Abstract
Background
Helicobacter pylori lipopolysaccharide (LPS) structures vary among strains of different geographic origin. The aim of this study was to characterize the LPS O-antigen profiles of H. pylori strains isolated from Southwest China, and to further analyze the association of Lewis antigen expression with clinical outcomes and antibiotic resistance.
Results
A total of 71 H. pylori isolates from Southwest China were included for LPS profiling by silver staining and Western blotting after SDS-PAGE electrophoresis. We demonstrated that all the clinical isolates had the conserved lipid A and core-oligosaccharide, whereas the O-antigen domains varied significantly among the isolates. Compared with the common presence of the glucan/heptan moiety in LPS O-antigen structure of European strains, the clinical isolates in this study appeared to lack the glucan/heptan moiety. The expression frequency of Lex, Ley, Lea, and Leb was 66.2% (47/71), 84.5% (60/71), 56.3% (40/71), and 31.0% (22/71), respectively. In total, the expression of type II Lex and/or Ley was observed in 69 (97.2%) isolates, while type I Lea and/or Leb were expressed in 49 (69.0%) isolates. No association of Lewis antigen expression with clinical outcomes or with antibiotic resistance was observed.
Conclusions
H. pylori strains from Southwest China tend to produce heptan-deficient LPS and are more likely to express type I Lewis antigens as compared with Western strains. This may suggest that H. pylori evolves to change its LPS structure for adaptation to different hosts.
Similar content being viewed by others
Introduction
Helicobacter pylori is a spiral, microaerobic, gram-negative bacterium that chronically colonizes the human gastric mucosa and infects approximately half of the global population [1]. Although most people with H. pylori infection have no obvious symptoms, almost all of them have histological chronic active gastritis, which may develop to gastric mucosa atrophy, intestinal metaplasia, and even gastric cancer. H. pylori infection has been accepted as the most important etiological factor for gastric cancer [2,3,4,5].
The outcomes of chronic H. pylori colonization depend on the interaction between bacterial, environmental, and host genetics factors. H. pylori lipopolysaccharide (LPS), localized in the outer leaflet of the bacterial outer membrane, plays essential roles in host‒pathogen interactions [6,7,8,9,10]. H. pylori LPS consists of three domains, including a hydrophobic lipid A domain embedded in the outer membrane, the outermost O-antigen, and the intermediate core-oligosaccharide domain [7,8,9,10]. H. pylori lipid A is constitutively modified into a tetra-acylated and mono-phosphorylated structure, which confers H. pylori intrinsic resistance to cationic antimicrobial peptides (CAMPs), and the ability to escape Toll-like receptor 4 (TLR4) recognition [7, 11]. Furthermore, H. pylori LPS O-antigen usually contains a distal Lewis antigen, which mimics the host Lewis blood group antigens expressed on gastric epithelial cells, camouflaging the bacterium to evade host immune surveillance [7]. Thus, the unique LPS structure plays an important role in chronic colonization of H. pylori within the host gastric niche.
Through systematic construction of glycosyltransferase gene mutants combined with LPS structural analysis by mass spectrometry and nuclear magnetic resonance spectroscopy, our group has recently elucidated the LPS structure and related glycosyltransferases in the European H. pylori reference strain G27 (Fig. 1A) [8, 10]. Our results redefined the core-oligosaccharide domain as a short conserved hexa-saccharide (KDO-LD-Hep I-LD-Hep II-DD-Hep III-Gal-Glc), whereas the O-antigen is a long linear alignment encompassing not only Lewis antigen but also the conserved trisaccharide (GlcNAc-Fuc-DD-Hep) termed as Trio, the glucan (homopolymer of glucose), and the heptan domains (homopolymer of DD-heptose) [10]. Further comparative genomic analysis among 177 diverse H. pylori strains revealed that the glycosyltransferase genes involved in the assembly of Core-Trio-Glc and the distal Lewis antigen motifs are conserved, whereas the heptan glycosyltransferase gene HP1283 (present in the genome of approximately 80% European strains), is completely absent in the included 74 East-Asian strains, suggesting the absence of heptan in LPS structure of East-Asian strains [8]. This is consistent with a previous study on structural analysis of LPS from 12 East-Asian strains, which has shown the absence of heptan and glucan in LPS from all these strains [12]. Of note, the Japanese strain CA2, one of the 12 East-Asian strains, has been fully sequenced to show the absence of the heptan transferase gene HP1283 [8] (Fig. 1B). Thus, the most striking difference in O-antigen structure of LPS between Western and East-Asian H. pylori strains is the presence and absence of the heptan-glucan structure [8, 9] (compare Fig. 1A and B).
LPS structural differences between Western and East-Asian H. pylori strains. A: LPS structure of Western H. pylori strains (represented by the European reference strain G27) [10]. The lipid A, core-oligosacchardie, and the O-antigen domains are indicated. G27 LPS O-antigen contains the Trio, glucan, heptan, and the distal Lewis antigen; (B): LPS structure of East-Asian H. pylori strains (represented by the Japanese strain CA2) [10, 12]. CA2 LPS O-antigen contains the Trio and the distal Lewis antigen, but lacks the intermediate glucan and heptan structures; (C): Structures of the type I and type II Lewis antigens in H. pylori
LPS O-antigen in Western and East Asian H. pylori strains are also found to be different in the expression of Lewis antigens [12, 13]. H. pylori LPS Lewis antigen is composed of a Gal-GlcNAc backbone, which can be divided into type I and type II chains based on the glycosidic linkage (Fig. 1C) [7]. The type I chain is a Gal-(β-1,3)-GlcNAc linkage, which generates Lewis a (Lea) with the decoration of Fuc residue to the backbone GlcNAc, and Lewis b (Leb) with the further addition of Fuc residue to the backbone Gal, while the type 2 chain is a Gal-(β-1,4)-GlcNAc linkage, which gives rise to Lewis x (Lex) and Lewis y (Ley) with the decoration of Fuc residues. It has been reported that the majority of Western H. pylori strains express type II Lewis antigens, while East-Asian strains are prone to express type I Lewis antigens [12, 14, 15].
LPS structural variations among different strains have been suggested to be related with different clinical outcomes [15, 16], as well as with antibiotic resistance [17,18,19]. The aim of this study was to characterize the LPS O-antigen profiles of 71 East Asian H. pylori strains isolated from Southwest China, and to further analyze the association of Lewis antigen expression with gastric diseases and antibiotic resistance.
Materials and methods
H. pylori strains and culture conditions
A total of 71 clinical H. pylori strains from gastric biopsies previously collected at West China Hospital in Southwest China were included in this study. These clinical strains, together with the reference strain G27, were sub-cultured onto commercial Columbia blood agar (CBA) plates (Autobio, China). The plates were incubated at 37 °C for 24–48 h under microaerobic conditions generated by the Anoxomat Mark-II system (Mart Microbiology B.V., the Netherlands).
LPS microextraction for silver staining and Western blotting
LPS microextraction was performed based on previously described procedures [20, 21]. Briefly, bacterial cells (OD600 = 3) collected from CBA plates were suspended in 100 μL LPS lysis buffer (2% SDS, 4% β-mercaptoethanol, 0.1% bromophenol blue, 10% glycerol, 1 M Tris–HCl (pH 6.8)) and then heated at 100 °C for 10 min. After that, the samples were cooled, and 5 μL proteinase K (PK) (20 mg/ml) was added, incubating the samples in a 55 °C water bath overnight. The obtained LPS samples were run on 15% SDS‒PAGE gels and visualized by silver staining and Western blotting using mouse anti-Lea (1:1500), anti-Leb (1:1500), anti-Lex (1:1500), and anti-Ley (1:1500) (Santa Cruz, USA). After incubation with a secondary rabbit anti-mouse antibody conjugated with peroxidase, HRP conjugates were applied to detect the expression of Lewis antigens.
Antimicrobial susceptibility testing
We determined the minimum inhibitory concentrations (MICs) for amoxicillin, clarithromycin, levofloxacin, metronidazole, tetracycline and rifampicin using the E-test strips (Liofilchem s.r.l, Italy). In brief, the freshly grown strains were suspended in sterile saline, and the culture suspension with a concentration of 1.0 OD600 was evenly inoculated onto commercial Columbia Blood Agar (CBA). Subsequently, the E-test strips were placed firmly onto the inoculated CBA plates and incubated for 3–5 days at 37℃ under microaerobic conditions. According to the recommendations from the European Committee on Antimicrobial Susceptibility Testing (EUCAST), resistance break points for amoxicillin, clarithromycin, levofloxacin, metronidazole, tetracycline and rifampicin were defined as MIC > 0.125 mg/L, > 0.5 mg/L, > 1 mg/L, > 8 mg/L, > 1 mg/L and > 1 mg/L, respectively.
Statistical analysis
All statistical analyses were performed with SPSS version 18.0 software (SPSS Inc., Chicago, USA). The chi-square test was used to assess the discrepancy in the frequency of endoscopic findings, histological findings and antimicrobial resistance in different Lewis antigen groups. Statistical significance was regarded as p < 0.05.
Results
Patient demographic and clinical characteristics
A total of 71 clinical H. pylori strains consecutively collected from patients of Chinese Han ethnicity were included for characterization of LPS O-antigen profiles and their association with the severity of patient gastric diseases and antibiotic resistance. The patient demographic and clinical characteristics were shown in Table 1 and Table S1. The number of men and women was 36 and 35, respectively, and their mean age was 47.23 years. Chronic gastritis or duodenitis was found in 54 (76.0%) patients, and peptic ulcer in 17 (24.0%) patients. Histological examination of the gastric mucosa showed that non-atrophic gastritis was present in 44 (61.9%) patients, and atrophic gastritis in 27 (38.1%) patients.
Antibiotic resistance profiles of the 71 H. pylori clinical isolates
Antibiotic resistance patterns of the 71 clinical strains were profiled by E-test. Antibiotic resistance rates to clarithromycin, metronidazole, and levofloxacin were high, being 83.1%, 92.9%, and 71.8%, respectively. Resistance rates to rifampicin and amoxicillin were 19.7% and 9.8%, respectively, while a negligible 2.8% resistance was found for tetracycline (Fig. 2, Table S1).
LPS profiles of the 71 H. pylori clinical isolates
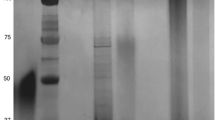
To characterize the LPS profiles, LPS samples extracted from the 71 clinical isolates were resolved on SDS‒PAGE for comparison of LPS general structure by silver staining, and for comparison of Lewis antigen expression by Western bloting (Figs. 3, 4 and 5). As LPS structure of strain G27 has been fully elucidated by our group, LPS extracted from G27 was included as a reference. Of note, G27 LPS is known to harbour type II Lex and Ley, but no type I Lea and Leb [8, 10]. For G27 LPS, after SDS-PAGE electrophoresis and silver staining, the stained bands located at 10–20 kDa correspond to LPS lipid A and core-oligosaccharide, while the bands above 20 kDa correspond to O-antigen domain including the Trio, glucan, heptan, and the capping Lewis antigens (Lex/y).
Silver staining revealed that G27 and all the 71 clinical isolates had the conserved lipid A and core-oligosaccharide. However, the O-antigen domains varied significantly among the isolates. For example, LPS of isolates 16#, 18#, 25#, and 50# appeared to lack the structure above 35 kDa. LPS of many strains including 41–56# appeared to have a “hollow” in the 15–35 kDa region (Fig. 4, silver staining), which is likely due to the absence of glucan and heptan structures in these strains. In addition, LPS samples from isolates 38# and 48# appeared to be stained uniquely different from the LPS profiles of other isolates (Fig. 4).
Western bloing using anti-Lex, and anti-Ley, anti-Lea, and anti-Leb revealed variations in Lewis antigen expression and chain length among the clinical isolates. Of the 71 H. pylori isolates, the expression of Lex, Ley, Lea, and Leb was found to be 66.2% (47/71), 84.5% (60/71), 56.3% (40/71), and 31.0% (22/71), respectively. In total, 97.2% (69/71) of the isolates expressed type II Lex and/or Ley, and 69.0% (49/71) expressed type I Lea and/or Leb. Interestingly, co-expression of the four Lewis antigens was detected in 6 isolates (12#, 25#, 26#, 31#, 62#, and 63#), while none of the four Lewis antigens was detected in isolate 39# (Figs. 3, 4 and 5, Table S1)).
Association of Lewis antigen expression with gastric diseases and antibiotic resistance
We further analyzed whether Lewis antigen expression in clinical H. pylori strains correlated with clinical outcomes and antibiotic resistance. As shown in Table 2, Lewis antigen expression was neither significantly different between isolates from patients with or without peptic ulcers, nor between isolates from patients with or without atrophic gastritis. Furthermore, our data showed no association between Lewis antigen expression and antibiotic resistance (Table 3).
Discussion
H. pylori LPS structure is unique and plays important roles in chronic and persistent colonization of H. pylori within the host gastric niche [10, 22]. In this study, we evaluated the LPS O-antigen profiles of 71 East Asian H. pylori clinical isolates by silver staining and Western bloting. We demonstrated that H. pylori LPS lipid A and core-oligosaccharide domains are conserved, while the O-antigen domain varies significantly among the clinical isolates. Furthermore, we demonstrated that both type I and II Lewis antigens are commonly expressed in these isolates, and no association of the Lewis antigen expression frequency with clinical outcomes or antibiotic resistance was found.
Through SDS-PAGE electrophoresis and silver staining of LPS, we observed that the LPS samples extracted from many of the clinical isolates had a “hollow” in the 15–35 kDa region compared with the obvious bands observed in LPS of G27. This “hollow” is likely to be explained by the absence of glucan and heptan motifs in LPS of East-Asian strains [8, 9]. This phenomenon is consistent with the previous LPS structural studies showing the common presence of glucan/heptan structure in Western H. pylori strains [12, 18, 23], whereas a complete absence of the heptan moiety in LPS structures of all 12 East-Asian strains [12]. Through comparative genomic analysis, our group has also recently demonstrated the common presence (78%) of the heptan transferase gene HP1283 in 78 European strains, while a complete absence of the HP1283 gene in 74 East-Asian strains [8]. The presence and absence of the heptan in LPS structure of Western and East-Asian strains are likely due to H. pylori adaptation to different hosts. In addition, the lack of heptan in LPS structure may also be associated with more severe pathogenesis of East-Asian H. pylori strains than that of the Westen strains [8].
In the present study, we demonstrated that the type II Lex and/or Ley antigens were dominantly expressed in the Chinese isolates (97.2%), which was comparable to the high expression rate of Lex/y previously reported in both East-Asian and Western clinical isolates (up to 95.4%) [15, 18, 23]. The expression rates of type 1 Lea and/or Leb in our local isolates were 56.3%/31.0%, which were much higher than that in isolates from America and Europe [18, 23, 24]. The expression rates of Lea and Leb in 50 isolates from Greek children [23], 38 isolates from Chileans [24], and 41 isolates from Canadians [18] were 0.02%/22%, 0%/24%, and 0.5%/19.5%, respectively. Thus, our study supports the previous findings that the type II Lex/y antigens are frequently expressed in LPS of both East-Asian and Western strains, whereas a tendency for the expression of type 1 Lea/b antigens in LPS from East-Asian hosts compared with Western populations [12, 15]. The difference of Lewis antigen expression in different populations has been suggested to be related to the host Lewis phenotype, suggesting bacterial host adaptation [25,26,27].
The molecular mimicry between H. pylori LPS Lewis antigens and host Lewis blood-group antigens has been suggested to be involved in the development of autoimmune gastric diseases [7, 22, 28]. It has been previously reported that Lewis antigen expression was significantly higher among isolates from patients who have peptic ulcer disease (PUD) than from those without the PUD [15, 29]. However, there are other studies reporting no association between Lewis antigen expression and gastric lesions [24, 30]. In the present study, we found no association between bacterial Lewis antigen expression and gastric lesions, either. These controversial results may be partly explained by the different Lewis antigen expression measuring methods (ELISA or Western bot) or different antibodies used in these studies. Furthermore, the relatively small number of isolates included is likely to be a small-sample bias for analyzing the association between Lewis antigen expression and gastric lesions. Future studies may need to enroll more subjects and more H. pylori isolates from different geographical patients and use the same method to detect Lewis antigen expression to further analyze the relationship between Lewis antigen expression and clinical outcomes.
In recent years, the increasing resistance of H. pylori against commonly used antibiotics has posed a great challenge to the success rate of H. pylori eradication [4, 31,32,33]. It is well known that H. pylori resistance to clarithromycin and levoflaxacin is mainly due to the point mutations in the 23S rRNA and gyrA gene, respectively [34,35,36]. However, gene mutations can’t well explain H. pylori resistance to other antibiotics including metronidazole, amoxicillin. Moreover, there are also clarithromycin and levofloxacin resistant strains without the presence of known point mutations in the 23S rRNA and gyrA gene, suggesting the existence of other mechanisms for clarithromycin and levofloxacin resistance [34, 37]. Several previous studies have reported the association of LPS structure with drug resistance [17,18,19]. Altman et al. reported that the expression of type II Lewis antigens was higher in clarithromycin-resistant strains than in clarithromycin-susceptible strains (95.7% vs 77.7%, p < 0.05) [18]. However, in the present study, we found no association between the frequency of Lewis antigen expression and resistance to all tested antibiotics (amoxicillin, clarithromycin, levofloxacin, metronidazole, tetracycline, and rifampicin). Our group has recently shown that the deletion of a series of LPS glycosyltransferase genes does not affect H. pylori susceptibility to the commonly used anti-H. pylori antibiotics [17].
Our study has limitations. Firstly, the size of the subjects or the number of clinical isolates included in this study was relatively small, which may affect the analysis of association of Lewis antigen expression frequency with antibiotic resistance and clinical outcomes. Secondly, LPS structural analysis by mass spectrometry was not performed for the clinical isolates, and therefore the accurate chemical structures or the absence of the heptan moiety in these Chinese strains can’t be determined.
In summary, our study characterized the LPS profiles of clinical H. pylori strains isolated from Southwest China, and analyzed the association of Lewis antigen expression frequency with gastric diseases and antibiotic resistance. We demonstrated that the LPS lipid A and core-oligosaccharide domains are conserved among H. pylori strains of different phylogeographic origin, while the LPS O-antigen heptan moiety (commonly present in European strains) appeared to be absent in the clinical isolates. Furthermore, we showed our clinical isolates had a propensity to express more type I Lewis antigens than the Western strains, suggesting bacterial host adaptation. We found no association of Lewis antigen expression with clinical outcomes or with antibiotic resistance.
Availability of data and materials
The authors confirm that the data and materials supporting the findings of this study are available within the article and its supplementary materials.
References
Li Y, Choi H, Leung K, Jiang F, Graham DY, Leung WK. 1 Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8(6):553–64. https://doi.org/10.1016/s2468-1253(23)00070-5. Epub 2023/04/23 PubMed PMID: 37086739.
Yang L, Kartsonaki C, Yao P, de Martel C, Plummer M, Chapman D, et al. 2 The relative and attributable risks of cardia and non-cardia gastric cancer associated with Helicobacter pylori infection in China: a case-cohort study. Lancet Public Health. 2021;6(12):e888–96. https://doi.org/10.1016/s2468-2667(21)00164-x. Epub 2021/11/29 PubMed PMID: 34838195; PubMed Central PMCID: PMCPMC8646857.
Rugge M, Genta RM, Di Mario F, El-Omar EM, El-Serag HB, Fassan M, et al. 3 Gastric Cancer as Preventable Disease. Clin Gastroenterol Hepatol. 2017;15(12):1833–43. https://doi.org/10.1016/j.cgh.2017.05.023. Epub 2017/05/24 PubMed PMID: 28532700.
Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, et al. 29 Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022. Epub 2022/08/10. https://doi.org/10.1136/gutjnl-2022-327745. PubMed PMID: 35944925.
Ding SZ, Du YQ, Lu H, Wang WH, Cheng H, Chen SY, et al. Chinese consensus report on family-based Helicobacter pylori infection control and management (2021 Edition). Gut. 2022;71(2):238–53. https://doi.org/10.1136/gutjnl-2021-325630. Epub 20211126 PubMed PMID: 34836916; PubMed Central PMCID: PMCPMC8762011.
Manilla V, Di Tommaso N, Santopaolo F, Gasbarrini A, Ponziani FR. 4 Endotoxemia and gastrointestinal cancers: insight into the mechanisms underlying a dangerous relationship. Microorganisms. 2023;11(2):267. https://doi.org/10.3390/microorganisms11020267. Epub 2023/02/26 PubMed PMID: 36838231; PubMed Central PMCID: PMCPMC9963870.
Li H, Liao T, Debowski AW, Tang H, Nilsson HO, Stubbs KA, et al. 5 Lipopolysaccharide structure and biosynthesis in Helicobacter pylori. Helicobacter. 2016;21(6):445–61. https://doi.org/10.1111/hel.12301. Epub 2016/03/05 PubMed PMID: 26934862.
Li H, Marceau M, Yang T, Liao T, Tang X, Hu R, et al. 8 East-Asian Helicobacter pylori strains synthesize heptan-deficient lipopolysaccharide. PLoS Genet. 2019;15(11):e1008497. https://doi.org/10.1371/journal.pgen.1008497. Epub 2019/11/21 PubMed PMID: 31747390; PubMed Central PMCID: PMCPMC6892558 Ondek Pty. Ltd. The remaining authors disclose no conflicts.
Li H, Tang H, Debowski AW, Stubbs KA, Marshall BJ, Benghezal M. 17 Lipopolysaccharide structural differences between Western and Asian Helicobacter pylori strains. Toxins (Basel). 2018;10(9):364. https://doi.org/10.3390/toxins10090364. Epub 2018/09/13 PubMed PMID: 30205541; PubMed Central PMCID: PMCPMC6162551.
Li H, Yang T, Liao T, Debowski AW, Nilsson HO, Fulurija A, et al. 7 The redefinition of Helicobacter pylori lipopolysaccharide O-antigen and core-oligosaccharide domains. PLoS Pathog. 2017;13(3):e1006280. https://doi.org/10.1371/journal.ppat.1006280. Epub 2017/03/18 PubMed PMID: 28306723; PubMed Central PMCID: PMCPMC5371381 Pty Ltd. The remaining authors disclose no conflicts.
Cullen TW, Giles DK, Wolf LN, Ecobichon C, Boneca IG, Trent MS. Helicobacter pylori versus the host: remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog. 2011;7(12):e1002454. https://doi.org/10.1371/journal.ppat.1002454. Epub 20111222 PubMed PMID: 22216004; PubMed Central PMCID: PMCPMC3245313.
Monteiro MA, Zheng P, Ho B, Yokota S, Amano K, Pan Z, et al. 10 Expression of histo-blood group antigens by lipopolysaccharides of Helicobacter pylori strains from asian hosts: the propensity to express type 1 blood-group antigens. Glycobiology. 2000;10(7):701–13. https://doi.org/10.1093/glycob/10.7.701. Epub 2000/07/27. 10.1093/glycob/10.7.701. PubMed PMID: 10910974.
Sijmons D, Guy AJ, Walduck AK, Ramsland PA. 9 Helicobacter pylori and the role of Lipopolysaccharide variation in innate immune evasion. Front Immunol. 2022;13:868225. https://doi.org/10.3389/fimmu.2022.868225. PubMed PMID: 35634347; PubMed Central PMCID: PMCPMC9136243.
Simoons-Smit IM, Appelmelk BJ, Verboom T, Negrini R, Penner JL, Aspinall GO, et al. 11 Typing of Helicobacter pylori with monoclonal antibodies against Lewis antigens in lipopolysaccharide. J Clin Microbiol. 1996;34(9):2196–200. https://doi.org/10.1128/jcm.34.9.2196-2200.1996. Epub 1996/09/01 PubMed PMID: 8862584; PubMed Central PMCID: PMCPMC229216.
Zheng PY, Hua J, Yeoh KG, Ho B. 23 Association of peptic ulcer with increased expression of Lewis antigens but not cagA, iceA, and vacA in Helicobacter pylori isolates in an Asian population. Gut. 2000;47(1):18–22. https://doi.org/10.1136/gut.47.1.18. Epub 2000/06/22 PubMed PMID: 10861258; PubMed Central PMCID: PMCPMC1727977.
Negrini R, Lisato L, Zanella I, Cavazzini L, Gullini S, Villanacci V, et al. 26 Helicobacter pylori infection induces antibodies cross-reacting with human gastric mucosa. Gastroenterology. 1991;101(2):437–45. https://doi.org/10.1016/0016-5085(91)90023-e. Epub 1991/08/01 PubMed PMID: 2065920.
Tang X, Yang T, Shen Y, Song X, Benghezal M, Marshall BJ, et al. 14 roles of Lipopolysaccharide Glycosyltransferases in maintenance of Helicobacter pylori Morphology, cell wall permeability, and antimicrobial susceptibilities. Int J Mol Sci. 2023;24(14):11381. https://doi.org/10.3390/ijms241411381.
Altman E, Harrison BA, Chandan V, Slinger R. 13 Lipopolysaccharide glycotyping of clarithromycin-resistant and clarithromycin-susceptible Canadian isolates of Helicobacter pylori. Can J Microbiol. 2014;60(1):35–9. https://doi.org/10.1139/cjm-2013-0747. Epub 2014/01/08 PubMed PMID: 24392924.
Hasegawa M, Kobayashi I, Saika T, Nishida M. Drug-resistance patterns of clinical isolates of Pseudomonas aeruginosa with reference to their lipopolysaccharide compositions. Chemotherapy. 1997;43(5):323–31. https://doi.org/10.1159/000239585. PubMed PMID: 9309365.
Apicella MA. 15 Isolation and characterization of lipopolysaccharides. Methods Mol Biol. 2008;431:3–13. https://doi.org/10.1007/978-1-60327-032-8_1. Epub 2008/02/22 PubMed PMID: 18287743.
Li H, Benghezal M. Crude Preparation of Lipopolysaccharide from Helicobacter pylori for Silver Staining and Western Blot. Bio Protoc. 2017;7(20):e2585. https://doi.org/10.21769/BioProtoc.2585. Epub 20171020 PubMed PMID: 34595267; PubMed Central PMCID: PMCPMC8438427.
Bertani B, Ruiz N. 16 Function and Biogenesis of Lipopolysaccharides. EcoSal Plus. 2018;8(1). Epub 2018/08/02. https://doi.org/10.1128/ecosalplus.ESP-0001-2018. PubMed PMID: 30066669; PubMed Central PMCID: PMCPMC6091223.
Altman E, Chandan V, Harrison BA, Panayotopoulou EG, Roma-Giannikou E, Li J, et al. 28 Helicobacter pylori isolates from Greek children express type 2 and type 1 Lewis and α1,6-glucan antigens in conjunction with a functional type IV secretion system. J Med Microbiol. 2012;61(4):559–66. https://doi.org/10.1099/jmm.0.038729-0. Epub 2011/12/14 PubMed PMID: 22160312.
Altman E, Fernandez H, Chandan V, Harrison BA, Schuster MW, Rademacher LO, et al. 22 Analysis of Helicobacter pylori isolates from Chile: occurrence of selective type 1 Lewis b antigen expression in lipopolysaccharide. J Med Microbiol. 2008;57(Pt 5):585–91. https://doi.org/10.1099/jmm.0.47783-0. PubMed PMID: 18436591.
Pohl MA, Romero-Gallo J, Guruge JL, Tse DB, Gordon JI, Blaser MJ. 6 Host-dependent Lewis (Le) antigen expression in Helicobacter pylori cells recovered from Leb-transgenic mice. J Exp Med. 2009;206(13):3061–72. https://doi.org/10.1084/jem.20090683. Epub 2009/12/17 PubMed PMID: 20008521; PubMed Central PMCID: PMCPMC2806470.
Broadberry RE, Lin-Chu M. 25 The Lewis blood group system among Chinese in Taiwan. Hum Hered. 1991;41(5):290–4. https://doi.org/10.1159/000154015. Epub 1991/01/01 PubMed PMID: 1778604.
Perepelov AV, Senchenkova SN, Knirel YA. Variations in the expression of terminal Oligosaccharide units and Glycosylation of Poly(N-acetyllactosamine) chain in the Helicobacter pylori Lipopolysaccharide upon colonization of Rhesus Macaques. Biochemistry (Mosc). 2020;85(2):234–40. https://doi.org/10.1134/s0006297920020108. PubMed PMID: 32093599.
Moran AP. Relevance of fucosylation and Lewis antigen expression in the bacterial gastroduodenal pathogen Helicobacter pylori. Carbohydr Res. 2008;343(12):1952–65. https://doi.org/10.1016/j.carres.2007.12.012. Epub 20071225 PubMed PMID: 18279843.
Wirth HP, Yang M, Karita M, Blaser MJ. 21 Expression of the human cell surface glycoconjugates Lewis x and Lewis y by Helicobacter pylori isolates is related to cagA status. Infect Immun. 1996;64(11):4598–605. https://doi.org/10.1128/iai.64.11.4598-4605.1996. Epub 1996/11/01 PubMed PMID: 8890213; PubMed Central PMCID: PMCPMC174419.
Wirth HP, Yang M, Peek RM Jr, Tham KT, Blaser MJ. 27 Helicobacter pylori Lewis expression is related to the host Lewis phenotype. Gastroenterology. 1997;113(4):1091–8. https://doi.org/10.1053/gast.1997.v113.pm9322503. Epub 1997/10/10 PubMed PMID: 9322503.
Yang T, Hu R, Tang X, Shen Y, Tay A, Pi X, et al. Susceptibility-guided bismuth quadruple therapies for resistant Helicobacter pylori infections. Precis Clin Med. 2020;3(2):127–35. https://doi.org/10.1093/pcmedi/pbaa010. Epub 20200317 PubMed PMID: 35692608; PubMed Central PMCID: PMCPMC8985787.
Li H, Yang T, Tang H, Tang X, Shen Y, Benghezal M, et al. Helicobacter pylori infection is an infectious disease and the empiric therapy paradigm should be changed. Precis Clin Med. 2019;2(2):77–80. https://doi.org/10.1093/pcmedi/pbz009. Epub 20190619 PubMed PMID: 35692450; PubMed Central PMCID: PMCPMC8985775.
Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in world health organization regions. Gastroenterology. 2018;155(5):1372-82.e17. https://doi.org/10.1053/j.gastro.2018.07.007. Epub 20180707 PubMed PMID: 29990487; PubMed Central PMCID: PMCPMC6905086.
Zhou J, Shen Y, Song X, Zhou L, Tang H, Li H. Evaluation of a molecular Mosprie assay for detection of Helicobacter pylori and resistance to Clarithromycin and Levofloxacin. J Infect Dis. 2022;226(Suppl 5):S503–9. https://doi.org/10.1093/infdis/jiac402. PubMed PMID: 36478246.
Li H, Shen Y, Song X, Tang X, Hu R, Marshall BJ, et al. Need for standardization and harmonization of Helicobacter pylori antimicrobial susceptibility testing. Helicobacter. 2022;27(2):e12873. https://doi.org/10.1111/hel.12873. Epub 20220212 PubMed PMID: 35151236.
Li Y, Lv T, He C, Wang H, Cram DS, Zhou L, et al. Evaluation of multiplex ARMS-PCR for detection of Helicobacter pylori mutations conferring resistance to clarithromycin and levofloxacin. Gut Pathog. 2020;12:35. https://doi.org/10.1186/s13099-020-00373-6. Epub 20200710 PubMed PMID: 32670416; PubMed Central PMCID: PMCPMC7350683.
Tshibangu-Kabamba E, Yamaoka Y. 30 Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2021;18(9):613–29. https://doi.org/10.1038/s41575-021-00449-x. PubMed PMID: 34002081.
Funding
This study was supported by the National Natural Science Foundation of China (grant 82072248), the International Cooperation Excellence Initiative Grant, West China Hospital, Sichuan University (grant 139220012), and the Fund for the transformation of science and technological achievements, West China Hospital, Sichuan University (grant CGZH19005).
Author information
Authors and Affiliations
Contributions
Conceptualization, Mohammed Benghezal, Hong Tang and Hong Li; Formal analysis, Xiaoqiong Tang, Peng Wang, Yalin Shen, Xiaona Song and Hong Li; Funding acquisition, Barry J. Marshall, Hong Tang and Hong Li; Investigation, Xiaoqiong Tang, Peng Wang, Mohammed Benghezal and Hong Li; Methodology, Xiaoqiong Tang, Peng Wang, Yalin Shen and Xiaona Song; Project administration, Barry J. Marshall, Hong Tang and Hong Li; Supervision, Mohammed Benghezal, Barry J. Marshall and Hong Tang; Writing – original draft, Xiaoqiong Tang; Writing – review & editing, Hong Tang and Hong Li.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
LPS profiles of clinical isolates No. 1-8. LPS samples from G27 wild-type and clinical isolates were analyzed by silver staining (A); and Western blot using anti-Lex (B), anti-Ley (C), anti-Lea (D), and anti-Leb (E). Figure S2. LPS profiles of clinical isolates No. 9-16. LPS samples from G27 wild-type and clinical isolates were analyzed by silver staining (A); and Western blot using anti-Lex (B), anti-Ley (C), anti-Lea (D), and anti-Leb (E). Figure S3. LPS profiles of clinical isolates No. 17-24. LPS samples from G27 wild-type and clinical isolates were analyzed by silver staining (A); and Western blot using anti-Lex (B), anti-Ley (C), anti-Lea (D), and anti-Leb (E). Figure S4. LPS profiles of clinical isolates No. 25-32. LPS samples from G27 wild-type and clinical isolates were analyzed by silver staining (A); and Western blot using anti-Lex (B), anti-Ley (C), anti-Lea (D), and anti-Leb (E). Figure S5. LPS profiles of clinical isolates No. 33-40. LPS samples from G27 wild-type and clinical isolates were analyzed by silver staining (A); and Western blot using anti-Lex (B), anti-Ley (C), anti-Lea (D), and anti-Leb (E). Figure S6. LPS profiles of clinical isolates No. 41-48. LPS samples from G27 wild-type and clinical isolates were analyzed by silver staining (A); and Western blot using anti-Lex (B), anti-Ley (C), anti-Lea (D), and anti-Leb (E). Figure S7. LPS profiles of clinical isolates No. 49-56. LPS samples from G27 wild-type and clinical isolates were analyzed by silver staining (A); and Western blot using anti-Lex (B), anti-Ley (C), anti-Lea (D), and anti-Leb (E). Figure S8. LPS profiles of clinical isolates No. 57-63. LPS samples from G27 wild-type and clinical isolates were analyzed by silver staining (A); and Western blot using anti-Lex (B), anti-Ley (C), anti-Lea (D), and anti-Leb (E). Figure S8. LPS profiles of clinical isolates No. 64-71. LPS samples from G27 wild-type and clinical isolates were analyzed by silver staining (A); and Western blot using anti-Lex (B), anti-Ley (C), anti-Lea (D), and anti-Leb (E).
Additional file 2: Table S1.
Patients characteristics, antimicrobial resistance pattern and Lewis expression among the 71 H. pylori isolates from Southwest China.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tang, X., Wang, P., Shen, Y. et al. Lipopolysaccharide O-antigen profiles of Helicobacter pylori strains from Southwest China. BMC Microbiol 23, 360 (2023). https://doi.org/10.1186/s12866-023-03116-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-023-03116-0