Environmental variables may also contribute to the pathogenesis of autism. Prenatal and postnatal factors such as maternal drug use, infections, and immunological activation have all been linked to an increased risk of ASD [8]. Additionally, environmental toxins, such as air pollutants and certain chemicals, may impact the development of autism [9].
Individuals with autism often exhibit signs of immune dysregulation and heightened immune activation [10]. Increased levels of pro-inflammatory cytokines, such as interleukin-17 A (IL-17 A), tumor necrosis factor-alpha (TNF-alpha), and interleukin-1 beta (IL-1β), have been observed in the blood and cerebrospinal fluid of individuals with autism [11,12,13,14]. Furthermore, maternal immune activation during pregnancy, characterized by an exaggerated immune response and heightened production of pro-inflammatory factors, has been suggested as a potential risk factor for autism in offspring [11].. These pro-inflammatory factors participate in diverse inflammatory pathways and can negatively impact neuronal function and development. The involvement of inflammation in autism is complex and multifactorial, with immune dysregulation and heightened inflammation hypothesized to potentially contribute to the development and progression of autism symptoms. Previous research suggests that children with ASD may exhibit higher food selectivity compared to control groups, often leading to nutritional deficiencies [15, 16]. Bandini and other researchers have reported that children with Autism Spectrum Disorder (ASD) exhibit increased aversive eating behaviors [16], leading to a limited variety of food consumption, which may contribute to nutritional deficiencies. However, there remains a necessity for comprehensive analyses encompassing all facets of nutritional intake in children with ASD. This includes examining the frequency of food consumption across different groups, assessing the extent of deficiencies in individuals with ASD, and exploring their dietary preferences. Additionally, aside from inflammatory factors, elevated blood homocysteine levels have been linked to various neurological and mental disorders, including autism [17, 18].. Homocysteine is an amino acid involved in methylation reactions and the metabolism of folate and vitamin B12 [19]. It can have neurotoxic effects, impacting neuronal function, neurotransmitter synthesis, and oxidative stress levels [20]. Additionally, homocysteine can disrupt DNA methylation, an essential epigenetic mechanism that regulates gene expression during brain development and function. High levels of homocysteine are associated with alterations in neurotransmitter systems, such as glutamate and gamma-aminobutyric acid (GABA), both crucial for brain function and potentially contributing to the core symptoms of autism [21]. While the association between homocysteine and autism is still being investigated, evidence suggests that reducing elevated homocysteine levels through nutritional interventions, such as folate, vitamin B12, and vitamin B6 supplementation, may have beneficial effects on behavioral and cognitive symptoms in certain individuals with autism [22, 23].
The aim of this study was to evaluate serum levels of interleukin-17 A (IL-17 A) and homocysteine (Hcy) in a cohort of children diagnosed with autism spectrum disorder (ASD) and examine the potential correlation between these serum biomarkers and the severity of ASD symptoms.
Materials and methods
Participants
Fifty individuals diagnosed with ASD were recruited for this study at the Center for Autism of Northwest Women’s and Children’s Hospital between September 2021 and June 2023. The Diagnosis was based on the criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) [24]. To ensure a comprehensive assessment of the clinical profile, in addition to DSM-V, the Autism Diagnostic Interview-Revised (ADI-R) and the Autism Diagnostic Observation Schedule (ADOS) were employed.
The eligible participants were children aged 3 to 12 years who had received a diagnosis of ASD, and whose parents provided consent for their participation in the research. Exclusions from the study comprised children with chronic diseases, infections, and other physical or neurological abnormalities. Also excluded were those currently taking medications, supplements, or vitamins.
Vitamin B12 deficiency
We also assessed the participants for vitamin B12 levels to explore potential associations with ASD. Blood samples were collected. Any participants found to have vitamin B12 deficiency were documented and considered in the analysis.
Gastrointestinal symptoms
Comprehensive medical histories, including the presence of gastrointestinal symptoms, were obtained from the participants and their parents or caregivers. This information was crucial in understanding the potential impact of gastrointestinal symptoms on the serum levels of analyzed parameters, such as folate and vitamin B12.
To provide a control group, 50 healthy candidates, matched for age and sex, were enrolled as volunteers with the permission of their parents.
The research was conceptualized and conducted in accordance with the principles outlined in the Helsinki Declaration. All participants were recruited within the same timeframe, and informed consent forms were completed by the primary caregivers. Ethical approval for this study was obtained from the ethics committee at Northwest Women’s and Children’s Hospital.
Clinical evaluation
The assessment of individuals with ASD involved a comprehensive review of the clinical record provided by the caregivers, a clinical examination, and a neuropsychiatric evaluation. The severity of ASD was quantified using the Childhood Autism Rating Scale (CARS) [25], which evaluates a child across fifteen domains on a scale of one to hour. Children scoring between 30 and36 on the scale were classified as having mild to moderate ASD, while those with values ranging from 37 to 60 points were categorized as having severe ASD.
Blood sampling
Collect 2 milliliters of venous blood sample into serum separation tubes and centrifuge immediately at 1500 g for 10 min. Store the serum samples at -80 °C until testing. Use human folate ELISA assay kit (Roche, cat. no. RAB0659 sensitivity 0.1 ng/mL, assay range 0.102-25 ng/mL), human homocysteine ELISA kit (Roche, cat. no. EY-01H807, sensitivity 0.5 ng/mL, assay range 0.5 ng/mL to 50 ng/mL), and Thermofisher human interleukin 17a (Hu IL-17 A) ELISA kit (cat. no. 88-7176-88, sensitivity 4 pg/mL, assay range 4-500 pg/mL), following the manufacturer’s instructions.
Statistics analysis
Graphpad 8.4 and Rstudio software were used. Continuous data were reported as mean standard deviation, whilst categorical data were presented as percentages.
The continuous variables were initially assessed for normal distribution using the Kolmogorov-Smirnov test. If both sets of data followed a normal distribution, the Student’s t-test was applied. In cases where non-normal distribution was observed, the Mann-Whitney U test was utilized. The simultaneous use of Levene’s test is employed to assess the homogeneity of variances for continuous variables. For quantitative data intergroup comparisons, the Chi-square test or Fisher’s exact Chi-square test was utilized. To evaluate the association between two measured values in the groups, Pearson’s and Spearman correlation tests were utilized.
Results
Characteristics of participants
The demographic and clinical characteristics of the study participants, along with the severity of autism spectrum disorder (ASD) as measured by the Childhood Autism Rating Scale (CARS) total score, are presented in Table 1. The mean age of all participants was 4.22 years (SD = 0.39). Children in the ASD group had a slightly higher mean age of 4.25 years (SD = 0.31) compared to the control group’s mean age of 4.19 years (SD = 0.45), although these differences were not statistically significant (p > 0.05). Additionally, there were no significant differences in age, gender distribution, or body mass index (BMI) between children with ASD and healthy subjects (p > 0.05). Among children with ASD, 54% exhibited mild to moderate symptoms, while the remaining 46% displayed severe symptoms. Detailed results of normality and homogeneity tests for all data are provided in Supplementary Table 1.
Comparison of serum cytokine, homocysteine, folate and vitamin B12 levels between children with ASD and control groups
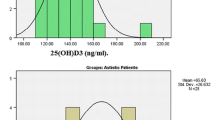
In Table 2, a comparative analysis of serum IL-17 A, homocysteine (Hcy), folate, and vitamin B12 levels among individuals with varying degrees of autism spectrum disorder (ASD) (mild, moderate, or severe) and age- and sex-matched control children is presented. In the ASD group, IL-17 A levels were significantly elevated (1.48 pg/ml) compared to the control group (0.44 pg/ml) with a p-value of less than 0.001. Vitamin B12 concentrations were notably reduced in children with ASD (502.69 pmol/L) compared to controls (626.41 pmol/L), demonstrating a statistically significant difference with a p-value less than 0.001. In comparison to the control group, the ASD group exhibited significantly elevated levels of IL-17 A and Hcy. Conversely, blood concentrations of folate and vitamin B12 were 1.6-fold and 1.2-fold higher in healthy children compared to those with ASD, respectively (Fig. 1 A, B, C and D). In the cohort of 50 ASD patients, 7 presented with gastrointestinal symptoms. There was no statistically significant difference in the levels of B12 between the two groups, those with and without gastrointestinal symptoms (see Supplementary Table 2).
Associations between serum IL-17 A and homocysteine levels with CARS total scores in the ASD group
An analysis of the association between blood levels of IL-17 A, Hcy, folate, and vitamin B12 with the Childhood Autism Rating Scale (CARS) total score in individuals with autism spectrum disorder (ASD) reveals significant findings. Higher IL-17 A levels (r = 0.65) and elevated homocysteine levels (r = 0.62) positively correlated with increased severity of ASD. Conversely, lower folate levels (r = -0.90) and reduced vitamin B12 levels (r = -0.79) negatively correlated with higher severity of ASD symptoms. These numerical associations provide insights into potential biomarkers for ASD (Table 3).
Associations of serum IL-17 A and homocysteine levels and their interaction with ASD severity
The association between serum IL-17 A and homocysteine (HCY) levels and the severity of Autism Spectrum Disorder (ASD) is categorized into mild-to-moderate symptoms (MASD) and severe symptoms (SASD) (Table 4). The results indicate a strong positive correlation (r = 0.68) between IL-17 A levels and ASD severity, with mASD showing 1.32 pg/ml (± 0.57) and sASD exhibiting 1.68 pg/ml (± 0.59). Similarly, Hcy levels also displayed a positive correlation (r = 0.63) with ASD severity, where mASD had 7.29 μmol/L (± 1.26) and sASD showed 8.37 μmol/L (± 2.90). Both correlations were statistically significant (p < 0.001), emphasizing the potential role of IL-17 A and Hcy in influencing the severity of ASD symptoms. Subgroup analysis was conducted based on gender for ASD patients and the control group. No statistically significant differences were observed in the levels of IL-17 A, Hcy, Folate, and VitB12 among ASD patients of different genders. Refer to Supplementary Table 3 for details.
Discussion
ASD is a neurodevelopmental disorder characterized by diverse clinical manifestations, including repetitive and stereotyped behaviors, impaired verbal communication skills, and deficits in social interaction [2]. The prevalence of ASD has been steadily increasing, highlighting the importance of understanding its underlying biological mechanisms and identifying potential biomarkers for diagnostic and therapeutic purposes. This study aimed to explore the association between blood levels of homocysteine (Hcy) and IL-17 A and the severity of ASD in children. Hcy, an amino acid containing sulfur, exerts cytotoxic effects by inducing apoptosis and disrupting normal cellular processes during embryonic development [26, 27]. Under physiological conditions, Hcy is maintained in dynamic equilibrium within the body. However, impairments in its metabolic pathways can lead to its accumulation, resulting in a condition called hyperhomocysteinemia [28]. Hcy is primarily metabolized through two pathways: the methylation pathway and the transsulfuration pathway [29]. The methylation pathway involves the utilization of vitamin B12 as a coenzyme and the formation of S-adenosylmethionine through the action of S-adenosylmethionine synthetase [30]. The transsulfuration pathway, catalyzed by cystathionine synthetase, is an alternate pathway for homocysteine (Hcy) metabolism. Elevated levels of Hcy can lead to the generation of reactive oxygen species and hydrogen peroxide, resulting in the inhibition of antioxidant enzymes and exacerbation of lipid peroxidation [31]. Our study observed higher levels of Hcy in children with ASD, suggesting an association with increased oxidative stress in ASD. B vitamins, including vitamin B6, vitamin B12, and folic acid, play essential roles in neurodevelopment. Deficiencies in these vitamins have been linked to an elevated risk of neurological diseases, neurodevelopmental disorders, and Alzheimer’s disease [32, 33]. As coenzymes, vitamin B12 promotes protein and nucleic acid biosynthesis and enhances the utilization of folic acid, thereby offering protection to the nervous system [34]. Findings from our study demonstrated folate and vitamin B12 levels were significantly lower in children with ASD compared to the healthy control. Similar to Li et al [35]., who compared multiple research groups and found that 5-HT, feeding practices, Hcy, vitamin B12 levels, and febrile seizures are major risk factors for autism in children, with significant correlations. However, there are conflicting findings as well. Wen-Xiong Chen et al [36]., in their study analyzing the plasma amino acid profile of 110 cases of autism spectrum disorder children in southern China, reported a decrease in Hcy levels in the plasma of ASD patients. These findings suggest an imbalance in the one-carbon metabolism pathway, which is involved in methylation reactions and essential for normal neurological development.
Research has consistently indicated that specific cytokines, produced by both immune and non-immune cells upon activation, can have detrimental effects on neurodevelopment and behavioral outcomes [37, 38]. Perturbations in cytokine levels have been observed in response to infections, diseases, and exposure to various toxic substances, suggesting that abnormal cytokine levels could result from environmental factors influencing autism [39, 40]. Several studies have reported alterations in cytokine profiles in children with ASD.
Alterations in cytokine profiles in children with ASD and notable changes in cytokines and their receptors are highlighted. Key cytokines and their receptors investigated in relation to childhood autism include IL-17 A, IL-1β, IL-2R, IL-4, IL-6, and various interleukins, each with diverse cellular origins and target cell types [41, 42]. Consistent with previous studies, our results revealed elevated levels of IL-17 A in children with ASD, indicative of increased inflammation associated with ASD.
In the ASD cohort, we observed a correlation between the severity of Autism Spectrum Disorder (ASD), as assessed by the Childhood Autism Rating Scale (CARS) score, and serum levels of IL-17 A, homocysteine (Hcy), folate, and vitamin B12. Higher CARS scores were specifically associated with elevated levels of IL-17 A and Hcy, while showing reduced levels of folate and vitamin B12. The CARS, developed by Schopler in the 1980s, serves as a widely used tool for screening and diagnosing autism [25]. Within our study, 23 out of the 50 children with severe ASD and 27 out of the 50 children with mild-to-moderate ASD were included based on the CARS criteria. Our findings revealed heightened serum IL-17 A and Hcy levels and decreased folate and vitamin B12 levels in children with severe ASD compared to those with mild-to-moderate ASD. Furthermore, correlation analyses unveiled significant negative associations between CARS scores and serum vitamin B12 levels, as well as significant positive associations between CARS scores and serum Hcy levels among children with ASD. The negative correlation between CARS scores and serum folate and vitamin B12 levels is consistent with previous studies demonstrating the importance of B vitamins in neurodevelopment. Deficiencies in these vitamins have been associated with an increased risk of neurological disorders. Furthermore, our study emphasizes the connection between IL-17 A, a pro-inflammatory cytokine, and the severity of ASD. Dysregulated cytokine levels, indicative of immune system dysfunction and potential neuroinflammation, have been reported in individuals with ASD [43]. Elevated IL-17 A levels in children with ASD provide additional support for the involvement of inflammatory processes in the pathogenesis of ASD. The increase in IL-17 A has been demonstrated to reflect the methylation of its mRNA. In the study by Wang et al [44]., an analysis of the activity of a reporter gene derived from pGL3 containing IL-17 A mRNA fragments revealed that the methylation by NSun2 promotes the translation of IL-17 A. In summary, NSun2 mediates the upregulation of IL-17 A expression induced by HHcy by methylating IL-17 A mRNA and facilitating its translation in T lymphocytes. Abdullah et al. also observed an increase in anti-inflammatory markers in the ASD patient group [45].
Additionally, our study acknowledges the prevalence of picky eating and resistance to consuming vegetables among individuals with ASD, which could contribute to low levels of B12 and folate. Previous research suggests that individuals with ASD may have heightened sensitivity to the taste, texture, and color of food, which could lead to aversion towards certain foods, such as vegetables. This aversion to vegetables, often accompanied by a preference for processed or specific food items, may result in a diet lacking in essential vitamins and minerals. Furthermore, individuals with ASD may experience sensory integration difficulties, affecting their perception and processing of external stimuli. The discomfort triggered by the texture and mouthfeel of vegetables may exacerbate their resistance to eating them, potentially contributing to nutritional deficiencies, including low B12 and folate levels [46].
This study has some limitations. Firstly, it is a single-center retrospective study, and future research would benefit from a larger sample size to enhance the validation of our conclusions. Secondly, the primary methodology employed in this study was ELISA. However, we acknowledge the importance of utilizing supplementary techniques for further validation. In subsequent research, we plan to incorporate Western blotting or flow cytometry to strengthen the robustness of our study findings. These considerations aim to address the current limitations and improve the overall reliability and generalizability of our research outcomes.
In summary, our investigation yields compelling evidence of disrupted serum levels of vitamin B12, homocysteine (Hcy), IL-17 A, and folate in children diagnosed with Autism Spectrum Disorder (ASD), suggesting potential implications in both the initiation and severity of the condition. These outcomes underscore the critical need for expanded research into the intricate connections between immune dysregulation, one-carbon metabolism, and neurodevelopment in the context of ASD.
Data availability
No datasets were generated or analysed during the current study.
References
Zeidan J, Fombonne E, Scorah J, Ibrahim A, Durkin MS, Saxena S, et al. Global prevalence of autism: a systematic review update. Autism Res. 2022;15(5):778–90.
Maenner MJ, Warren Z, Williams AR, Amoakohene E, Bakian AV, Bilder DA, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2020. MMWR Surveill Summ. 2023;72(2):1–14.
Hughes HK, Moreno RJ, Ashwood P. Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD). Brain, behavior, and immunity. 2023;108:245–54.
Main PA, Angley MT, Thomas P, O’Doherty CE, Fenech M. Folate and methionine metabolism in autism: a systematic review. Am J Clin Nutr. 2010;91(6):1598–620.
Chaste P, Leboyer M. Autism risk factors: genes, environment, and gene-environment interactions. Dialogues Clin Neurosci. 2012;14(3):281–92.
Roufael M, Bitar T, Sacre Y, Andres C, Hleihel W. Folate-Methionine cycle disruptions in ASD patients and possible interventions: a systematic review. Genes. 2023;14(3).
Vorstman JAS, Parr JR, Moreno-De-Luca D, Anney RJL, Nurnberger JI Jr., Hallmayer JF. Autism genetics: opportunities and challenges for clinical translation. Nat Rev Genet. 2017;18(6):362–76.
Cheng J, Eskenazi B, Widjaja F, Cordero JF, Hendren RL. Improving autism perinatal risk factors: a systematic review. Med Hypotheses. 2019;127:26–33.
Bai D, Yip BHK, Windham GC, Sourander A, Francis R, Yoffe R, et al. Association of genetic and environmental factors with autism in a 5-country cohort. JAMA Psychiatry. 2019;76(10):1035–43.
Robinson-Agramonte MLA, Noris García E, Fraga Guerra J, Vega Hurtado Y, Antonucci N, Semprún-Hernández N et al. Immune dysregulation in autism spectrum disorder: what do we know about it? Int J Mol Sci. 2022;23(6).
Fujitani M, Miyajima H, Otani Y, Liu X. Maternal and adult Interleukin-17A exposure and autism spectrum disorder. Front Psychiatry. 2022;13.
Gumusoglu SB, Hing BWQ, Chilukuri ASS, Dewitt JJ, Scroggins SM, Stevens HE. Chronic maternal interleukin-17 and autism-related cortical gene expression, neurobiology, and behavior. Neuropsychopharmacology. 2020;45(6):1008–17.
Majerczyk D, Ayad Elizabeth G, Brewton Kari L, Saing P, Hart Peter C. Systemic maternal inflammation promotes ASD via IL-6 and IFN-γ. Biosci Rep. 2022;42(11).
Xie J, Huang L, Li X, Li H, Zhou Y, Zhu H, et al. Immunological cytokine profiling identifies TNF-α as a key molecule dysregulated in autistic children. Oncotarget. 2017;8(47):82390–8.
Bandini LG, Anderson SE, Curtin C, Cermak S, Evans EW, Scampini R, et al. Food selectivity in children with autism spectrum disorders and typically developing children. J Pediatr. 2010;157(2):259–64.
Bandini LG, Curtin C, Phillips S, Anderson SE, Maslin M, Must A. Changes in food selectivity in children with autism spectrum disorder. J Autism Dev Disord. 2017;47(2):439–46.
Rossignol DA, Frye RE. The effectiveness of cobalamin (B12) treatment for autism spectrum disorder: a systematic review and meta-analysis. J Pers Med. 2021;11:8.
Li B, Xu Y, Pang D, Zhao Q, Zhang L, Li M, et al. Interrelation between homocysteine metabolism and the development of autism spectrum disorder in children. Front Mol Neurosci. 2022;15:947513.
Ulloque-Badaracco JR, Hernandez-Bustamante EA, Alarcon-Braga EA, Al-Kassab-Córdova A, Cabrera-Guzmán JC, Herrera-Añazco P, et al. Vitamin B12, folate, and homocysteine in metabolic syndrome: a systematic review and meta-analysis. Front Endocrinol. 2023;14:1221259.
Martínez-Lazcano JC, González-Guevara E, Boll C, Cárdenas G. Gut dysbiosis and homocysteine: a couple for boosting neurotoxicity in Huntington disease. Rev Neurosci. 2022;33(7):819–27.
Wang SD, Wang X, Zhao Y, Xue BH, Wang XT, Chen YX, et al. Homocysteine-induced disturbances in DNA methylation contribute to development of stress-associated cognitive decline in rats. Neurosci Bull. 2022;38(8):887–900.
Kaye AD, Jeha GM, Pham AD, Fuller MC, Lerner ZI, Sibley GT, et al. Folic acid supplementation in patients with elevated homocysteine levels. Adv Ther. 2020;37(10):4149–64.
Markišić M, Pavlović AM, Pavlović DM. The impact of homocysteine, vitamin B12, and vitamin D levels on functional outcome after first-ever ischaemic stroke. Biomed Res Int. 2017;2017:5489057.
American Psychiatric Association, Association D. AP. Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association: Washington, DC; 2013.
Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: childhood autism rating scale (CARS). J Autism Dev Disord. 1980;10(1):91–103.
Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26(3):137–46.
Hermann A, Sitdikova G, Homocysteine. Biochemistry, molecular biology and role in disease. Biomolecules. 2021;11(5).
Reddy VS, Trinath J, Reddy GB. Implication of homocysteine in protein quality control processes. Biochimie. 2019;165:19–31.
Brustolin S, Giugliani R, Félix TM. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1–7.
Stanger O, Fowler B, Piertzik K, Huemer M, Haschke-Becher E, Semmler A, et al. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: review and treatment recommendations. Expert Rev Neurother. 2009;9(9):1393–412.
Manivasagam T, Arunadevi S, Essa MM, SaravanaBabu C, Borah A, Thenmozhi AJ, et al. Role of oxidative stress and antioxidants in autism. Adv Neurobiol. 2020;24:193–206.
Martins LB, Malheiros Silveira AL, Teixeira AL. The link between nutrition and Alzheimer’s disease: from prevention to treatment. Neurodegener Dis Manag. 2021;11(2):155–66.
Kumar RR, Singh L, Thakur A, Singh S, Kumar B. Role of vitamins in neurodegenerative diseases: a review. CNS Neurol Disord Drug Targets. 2022;21(9):766–73.
Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5(11):949–60.
Li X. Correlation between 5-HT, Hcy and the incidence and severity of autism in children. Cell Mol Biol. 2023;69(1):54–60. (Noisy-le-Grand, France).
Chen WX, Chen YR, Peng MZ, Liu X, Cai YN, Huang ZF et al. Plasma amino acid profile in children with autism spectrum disorder in Southern China: analysis of 110 cases. J Autism Dev Disord. 2023.
Jiang NM, Cowan M, Moonah SN, Petri WA, Jr. The impact of systemic inflammation on neurodevelopment. Trends Mol Med. 2018;24(9):794–804.
Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64(1):61–78.
Moaaz M, Youssry S, Elfatatry A, El Rahman MA. Th17/Treg cells imbalance and their related cytokines (IL-17, IL-10 and TGF-β) in children with autism spectrum disorder. J Neuroimmunol. 2019;337:577071.
Masi A, Glozier N, Dale R, Guastella AJ. The immune system, cytokines, and biomarkers in autism spectrum disorder. Neurosci Bull. 2017;33(2):194–204.
Nour-Eldine W, Ltaief SM, Abdul Manaph NP, Al-Shammari AR. In search of immune cellular sources of abnormal cytokines in the blood in autism spectrum disorder: a systematic review of case-control studies. Front Immunol. 2022;13:950275.
Zhao H, Zhang H, Liu S, Luo W, Jiang Y, Gao J. Association of peripheral blood levels of cytokines with autism spectrum disorder: a meta-analysis. Front Psychiatry. 2021;12:670200.
Than UTT, Nguyen LT, Nguyen PH, Nguyen XH, Trinh DP, Hoang DH, et al. Inflammatory mediators drive neuroinflammation in autism spectrum disorder and cerebral palsy. Sci Rep. 2023;13(1):22587.
Wang N, Tang H, Wang X, Wang W, Feng J. Homocysteine upregulates interleukin-17A expression via NSun2-mediated RNA methylation in T lymphocytes. Biochem Biophys Res Commun. 2017;493(1):94–9.
Aldossari AA, Ansari MA, Nadeem A, Attia SM, Bakheet SA, Al-Ayadhi LY et al. Upregulation of inflammatory mediators in peripheral blood CD40(+) cells in children with autism spectrum disorder. Int J Mol Sci. 2023;24(8).
Baraskewich J, von Ranson KM, McCrimmon A, McMorris CA. Feeding and eating problems in children and adolescents with autism: a scoping review. Autism: Int J Res Pract. 2021;25(6):1505–19.
Acknowledgements
Thanks to the nurses in the department for their help with the project.
Funding
N/A.
Author information
Authors and Affiliations
Contributions
HL wrote the paper. YY provided the ideas. HL, YY and YHD reviewed the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participation
Written informed consent was obtained from a parent and/or legal guardian. This research was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Northwest Women’s and Children’s Hospital.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, H., Dang, Y. & Yan, Y. Serum interleukin-17 A and homocysteine levels in children with autism. BMC Neurosci 25, 17 (2024). https://doi.org/10.1186/s12868-024-00860-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12868-024-00860-5