Abstract
Background
Alternaria alternata is the primary pathogen of potato leaf spot disease, resulting in significant potato yield losses globally. Endophytic microorganism-based biological control, especially using microorganisms from host plants, has emerged as a promising and eco-friendly approach for managing plant diseases. Therefore, this study aimed to isolate, identify and characterize the endophytic fungi from healthy potato leaves which had great antifungal activity to the potato leaf spot pathogen of A. alternata in vitro and in vivo.
Results
An endophytic fungal strain SD1-4 was isolated from healthy potato leaves and was identified as Talaromyces muroii through morphological and sequencing analysis. The strain SD1-4 exhibited potent antifungal activity against the potato leaf spot pathogen A. alternata Lill, with a hyphal inhibition rate of 69.19%. Microscopic and scanning electron microscope observations revealed that the strain SD1-4 grew parallel to, coiled around, shrunk and deformed the mycelia of A. alternata Lill. Additionally, the enzyme activities of chitinase and β-1, 3-glucanase significantly increased in the hyphae of A. alternata Lill when co-cultured with the strain SD1-4, indicating severe impairment of the cell wall function of A. alternata Lill. Furthermore, the mycelial growth and conidial germination of A. alternata Lill were significantly suppressed by the aseptic filtrate of the strain SD1-4, with inhibition rates of 79.00% and 80.67%, respectively. Decrease of leaf spot disease index from 78.36 to 37.03 was also observed in potato plants treated with the strain SD1-4, along with the significantly increased plant growth characters including plant height, root length, fresh weight, dry weight, chlorophyll content and photosynthetic rate of potato seedlings.
Conclusion
The endophyte fungus of T. muroii SD1-4 isolated from healthy potato leaves in the present study showed high biocontrol potential against potato leaf spot disease caused by A. alternata via direct parasitism or antifungal metabolites, and had positive roles in promoting potato plant growth.
Similar content being viewed by others
Background
Potato (Solanum tuberosum L.) is one of the four important economic and food crops, extensively cultivated worldwide [1]. However, with the rapid expansion of cultivation areas, the potato crop faces threats from various diseases, leading to significant yield losses. Among these, potato leaf spot, primarily caused by Alternaria alternata, is a prominent fungal disease [2]. A. alternata can not only reduce the yield but also the quality and safety of potato as the fungus can produce mycotoxins that may be detrimental to human and animal health [3, 4]. Therefore, it is essential to control leaf spot disease caused by A. alternata in potato production. At present, use of fungicides is the principal means to control plant fungal disease and their widespread use has led to growing problems like the emergence of new resistant mutant strains and toxic residues in food and the environment [5]. Biocontrol strategy, as an eco-agricultural way to control plant diseases, is much safer and a sustainable alternative to chemical agents and hence has attracted more attention in recent years [6,7,8].
Biocontrol efficacy is affected by several factors in practical applications, including light, temperature, humidity and the affinity of biocontrol microorganisms to plants. Among these factors, the affinity of biocontrol microorganisms to plants often contributes significantly to the instability of biocontrol agents in the field [9,10,11,12,13]. Therefore, the colonization ability of biocontrol microorganisms in plants is an important index to evaluate whether it has industrialization prospect [14]. Recently, endophytic microorganisms are gained more and more popularity due to their excellent colonization efficacy and better acclimatizing potential [15,16,17,18]. When colonized throughout plant tissues, endophytic microorganisms can provide systemic tolerance to many plant pathogens and as endophytes interact closely with the host plant, they show great potential to promote plant growth through phytohormone regulation, phosphate solubilization and iron carrier production [19,20,21]. The endophyte Streptomyces sitangensis 136 was found to not only inhibit the growth of Sclerotium cepivorum and reduce the incidence of white rot disease, but also significantly promoted the growth of garlic plants [22]. The fungal endophytes of Trichoderma asperellum, Epicoccum nigrum and A. longipes showed broad-spectrum antifungal activity against Fusarium thapsinum, E. sorghinum, A. alternata and Curvularia lunata and plant growth-promoting traits under in vitro and in vivo field conditions as well as effective colonization [23]. Therefore, use of endophytic microorganisms, especially isolated from host plants to improve the biocontrol efficacy is advocated and promising [24].
Talaromyces muroii belongs to Talaromyces, species of which are widely distributed in sponges, plants and soil and have great potential in agriculture, food, cosmetics, medicine and environmental protection [25]. In the field of agriculture, Talaromyces species can inhibit pathological changes in crops and promote crop growth [25]. The strain T. muroii EU18 inhibited the growth of Colletotrichum coffeanum causing coffee anthracnose and C. musae causing banana anthracnose effectively [26]. The cytochalasans (CYTs) from T. muroii sp. displayed a variety of biological activities such as antitumor, phytotoxins, virulence factors, antimicrobials and cytotoxins [27, 28]. Furthermore, T. muroii strain TM28, isolated from panax pseudoginseng roots, not only controls wheat Fusarium crown rot caused by F. pseudograminearum but also significantly enhances the fresh weight and height of wheat plants [29]. Therefore, T. muroii may have great biocontrol potential in disease prevention and plant growth promotion.
In the present study, endophytic fungi from healthy potato leaves were isolated aiming to screen antifungal activity strains to inhibit the pathogen of A. alternata, the causal agent of potato leaf spot disease. The isolate T. muroii SD1-4 was obtained after in vitro antifungal analysis of co-cultural incubation test, microscopic observation and activity determination of fungal cell wall chitinases and glucanases. Intriguingly, T. muroii SD1-4 strain also showed great potential on protecting potato plants from leaf spot disease and promoting the growth of potato seedlings in vivo. Results in the current study not only enriched the endophytic microbial resources for biological control of potato leaf spot disease but also provided a new insight into using endophytes for promoting plant growth.
Results
Screening and identification of endophytic fungus SD1-4
Six fungal strains were obtained from healthy potato leaves in Sandu. Among them, one fungus labeled SD1-4 showed strong inhibitory activity against A. alternata Li11 in vitro and the inhibition rate of mycelia growth was 69.19% in the dual culture experiment (Table 1; Fig. 1). So, the endophytic fungus SD1-4 was used in all subsequent experiments in this study.
The strain SD1-4 was cultured on PDA media at 25ºC in dark for 7 days. The colony diameter was 47.55 ± 1.2 mm, with sparse yellow mycelia on the front of the colony and light-yellow color on the back of the colony. A large number of conidia were observed under a microscope. The conidium is short, smooth, single-or double-ringed, and conidiophores are smooth, oval to spindle-shaped, linear strung at the top of the phialides, with a size of 3–5 μm × 2–3.5 μm (Fig. 2).
To identify the strain SD1-4, the ITS and β-tubulin gene were amplified by PCR and sequenced by Tsingke Biotech Co., Ltd. (Beijing, China). The similarity of the resulting nucleotide sequences was analyzed using the NCBI BLAST tool respectively. The ITS sequence of the strain SD1-4 exhibited a 99.58% identity with T. muroii (MK450747) and the β-tubulin gene sequence showed 99.45% identity with that of T. muroii (KM066151). A multigene phylogenetic tree was constructed using the maximum likelihood (ML) method in the MEGA 7.0 software. Phylogenetic analysis showed that the strain SD1-4 and T. muroii were clustered together, with approval rating of 100% (Fig. 3). Together with the morphological characteristics, the strain SD1-4 was identified as T. muroii.
Microexamination and scanning electron microscope observation
Changes in the mycelial morphology of A. alternata Lill after T. muroii SD1-4 treatment were observed under optical microscopy that T. muroii SD1-4 grew in parallel with and coiled around the mycelia of A. alternata Li11, and caused mycelial deformation (Fig. 4a-b). Scanning electron microscope analysis further confirmed the antifungal action of T. muroii on A. alternata (Fig. 4c-d). Untreated A. alternata mycelia were smooth and full (Fig. 4c), while the co-cultured mycelia of A. alternata Lill were shrinkage and collapse with the mycelia of T. muroii SD1-4 parallel to or interwoven them (Fig. 4d). These results were consistent with the change in mycelial morphology observed under optical morphology, suggesting that mycelial parasitism would be an antibacterial mechanism by which T. muroii SD1-4 controled the A. alternata Lill.
Micro-examination and scanning electron micrographs of mycelia of Talaromyces muroii SD1-4 in the co-cultures. (a-b) Optical morphology of T. muroii SD1-4 mycelia (blue arrow) and abnormal mycelia of Alternaria alternata Lill (yellow arrow) intertwined together. (c) Scanning electron micrographs of A. alternata Li11 mycelia and the co-cultures with the blue arrow represented for T. muroii SD1-4 mycelia and the yellow one for A. alternata Li11 mycelia (d)
Chitinase and β-1, 3-glucanase enzyme activities
β-glucan and chitin are important components of fungal cell wall. Chitinase and β-1, 3-glucanase are important cell wall-degrading enzymes, and the activities of them can be evaluated the integrity of fungal cell wall. Compared with the individually cultured samples control, both the chitinase and β-1, 3-glucanase enzyme activities in the contact zone were significantly increased, suggesting that the mycelial cell wall of A. alternata Li11 maybe impaired after co-cultured with T. muroii SD1-4 (Fig. 5).
Aseptic filtrate effects on the mycelial growth of A. alternata
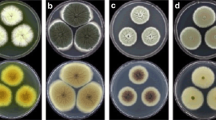
To explore whether the secondary metabolites of T. muroii SD1-4 have antifungal activity, aseptic filtrate of T. muroii SD1-4 with different concentration were added to PDA plates to maintain the A. alternata Lill colony. Results showed that the colony diameter was significantly reduced along with the AF concentration increasing (Fig. 6a). The mycelial growth of A. alternata Lill was almost completely suppressed at the 400 µL/mL aseptic filtrate concentration, with an inhibition rate of 79.00% (Fig. 6b).
Aseptic filtrate effects of Talaromyces muroii SD1-4 on the mycelial growth of Alternaria alternata Lill. (a) Antifungal activity of different concentration aseptic filtrates against A. alternata Lill on PDA plates after 7 days. (b) Inhibition rate of different concentrations of aseptic filtrates against A. alternata Lill. The different lowercase letters indicated significant difference between treatments (p < 0.05)
Aseptic filtrate effects on the conidial germination of A. alternata
Conidia are important reproductive structures for most pathogenic fungi in adverse environments and inhibition of conidial germination can reduce the reproduction and spread of fungi. As shown in Fig. 7, after treatment with aseptic filtrate, the conidia were deformed germinated and the germ tubes can not extend normally, which indicated the decrease in conidial infection ability. The conidial germination rate was 100.00% in the blank treatment without aseptic filtrate, whereas it was decreased to 50.00% or 19.33% under the treatment of aseptic filtrate with the concentration of 200 or 400 µL/mL, causing the inhibition rates of 50.00% and 80.67%, respectively (Table 2). These results indicated that conidia of A. alternata Lill were extremely sensitive to the aseptic filtrate of T. muroii SD1-4 in a concentration-dependent manner.
Determination of conidial germination of Alternaria alternata Lill treated with aseptic filtrates of Talaromyces muroii SD1-4. (a) Treated with sterile PDB filtrate as control. (b) Treated with 100 µL/mL aseptic filtrate of the strain SD1-4. (c) Treated with 200 µL/mL aseptic filtrate of the strain SD1-4. (d) Treated with 400 µL/mL aseptic filtrate of the strain SD1-4
Thermal stability of aseptic filtrate
The effects of different temperatures on the antifungal activities of the aseptic filtrate were evaluated and no significant changes were observed (Fig. 8), indicating that different temperature treatments had no effect on the antifungal components produced by T. muroii SD1-4. Thus, T. muroii SD1-4 had strong thermal stability.
Pot experiments and assessment of the growth-promoting effect
Results of the pot experiments showed the potent ability of T. muroii SD1-4 to reduce the disease severity and its biocontrol potential against potato leaf spot disease (Fig. 9a). At 12 days post inoculation, potato seedlings inoculated with the ddH2O (mock) or T. muroii SD1-4 only had no concentric brown spots while those inoculated with conidial suspension of A. alternata Lill showed typically leaf spot symptoms like brownish spots with concentric rings and chlorosis of potato leaves and the leaf spot disease index was 78.36 (Fig. 9b). However, the disease index of potato leaf spot via inoculating T. muroii SD1-4 for 2 days followed by conidia of A. alternata Lill was significantly reduced, which was 37.03 (Fig. 9b). These results indicated that the strain of T. muroii SD1-4 had the biocontrol potential to protect potato leaves from infection of A. alternata Lill.
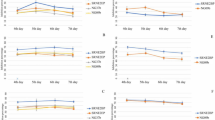
In addition to the biocontrol effect of T. muroii SD1-4 on potato leaf spot disease, the plant growth promoting ability was also assessed by detection of the plant height, root length, fresh weight and dry weight of potato plants inoculated with the T. muroii SD1-4 only or mock after inoculation for 12 days. Results showed that above indicators of T. muroii SD1-4 treated potato plants was significantly increased by 24.73%, 45.65%, 33.67%, and 47.38%, respectively (Fig. 9c-f), suggesting that T. muroii SD1-4 had a pro-growth effect on plant morphology. Further analysis found that the chlorophyll content and photosynthetic rate of single T. muroii SD1-4 treated potato plants were highest, followed by the mock with 35.30 SPAD, 6.83 µmol·m− 2s− 1 and 32.87 SPAD, 4.33 µmol·m− 2s− 1 separately. In contrast, potato leaf inoculated with A. alternata Lill only had the lowest chlorophyll content and photosynthetic rate at 25.5 SPAD, 3.20 µmol·m− 2s− 1 (Fig. 9g-h), indicating that the chlorophyll synthesis and photosynthesis efficiency in T. muroii SD1-4 treated potato plants were significantly increased.
Effect of Talaromyces muroii SD1-4 and Alternaria alternata Lill on growth of potato plants. (a) Roots and phenotypes of potato plants treated with different strains or sterilized distilled water as mock after inoculation for 12 days. (b) Disease index of potato leaf spot disease. (c-h) Plant height, root length, fresh weight, dry weight, photosynthetic rate and chlorophyll content of potato plants treated with single strain of SD1-4, Li11 or mock. Bars with the different lowercase letters indicated significant difference between treatments (p < 0.05)
Discussion
Potato leaf spot is a fungal disease on foliage that seriously affects potato production. Currently, chemical fungicides represent the primary method for disease management. However, given the goal of environmentally friendly disease control in agricultural settings, there is a continual need to develop novel approaches [30]. Biological control has become increasingly popular due to its advantages in pest, disease and weed management and ecological protection [6, 31,32,33]. A strong guarantee of safe crop production can be provided through biological control mechanisms, including parasitism and antibiotics [34]. In order to improve the colonization efficiency of biocontrol microorganisms in practical applications, use of endophytes from host plants in disease control is increasingly advocated [35]. Although an endophytic bacterial strain B. velezensis SEB1, isolated from the stem of Vigna mungo, showed good inhibitory activity on potato leaf spot pathogen of A. alternata, such as its reduced conidial germination and mycelial growth, isolation and screening endophytes with better antibiotic effect from the host potato leaf should be encouraged [36]. In the current study, the strain of endophytic fungus T. muroii SD1-4 was obtained, which exhibited good biocontrol effects on potato leaf spot disease as well as great potential of growth promotion on potato plants.
Cell wall integrity plays an important role in the normal growth and development of fungi and can improve fungal resistance to adverse environments [36]. In biological control, microorganisms tangle and penetrate the pathogens, resulting in abnormal perforation of the pathogenic cell wall, leakage of the cell contents, and thus the death of the fungus, which is a disease biological control principle known as parasitics [16, 37,38,39]. In this study, both optical microscopy and scanning electron microscope observation revealed that when T. muroii SD1-4 and A. alternata Lill were co-cultured, the mycelium of A. alternata Lill was entangled by T. muroii SD1-4, resulting in dried and crumpled deformities of the mycelium, so it was hypothesized that A. alternata Lill could be parasitized by T. muroii SD1-4. Subsequently, the enzyme activities of chitinase and β-1, 3-glucanase of mycelia collected from the co-cultivated area of T. muroii SD1-4 and A. alternata Lill or from the monoculture of both strains were conducted. Chitinase and β-1, 3-glucanase are considered to be the main components of fungal cell walls, and the rupture of fungal cell walls is often accompanied by an increase in the activities of chitinase and β-1, 3-glucanase enzymes [36]. The results of this study showed that chitinase and β-1, 3-glucanase activities of co-cultured mycelia were significantly increased than those of monoculture of both strains, suggesting the co-cultivation of A. alternata Lill and T. muroii SD1-4 may lead to the deformity and lysis of cell wall, suggesting that T. muroii SD1-4 could control potato leaf spot disease caused by A. alternata through parasitism. The parasitic mechanism employed by T. muroii SD1-4 in the present study was consistent with previously reported species in Talaromyces, such as T. flavus [40] and T. pinophilus [38].
Endophytic fungi may be used individually or in combination with different biocontrol mechanisms to inhibit plant diseases, among which production of antibiotics is an important mechanism in biological control [41]. Antibiotic action refers to the production of various antimicrobial compounds by biocontrol agents that inhibit or reduce the growth or proliferation of plant pathogens [41]. Talaromyces can produce many structurally diverse compounds with antagonistic properties, including esters, terpenes, steroids, alkaloids, polyketides, anthraquinones, glucanase, and others [25]. Hexane, ethyl acetate and methanol crude extracts of T. muroii EU18 at 1000 ppm inhibited the growth of colonies and sporulation of C. coffeanum [26]. The major bioactive compounds, eicosane, oleic acid, n-hexadecanoic acid, ethyl oleate, cis-vaccenic acid, heptacosane identified from T. trachyspermus have been reported to possess antioxidant, antibacterial and antifungal properties [42]. In the present study, the aseptic filtrate of T. muroii SD1-4 significantly suppressed the growth of A. alternata Lill and its mycelial growth and conidial germination was significantly inhibited by 79.00% and 80.67% respectively under the treatment of 400 µL/mL of the aseptic filtrate of T. muroii SD1-4, which may be due to some antimicrobial substances in the media produced by T. muroii SD1-4. Further study will focus on the identification of these antibiotic substances and confirmation of their biocontrol roles.
The efficacy of biocontrol agents can be affected by a variety of environmental factors resulting in reduced or increased antagonism [43]. Among these factors, temperature seems to be an important factor for Talaromyces strains, which are mostly thermophilic, and whether their secondary metabolites are also active at high temperatures [44], therefore thermal stability detection was performed on aseptic filtrates of T. muroii SD1-4 in the current study. Results showed that the aseptic filtrates can retain their stability between − 80ºC and 121ºC, which may provide an important implication for the utilization and storage of antibiotics of T. muroii SD1-4 in future.
Applying beneficial microorganisms as biocontrol agents is a promising and environmentally friendly method to protect plants against diseases and reduce the use of harmful chemicals [21, 30]. In the present study, T. muroii SD1-4, isolated from potato leaves, reduced the index of potato leaf spot disease by 41.33 when inoculated with the strain of SD1-4 compared with those only inoculated with A. alternata Li11. The decrease in the disease index may be due to the parasitism or antifungal metabolites of the strain SD1-4.
Talaromyces species can promote plant growth and improve crop quality through plant’s own action or production of various metabolites besides mitigating diseases [45,46,47]. The growth of cotton and potato can be promoted by T. flavus [48]. The antibiotic 3-O-methylfunicone, isolated from T. pinophilus, shows promising effects in protecting plants against pathogens and promoting plant growth [49]. The positive impact of T. pinophilus was observed through enhanced phosphorus uptake, leading to increased growth and yield of potatoes [47]. In the present study, compared with mock, the plant growth characteristics including plant height, root length, fresh weight, and dry weight of potato plants treated solely with T. muroii SD1-4 were significantly increased by 24.73%, 45.65%, 33.67%, and 45.65%, respectively. These results suggest that T. muroii SD1-4 has a positive pro-growth effect on morphological indicators. Green leaves are the main organs for photosynthesis, an important pathway for the synthesis of organic compounds, which plays a key role in plant growth. Photosynthetic pigments are essential for photosynthesis, and their content is closely related to the photosynthetic capacity of plants [50]. Hence, this study investigated the chlorophyll content and photosynthetic rate of potato leaves, revealing a significant increase following treatment with T. muroii SD1-4. The increase of photosynthetic parameters maybe benefited from the reduction of potato leaf spot disease or stimulated by potato plant own after application of T. muroii SD1-4. Anyway, the results of the present study provide evidence that the growth of potato plants can be promoted either indirectly or directly by the strain of T. muroii SD1-4.
Conclusion
In this study, an endophytic fungus T. muroii SD1-4 was isolated from healthy potato leaves and evaluated as a promising biocontrol agent for potato leaf spot disease caused by A. alternata. The strain SD1-4 showed typical parasitism and antifungal activity against the pathogen of A. alternata Li11 in intro and exhibited great potential for protecting potato plants from leaf spot disease and promoting the growth of potato seedlings in vivo. These results provided valuable insights into utilization of endophytic fungi to bio-control potato leaf spot disease and enhance growth of potato plants.
Materials and methods
Pathogen and endophytic fungal strains
The pathogen A. alternata Li11 was isolated from potato leaves showed typical leaf spot lesions and the endophytic fungi strain SD1-4 was isolated from potato healthy leaves. Both of the potato leaf samples were collected from potato fields at San du county (26°02′N, 107°52′E), Guizhou province, China, in 2022. The healthy leaves were properly washed with ddH2O, and then cut into small pieces (ca. 5 mm) in 1.5% NaClO surface sterilisation for 45 s followed with triple washes using ddH2O, and dried with sterilized filter paper. Finally, these leaves were placed on PDA plates and cultured in the dark at 25 °C for 7–14 days until the ideal fungal growth was achieved [51]. The pure colonies were soaked in 25% glycerol and stored at − 80ºC until use.
Endophytic fungi screening
The isolated strains were tested in vitro for their antifungal effect against A. alternata Lill as reported method per [38] with slight modifications. In brief, a 5-mm-diameter disk from the margin of each actively growing colony of A. alternata Lill was transferred to PDA plate. Then, a disk of the isolated strains was inoculated at the symmetrical points at 25 mm on the other side of the pathogen. Control tests were also performed using the pathogen alone. All plates were incubated for 7 days at 25ºC in darkness. The inhibition rate was calculated using the formula: Inhibition rate (%) = (R1-R2)/(R1-5) × 100, where R1 and R2 was the radial growth of A. alternata Lill on the control and dual culture plate, respectively. All experiments were performed in triplicate and the means were used.
Identification of the endophyte strain SD1-4
The morphologies of the mycelia and conidia of strain SD1-4 incubated at 25ºC for 7 days were observed under an optical microscope (LEICA ICC50 W, Leica Microsystems Co., Ltd., Shanghai, China) after staining with lactophenol-cotton blue and identification of fungi was based on previous method [26].
In addition, the sequences of ITS region and β-tubulin of the strain SD1-4 were amplified by PCR [52]. After sequencing and alignment of these sequences, a multigene phylogenetic tree was constructed using the maximum likelihood (ML) method in MEGA7.0 software with bootstrap values based on 1000 replications. The sequences of ITS and β-tubulin obtained in this study were deposited under GenBank accession numbers OR835849 and OR854814, respectively.
Micro-examination of the co-cultures
The interaction between the strain SD1-4 and A. alternata Lill was examined using the slide culture method [53]. After incubation at 25ºC and 75% humidity until the colonies overlapped, the mycelia in overlapping areas or in single incubations was respectively selected for optical microscope (digital microscope system Keyence VHX-7000) and scanning electron microscope (SU-8010, Hitachi, Tokyo, Japan) observation at 3.0 kV at 10,000× of magnification [51, 54].
Detection of chitinase and β-1, 3-glucanase activities
To assess the effect of strain SD1-4 on the cell wall of the strain A. alternata Lill, chitinase and β-1, 3-glucanase activities of the strain SD1-4 or A. alternata Lill in single cultures and in overlapping areas of co-cultures were determined respectively using commercially available kits (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) following the manufacturers’ instructions. All experiments were performed in triplicate.
Antifungal activity of the aseptic filtrate of the strain SD1-4
Aseptic filtrate was collected by filtration of the liquid PDB cultures of SD1-4 at 25ºC for 5 days through a sterilized filter gauze and a 0.22 μm membrane filter, and then its efficacy against A.alternata Lill was assessed according to the previous study [51]. The pathogen disks were inoculated on the center of PDA plates containing 50 µL/mL, 100 µL/mL, 200 µL/mL and 400 µL/mL (v/v) aseptic filtrate, respectively. After incubation for 7 days at 25ºC, the colony diameters were measured in two perpendicular directions. The treatment using sterilized PDB filtrate was set as control. All experiments were repeated for three times. The inhibition rate was calculated as follows: Inhibition rate (%) = [(Dc-Dt)/(Dc-5)] × 100, where Dc and Dt represents the colony diameter of the strain A. alternata Lill on the control and under the treatment of different aseptic filtrate concentrations.
Aseptic filtrate effects on the conidial germination of A. alternata
The conidial germination of A. alternata Lill was detected after treatment with the aseptic filtrate of strain SD1-4. In brief, the aseptic filtrate and conidial suspension of A. alternata Lill were mixed in a 1: 1 (v: v) ratio so that the final concentrations of aseptic filtrate were 100 µL/mL, 200 µL/mL and 400 µL/mL, respectively, and the conidial concentration was 1.0 × 107 conidia/mL [51, 55]. Then, 20 µL mixture was dripped onto the center of a concave slide and covered with a cover slip. The mixture was incubated in a petri dish with moist filter paper at 25ºC for 12 h and conidial germination was tested under an optical microscope. Conidial germination was judged on the basis that the length of the shoot tube was more than half the length of the conidia. Three fields of view of each treatment with at least 50 conidia in each field of view were used. The experiment was performed in triplicate and the treatment of the liquid PDB was used as a control.
Thermal stability of the aseptic filtrate
The stability of the aseptic filtrate was evaluated under the treatment of different thermal conditions as previously described with minor modifications [51]. The aseptic filtrate was treated at − 80ºC, − 20ºC, 0ºC, 20ºC, 40ºC, 80ºC and 121ºC for 30 min respectively, and then returned to room temperature. After the different treatments, the effects of 200 µL/mL aseptic filtrate on the inhibition of mycelial growth of A. alternata Lill were determined to evaluate the filtrate stability. All experiments were performed in triplicate.
Pot experiments and assessment of the growth-promoting effect
Protective effect of the strain SD1-4 against potato leaf spot disease and its growth-promoting effect were investigated in pot experiments. The conidial suspensions of strain SD1-4 and A. alternata Lill were collected by incubation in liquid PDB at 25℃, 150 rpm for 5 days, respectively. Conidia were harvested by centrifuging at 5000 g for 1 min and suspending in ddH2O to the concentration of 1.0 × 107conidia/mL [55].
Uniformly grown healthy potato seedlings at the 5-leaf-stage were used for the pot experiments. The 5-leaf-stage potato seedlings were treated by foliar spraying with 30 mL of ddH2O (mock), 30 mL of the conidial suspension of the strain SD1-4, 30 mL of the conidial suspension of A. alternata Lill, and 30 mL of the conidial suspension of the strain SD1-4 sprayed 2 days before 30 mL of the A. alternata Lill conidial suspension, respectively [30, 56]. All the potato plants were kept in a greenhouse at 25℃, 16 h light: 8 h dark and 80% relative humidity. Six plants were employed in each treatment and the pot experiment was repeated for three times.
Disease severity of each seedling was recorded at 12 days following nine categories [30]. Disease index was calculated according to the formula: Disease index= (Sum of all disease rating/Total number of ratings × Maximum disease grade) × 100 [57]. In addition, to evaluate the influence of the treatment on growth index and physiological index of potato seedlings, the plant height (cm), root length (cm), fresh weight of potato plant (g), dry weight of potato plant (g), photosynthetic rate (µmol·m− 2s− 1) and chlorophyll content (SPAD) of potato plants were measured following the published method [58].
Statistical analyses
Data was analyzed using the one-way ANOVA performed as per LSD multiple range test to determine the significant difference at p < 0.05. Charts were plotted with Origin 2021. Statistical analyses were performed using Excel 2010 and SPSS v. 23.0 software.
Data availability
Sequence data that support the findings of this study have been deposited in the NCBI Nucleotide database with the GenBank accession numbers of OR835849 and OR854814.
Abbreviations
- PDA:
-
Potato Dextrose Agar (Medium)
- PDB:
-
Potato Dextrose Broth
- ANOVA:
-
Analysis of Variance
- ddH2O:
-
Sterilized Distilled Water
- LSD:
-
Least Significant Difference
References
Zhang H, Xu F, Wu Y, Hu H-h. Dai X-f. Progress of potato staple food research and industry development in China. J Integr Agr. 2017;16(12):2924–32. https://doi.org/10.1016/s2095-3119(17)61736-2
Nasr Esfahani M. Analysis of virulence and genetic variability of Alternaria alternata associated with leaf spot disease in potato plants in Iran. Acta Mycologica. 2018;53(1). https://doi.org/10.5586/am.1105
Xing L, Zou L, Luo R, Wang Y. Determination of five Alternaria toxins in wolfberry using modified QuEChERS and ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2020;311. https://doi.org/10.1016/j.foodchem.2019.125975
Wang B, Lou T, Wei L, Chen W, Huang L, Ding L, et al. Biochemical and molecular characterization of Alternaria alternata isolates highly resistant to procymidone from broccoli and cabbage. Phytopathol Res. 2021;3(1). https://doi.org/10.1186/s42483-021-00092-z
Petit AN, Fontaine F, Clement C, Vaillant-Gaveau N. Photosynthesis limitations of grapevine after treatment with the fungicide fludioxonil. J Agr Food Chem. 2008;56:6761–7.
Zhang N, Wang Z, Shao J, Xu Z, Liu Y, Xun W, et al. Biocontrol mechanisms of Bacillus: improving the efficiency of green agriculture. Microb Biotechnol. 2023;16(12):2250–63. https://doi.org/10.1111/1751-7915.14348
Harish BN, Nagesha SN, Ramesh BN, Shyamalamma S, Nagaraj MS, Girish HC, et al. Molecular characterization and antifungal activity of lipopeptides produced from Bacillus subtilis against plant fungal pathogen Alternaria alternata. Bmc Microbiol. 2023;23(1). https://doi.org/10.1186/s12866-023-02922-w
Safari Motlagh MR, Abolghasemi M. The effect of Trichoderma spp. isolates on some morphological traits of canola inoculated with Sclerotinia sclerotiorum and evaluation of their efficacy in biological control of pathogen. J Saudi Soc Agric Sci. 2022;21(4):217–31. https://doi.org/10.1016/j.jssas.2021.08.004
Carro-Huerga G, Compant S, Gorfer M, Cardoza RE, Schmoll M, Gutiérrez S, et al. Colonization of Vitis vinifera L. by the endophyte Trichoderma sp. strain T154: Biocontrol activity against Phaeoacremonium minimum. Front Plant Sci. 2020;11. https://doi.org/10.3389/fpls.2020.01170
Han L, Zhou X, Zhao Y, Zhu S, Wu L, He Y, et al. Colonization of endophyte Acremonium sp. D212 in panax notoginseng and rice mediated by auxin and jasmonic acid. J Integr Plant Biol. 2020;62(9):1433–51. https://doi.org/10.1111/jipb.12905
Li CH, Shi L, Han Q, Hu HL, Zhao MW, Tang CM, et al. Biocontrol of verticillium wilt and colonization of cotton plants by an endophytic bacterial isolate. J Appl Microbiol. 2012;113(3):641–51. https://doi.org/10.1111/j.1365-2672.2012.05371.x
Oku S, Komatsu A, Tajima T, Nakashimada Y, Kato J. Identification of chemotaxis sensory proteins for amino acids in Pseudomonas fluorescens Pf0-1 and their involvement in chemotaxis to tomato root exudate and root colonization. Microbes Environ. 2012;27:462–9. https://doi.org/10.1264/jsme2.ME12005
Safari Motlagh MR, Kulus D, Kaviani B, Habibollahi H. Exploring fungal endophytes as biocontrol agents against rice blast disease. Acta Agrobot. 2024;76:1–13. https://doi.org/10.5586/aa/182943
Salvatierra-Martinez R, Arancibia W, Araya M, Aguilera S, Olalde V, Bravo J, et al. Colonization ability as an indicator of enhanced biocontrol capacity—An example using two Bacillus amyloliquefaciens strains and Botrytis Cinerea infection of tomatoes. J Phytopathol. 2018;166(9):601–12. https://doi.org/10.1111/jph.12718
Kashyap N, Singh SK, Yadav N, Singh VK, Kumari M, Kumar D, et al. Biocontrol screening of endophytes: applications and limitations. Plants. 2023;12(13). https://doi.org/10.3390/plants12132480
Wulandari AP, Triani E, Sari K, Prasetyani M, Nurzaman M, Purwati RD, et al. Endophytic microbiome of Boehmeria nivea and their antagonism against latent fungal pathogens in plants. BMC Microbiol. 2022;22(1). https://doi.org/10.1186/s12866-022-02737-1
El-Hasan A, Ngatia G, Link TI, Voegele RT. Isolation, identification, and biocontrol potential of root fungal endophytes associated with solanaceous plants against potato late blight (Phytophthora infestans). Plants. 2022;11(12). https://doi.org/10.3390/plants11121605
Safari Motlagh MR, Jahangiri B, Kulus D, Tymoszuk A, Kaviani B. Endophytic fungi as potential biocontrol agents against Rhizoctonia Solani J.G. Kühn, the causal agent of rice sheath blight disease. Biology. 2022;11(9). https://doi.org/10.3390/biology11091282
Li H, Parmar S, Sharma VK, White JF. Seed endophytes and their potential applications. Seed Endophytes: Biology Biotechnol. 2019:35–54.
Morales-Cedeño LR, Orozco-Mosqueda MdC, Loeza-Lara PD, Parra-Cota FI, de los Santos-Villalobos S, Santoyo G. Plant growth-promoting bacterial endophytes as biocontrol agents of pre- and post-harvest diseases: fundamentals, methods of application and future perspectives. Microbiol Res. 2021;242. https://doi.org/10.1016/j.micres.2020.126612
Motlagh MRS, Jafari N. Biological control of Botrytis Cinerea, the causal agent of rose gray mold disease by antagonistic fungi. Int J Pest Manage. 2020;68(2):167–74. https://doi.org/10.1080/09670874.2020.1807654
Wang J, Shi L, Wang D, Li L, Loake GJ, Yang X, et al. White rot disease protection and growth promotion of garlic (Allium sativum) by endophytic bacteria. Plant Pathol. 2019;68(8):1543–54. https://doi.org/10.1111/ppa.13066
Rajini SB, Nandhini M, Udayashankar AC, Niranjana SR, Lund OS, Prakash HS. Diversity, plant growth-promoting traits, and biocontrol potential of fungal endophytes of sorghum bicolor. Plant Pathol. 2020;69(4):642–54. https://doi.org/10.1111/ppa.13151
Limdolthamand S, Songkumarn P, Suwannarat S, Jantasorn A, Dethoup T. Biocontrol efficacy of endophytic Trichoderma spp. in fresh and dry powder formulations in controlling northern corn leaf blight in sweet corn. Biol Control. 2023;181. https://doi.org/10.1016/j.biocontrol.2023.105217
Lei L, Gong L, Jin M, Wang R, Liu R, Gao J, et al. Research advances in the structures and biological activities of secondary metabolites from Talaromyces. Front Microbiol. 2022;13. https://doi.org/10.3389/fmicb.2022.984801
Soytong M, Poeaim S. Antifungal activity of Talaromyces muroii against coffee anthracnose. J Agricultural Technol. 2015;11:1941–8.
Hu Z, Ye Y, Zhang Y. Large-scale culture as a complementary and practical method for discovering natural products with novel skeletons. Nat Prod Rep. 2021;38(10):1775–93. https://doi.org/10.1039/d0np00069h
Pattarasaikul W, Soytong K, Poeaim S. Biological control of anthracnose disease on banana var ‘Namwa Mali-Ong’ by Neosartorya species. Int J Agricultural Technol. 2018;14:1589–98.
Yang H, Cui S, Wei Y, Li H, Hu J, Yang K, et al. Antagonistic effects of Talaromyces Muroii TM28 against Fusarium crown rot of wheat caused by Fusarium pseudograminearum. Front Microbiol. 2024;14. https://doi.org/10.3389/fmicb.2023.1292885
Ahmed AIS. Biological control of potato brown leaf spot disease caused by Alternaria alternata using Brevibacillus formosus strain DSM 9885 and Brevibacillus brevis strain NBRC 15304. J Plant Pathol Microbiol. 2017;08(06). https://doi.org/10.4172/2157-7471.1000413
Motlagh MRS, Javadzadeh A. Study of Alternaria pellucida as a promising mycoherbicide for controlling Arrowhead (Sagitaria Trifolia) in paddy fields. Plant Omics. 2010;3(6):172–6.
Motlagh MRS. Reaction of major weeds and some rice cultivars to Alternaria pellucida, a potential biocontrol agent. Plant Omics. 2011;4(3):163–8.
Motlagh MRS. Comparison of pathogenicity of Alternaria pellucida and curvularia lunata on weed Echinochloa species. J Environ Biol. 2015;36(4):963–7.
Zhang H, Kong N, Liu B, Yang Y, Li C, Qi J, et al. Biocontrol potential of Trichoderma Harzianum CGMCC20739 (Tha739) against postharvest bitter rot of apples. Microbiol Res. 2022;265. https://doi.org/10.1016/j.micres.2022.127182
Giordano DF, Pastor NA, Rouws LFM, de Freitas KM, Erazo JG, Del Canto A, et al. Trichoderma Harzianum ITEM 3636 colonizes peanut roots as an endophyte and protects the plants against late leaf spot. Symbiosis. 2023;89(3):337–52. https://doi.org/10.1007/s13199-023-00913-z
Gorai PS, Ghosh R, Konra S, Mandal NC. Biological control of early blight disease of potato caused by Alternaria alternata EBP3 by an endophytic bacterial strain Bacillus velezensis SEB1. Biol Control. 2021;156. https://doi.org/10.1016/j.biocontrol.2021.104551
Pandey V, Shukla A, Kumar J. Physiological and molecular signalling involved in disease management through Trichoderma: an effective biocontrol paradigm. Current trends in Plant Disease Diagnostics and Management practices. 2016: 317–46.
Abdel-Rahim IR, Abo-Elyousr KAM. Talaromyces pinophilus strain AUN-1 as a novel mycoparasite of Botrytis Cinerea, the pathogen of onion scape and umbel blights. Microbiol Res. 2018;212:1–9. https://doi.org/10.1016/j.micres.2018.04.004
Safari Motlagh MR, Farokhzad M, Kaviani B, Kulus D. Endophytic fungi as potential biocontrol agents against Sclerotium Rolfsii Sacc. —The causal agent of peanut white stem rot disease. Cells. 2022;11(17). https://doi.org/10.3390/cells11172643
Madi L, Katan T, Katan J, Henis Y. Biological control of Sclerotium rolfsii and verticillium dahliae by Talaromyces Flavus is mediated by different mechanisms. Phytopathology. 1997;87:1054–60.
Ghorbanpour M, Omidvari M, Abbaszadeh-Dahaji P, Omidvar R, Kariman K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol Control. 2018;117:147–57. https://doi.org/10.1016/j.biocontrol.2017.11.006
Farhat H, Urooj F, Sohail N, Hameedi SF, Ali MS, Ehteshamul-Haque S. Evaluation of antibacterial potential of endophytic fungi and GC-MS profiling of metabolites from Talaromyces Trachyspermus. S Afr J Bot. 2022;150:240–7. https://doi.org/10.1016/j.sajb.2022.07.004
Sharma M, Manhas RK. Biocontrol potential of Streptomyces sp. M4 and salvianolic acid B produced by it against Alternaria black leaf spot. Microb Pathogenesis. 2022;173. https://doi.org/10.1016/j.micpath.2022.105869
Houbraken J, Spierenburg H, Frisvad JC. Rasamsonia, a new genus comprising thermotolerant and thermophilic Talaromyces and Geosmithia species. Antonie van Leeuwenhoek. 2011;101(2):403 – 21; https://doi.org/10.1007/s10482-011-9647-1
Farhat H, Urooj F, Irfan M, Sohail N, Majeed S, Ullah S, et al. Biological control potential of endophytic fungi with amelioration of systemic resistance in sunflower and GC-MS metabolic profiling of Talaromyces assiutensis. Curr Microbiol. 2023;80(2). https://doi.org/10.1007/s00284-022-03161-4
Ou T, Zhang M, Gao H, Wang F, Xu W, Liu X, et al. Study on the potential for stimulating mulberry growth and drought tolerance of plant growth-promoting fungi. Int J Mol Sci. 2023;24(4). https://doi.org/10.3390/ijms24044090
Sembiring M, Elfiati D, Sutarta ES, Sabrina T. Phosphate solubilization agents in increasing potatoes production on andisol sinabung area. Asian J Plant Sci. 2017;16:141–8. https://doi.org/10.3923/ajps.2017.141.148
Naraghi L, Heydari A, Rezaee S, Razavi M. Biocontrol agent Talaromyces Flavus stimulates the growth of cotton and potato. J Plant Growth Regul. 2012;31(4):471–7. https://doi.org/10.1007/s00344-011-9256-2
Vinale F, Nicoletti R, Lacatena F, Marra R, Sacco A, Lombardi N, et al. Secondary metabolites from the endophytic fungus Talaromyces Pinophilus. Nat Prod Res. 2017;31(15):1778–85. https://doi.org/10.1080/14786419.2017.1290624
Zhang M, Hua M, Guo D, Xue Y, Chen X, Rui L, et al. Effects of plant growth-promoting rhizobacteria on growth indicators and physiological characteristics of Peucedanum Praeruptorum Dunn leaves. Plant Signal Behav. 2023;18(1). https://doi.org/10.1080/15592324.2023.2203571
Li W, Long Y, Mo F, Shu R, Yin X, Wu X, et al. Antifungal activity and biocontrol mechanism of Fusicolla Violacea J-1 against soft rot in kiwifruit caused by Alternaria alternata. J Fungi. 2021;7(11). https://doi.org/10.3390/jof7110937
Yue Y, Jiang M, Hu H, Wu J, Sun H, Jin H, et al. Isolation, identification and insecticidal activity of the secondary metabolites of Talaromyces Purpureogenus BS5. J Fungi. 2022;8(3). https://doi.org/10.3390/jof8030288
Abdel-Fattah GM, Shabana YM, Ismail AE, Rashad YM. Trichoderma Harzianum: a biocontrol agent against Bipolaris oryzae. Mycopathologia. 2007;164(2):81–9. https://doi.org/10.1007/s11046-007-9032-9
Motlagh MRS, Rad SA, Mohesien MT, Mossa MI, Seidavi A, Ghosh S, et al. Potential of selected tobacco endophytic fungi against Sclerotinia Sclerotiorum, the causal agent of tobacco collar rot disease. Sydowia. 2023;75:221–32. https://doi.org/10.12905/0380.sydowia75-2023-0221
Wang F, Saito S, Michailides TJ, Xiao CL. Baseline sensitivity of Alternaria alternata and A. arborescens to natamycin and control of Alternaria rot on stored mandarin fruit. Plant Dis. 2021;105(11):3653–6. https://doi.org/10.1094/pdis-04-21-0809-re
van der Waals JE, Denner FDN, van Rij N, Korsten L. Evaluation of plant-plus, a decision support system for control of early blight on potatoes in South Africa. Crop Prot. 2003;22(6):821–8. https://doi.org/10.1016/s0261-2194(03)00049-8
Zhang J, Zhao G, Chai J, Zeng L, Gong W, Ran F, et al. Occurrence of Cladosporium herbarum causing leaf spot on Avena sativa in China. Crop Prot. 2024;177. https://doi.org/10.1016/j.cropro.2023.106555
Juntahum S, Ekprasert J, Boonlue S. Efficiency of arbuscular mycorrhizal fungi for the growth promotion of sugarcane under pot conditions. Sugar Tech. 2022;24(6):1738–47. https://doi.org/10.1007/s12355-022-01129-z
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (No. 31901836, 32260658) and Guizhou Provincial Science and Technology Project ([2020]1Y104).
Author information
Authors and Affiliations
Contributions
R.F. did conceptualization and revised the manuscript. L.Z. and W.X. conducted the experiments, analyzed data and drafted the manuscript. Z.Z. and Y.L. assisted in writing the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, L., Xu, W., Zhao, Z. et al. Biocontrol potential and growth-promoting effect of endophytic fungus Talaromyces muroii SD1-4 against potato leaf spot disease caused by Alternaria alternata. BMC Microbiol 24, 255 (2024). https://doi.org/10.1186/s12866-024-03411-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-024-03411-4