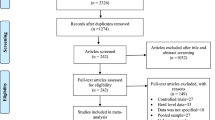
Abstract
Background
Aeromonas hydrophila is an opportunistic pathogen. Thus, it has received significant attention mainly in the fish sectors with high production scales. Nile tilapia broodstock confined in the environment of fish hatcheries can be stressed. Hence, they are vulnerable to A. hydrophila.
Results
Sequencing of the gyr B gene revealed the presence of 18 different A. hydrophila strains (kdy 10,620–10,637), which were deposited in the NCBI under accession numbers ON745861–ON745878. The median lethal doses of the isolates ranged from 2.62 × 104 to 3.02 × 106 CFU/mL. Antibiotic resistant genes, sulfonamide (sul1) and tetracycline (tetA) were found in the eighteen isolates. Approximately 83.3% of A. hydrophila strains were sensitive to ciprofloxacin and florfenicol. Further, eight A. hydrophila strains had high MDR indices at 0.27–0.45. All isolates presented with hemolysin activity. However, only 72.22% of them had proteolytic activity, and only 61.11% could form biofilms. Bacterial isolates harbored different pattern virulence genes, the heat-stable cytotonic enterotoxin (ast), cytotoxic enterotoxin (act), and hemolysin (hly) genes were the most prevalent. Also, a trial to inhibit bacterial growth was conducted using titanium dioxide nanoparticles (TiO2 NPs) with three sizes (13, 32, and 123 nm). If A. hydrophila strains with a high MDR index were tested against TiO2 NPs (20 µg/mL) for 1, 12, and 24 h, those with a small size had a greater bactericidal action than large ones. Bacterial strains were inhibited at different percentages in response to TiO2 NP treatment.
Conclusions
Nile tilapia broodstock, mortality is associated with different A. hydrophila strains, which harbored virulent and MDR genes. Furthermore, TiO2 NPs had bactericidal activity, thereby resulting in a considerable reduction in bacterial load.
Similar content being viewed by others
Background
Globally, the growing demand for fish and fish products caused by the rapid population growth and the increased preference for the consumption of healthier foods. Nile tilapia (Oreochromis niloticus) is one of the most produced species in freshwater aquaculture in several countries worldwide, Egypt is among the countries with a high production rate, and it ranks third in Nile tilapia aquaculture [1].
Aeromonas species are ubiquitous gram-negative, rod-shaped microbes that are commonly found in freshwater and estuary environments [2]. Different aquatic ecosystems are inhabited by a wide range of Aeromonas spp., these species can be isolated from water, soil, and food, such as meat, ham, raw milk, offal, sausage, vegetables, poultry, fish and shellfish [3, 4]. Further, they are normal inhabitants of the gastrointestinal tract of fish [5]. Due to excessive stocking density and poor farming management, farmed freshwater fish are more vulnerable to outbreaks of motile aeromonas septicemia. The pathogenesis of Aeromonas is attributed to the different genes that encode a wide range of virulence factors responsible for disease development in the target host. The common virulence factors of pathogenic Aeromonas spp. include three different enterotoxins (act, alt, and ast), hemolysin (hlyA), aerolysin (aerA), flagella (fla), lipase (lip), and elastase (ela) [6]. Aeromonas produces different toxins, such as hemolysin, aerolysin, and cytotonic enterotoxins, which are harmful to its hosts [7, 8]. Aeromonas hydrophila infection is one of the most devastating bacterial infections, accounting for millions of dollars in losses in the global freshwater aquaculture sector [9, 10]. Antibiotic is a key-stone in bacterial disease control. Consequently, there is a global issue regarding multidrug resistance (MDR), even the World Health Organization (WHO) named 2011 as the year of antibiotic resistance [11]. However, Chilean scallop Argopecten purpuratus larvae are produced under hatchery-controlled conditions, they are affected by bacterial diseases outbreaks [12, 13]. Treatment with antibiotics, such as chloramphenicol, which was substituted with florfenicol in A. purpuratus production, is associated with an increased risk of developing antibiotic resistance [14]. The mechanism or function of antibiotic resistance in pathogenic bacteria should be determined to choose the best option for treatment [15]. Five common mechanisms of antibiotic resistance for Gram-negative and Gram-positive bacteria are enzymatic hydrolysis, enzymatic modifications of antibiotics by group transfer and redox process, modifications of antibiotic targets, reduced permeability to antibiotics by modifications of porins, and active extrusion of antibiotics by membrane efflux pumps [16]. Egypt is among the countries where antimicrobials are regularly used in aquaculture without veterinarian prescriptions [17]. Furthermore, some antimicrobials are used irresponsibly as growth promotors and as preventive treatments to reduce the incidence of diseases in fish farming [18, 19]. In turn, Aeromonas develops an adaptive response to respective antibiotics [20]. Antibiotic resistance is transferred via plasmids, integrons, prophages, and transposons, which can carry virulence genes facilitating the development of antibiotic resistance among Aeromonas with multiple virulence genes [19, 21, 22]. Aeromonas spp. is resistant to several types of antibiotics, which pose a hazard to human health since these isolates can spread to people via the food chain or direct contact with the aquatic environment [23, 24].
Environmental factors, such as metal availability, salinity, dissolved oxygen, pH, and temperature, and potentially bad management (malnutrition, overfeeding, and overcrowding) in hatchery facilities can cause stress among aquatic animals. Thus, they become more vulnerable to infectious diseases. In contrast, natural disease outbreaks are seldom observed in wild aquatic species because they normally coexist with pathogens until there are significant environmental changes [25]. Moreover, disinfections are regularly used in hatcheries affecting microbial balance, thereby restricting the opportunistic ability of infections to be controlled by natural biological processes [26]. Research on more eco-friendly methods of disease management has been based on the growing political and environmental pressure that can limit the use of antibiotics and other therapeutic agents in aquaculture [27].
The exposure to nano metals resulted in bacterial cells deaths which were due to bioaccumulation within the cell membrane [28,29,30]. Bacterial deaths were proportionally related to nano metals concentration, size, and exposure time as 50 nm or less may pass through the bacteria cell wall if given enough time [31,32,33,34]. Titanium dioxide nanoparticles (TiO2 NPs) were prepared and used as antibacterial agents for eliminating human pathogens, such as gram-negative and gram-positive bacteria, and as antifungal agents [35]. Among the different metal oxide nanoparticles, TiO2 NPs are economical, stable, and safe for people and the environment [36]. The Food and Drug Administration has recommended the use of TiO2 in human nutrition [37].
The current study aimed to evaluate the failure of antibiotic treatments against pathogenic A. hydrophila in Nile tilapia to eliminate some highly pathogenic strains of isolated strains using TiO2 NPs.
Materials and methods
Fish hatcheries
Six tilapia fish hatcheries (TH1-6) are in Kafrelsheikh governorate, Egypt. During the normal hatching production process, moribund broodstock fish with clinical signs were collected and aseptically transferred in transporting clear bags with dechlorinated water. The bags were then placed in containers supplied with ice. The samples were transported to the bacteriology laboratory (Animal Health Research Institute) within 1–2 h for further examination using a tranquilizer (MS-222) and antiseptic iodine, according to a previous study [38].
Clinical examination
Upon the arrival of the samples, the fish were examined using the standard protocols for the assessment of external and internal pathological lesions based on a previous study [39].
Bacteriological examination
A. hydrophila isolation swab samples were collected from the kidney, liver, spleen, and brain of O. niloticus, inoculated into tryptic soy broth (Difco, Detroit, USA), and incubated at 30 °C for 24 h. Then, the inoculum was spread onto Aeromonas agar and then incubated at 30 °C for 24 h. Representative colonies that were selected randomly were purified by subculturing onto tryptic soy agar under the same conditions. The isolates were stored at − 80 °C in TS broth with glycerol for further analysis.
Biochemical profiles
The phenotypic and biochemical features of bacterial isolates were validated according to a previous study [40].
-
a. Biochemical tests were performed in triplicate using API20 E based on the manufacturer’s instructions (BioMérieux, Marcy l' Etoile, France).
-
b. Biofilm production was investigated using the tube adherence method, according to the study of Christensen et al. [41]. Briefly, bacterial isolates were incubated in plastic conical falcon tubes with TSB at 30 °C for 48 h. The tubes were then emptied, dyed with 0.1% solution of safranin, rinsed with distilled water, and dried. Coating of stained material stuck to the tube revealed biofilm formation.
-
c. Hemolytic activity was evaluated by streaking bacterial strains onto tryptic soy agar (Oxoid™) plates supplemented with 5% sheep erythrocytes and incubated for 24 h at 30 °C based on the study of Chen and Huang [42]. The appearance of a clear lytic zone on the surface of agar plates indicated a positive result.
-
d. According to the study of Arai et al. [43], proteolytic activity was evaluated by plating bacterial culture onto Brain Heart Infusion Agar (HiMedia) supplemented with 1% fresh egg yolk and incubated at 30 °C for 48 h. The evident proteolytic zone surrounding the cells was used to identify proteolytic activity.
Partial sequences of the gyrB gene
The Aeromonas strains were streaked onto Brain Heart Infusion Agar (Difco, the USA) and aerobically incubated at 28 °C for 24 h. The genomic DNA of Aeromonas isolates was extracted using the DNA extraction kit (DNeasy Kit, Qiagen, the USA), based on the manufacturer’s protocol. To validate the identity of Aeromonas spp., a genus-specific primer pair (gyrB F: 5′-TCCGGCGGTCTGCACGGCGT-3′ and R 5′-TTGTCCGGGTTGTACTCGTC-3′) was used in the polymerase chain reaction (PCR) amplification [44]. Briefly, a reaction mixture containing 12.5 μl of Dream Taq Green PCR Mix (2X) (Thermo-Scientific, the USA), 2 µl of extracted DNA, 1 µl of each primer, and 8.5 µl of distilled water. The thermal cycler program was adjusted as follows: 95 °C for 4 min (initial denaturation), followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 90 s. The reaction was ended at 72 °C for 10 min (as the final extension). The amplicons were purified using the A GeneJET™ PCR Purification Kit (Thermo Fisher Scientific, the USA). PCR products were electrophoresed in 1.5% agarose and visualized under ultraviolet light.
The amplified gyrB gene was sequenced in two directions using the ABI 3730xl DNA sequencer (Applied Biosystems, the USA). The raw sequences were edited and assembled using BioEdit version 7.0 [45]. The assembled gyrB genes were submitted to GenBank. The phylogenetic tree was constructed using MEGA version X [46]. Neighbor-joining phylogenetic analysis was performed using the Kimura two-step algorithm with 1,000 bootstrap replicates.
Examination of virulence and antibiotic-resistant genes
The virulence genes were cytotoxic enterotoxin (act), heat-stable cytotonic enterotoxin (ast), cytotoxic enterotoxin (alt), hly, serine protease (ser), and extracellular lipase (lip). Some antibiotic resistant genes namely sul1, tetA, quinolone (qnrs), and erythromycin (ermB) were screened in all Aeromonas isolates using PCR, as described in the study of Randall et al. [47]. All primers used in this work are inserted in (Table 1).
Antibiogram of Aeromonas strains
The disc diffusion method was used to assess the antibiotic susceptibility of bacterial isolates using Mueller–Hinton agar (Oxoid™) based on a previous study [53]. The antimicrobial agents tested were tetA 30 μg, trimethoprim/sulfamethoxazole (SXT) 1.25/23.75 μg), ciprofloxacin (CIP) 5 μg, florfenicol 30 μg, erythromycin (E, 15 μg), gentamicin 10 μg, amoxicillin 10 μg, ampicillin (AMP) 10 μg, kanamycin 30 μg, cefotaxime 30 μg, and streptomycin 30 μg). The test was performed in triplicates. Isolates were subcultured in tryptic soy broth, incubated overnight at 30 °C, and streaked onto Mueller–Hinton agar plates using a cotton swab. The antibiotic discs were adjusted on the agar surface and incubated for 24 h at 30 °C. The diameter of the inhibition zones was determined, and the results were interpreted according to the criteria of the Clinical Laboratory Standards Institute,.MDR index was calculated as X/Y, where X is all types of antibiotics wherein the isolates were resistant to and Y is all antibiotics used in the study. An MDR index of > 0.2 indicated resistance to multiple antibiotics [54].
Median lethal dose (LD50)
The median lethal dose (LD50) of A. hydrophila was evaluated according to the procedure of Reed & Muench [55]. Briefly, O. niloticus (40 ± 3 g b.w.) was acclimated at the wet laboratory of the Animal Health Research Institute. After anesthetizing the fish using tricaine methanesulfonate (MS222; Sigma, St. Louis, MO, the USA), groups of 10 fish were intraperitoneally injected with serial tenfold dilutions of A. hydrophila cultured in Brain Heart Infusion Broth at 30 °C for 24 h. First, 100 μl of A. hydrophila suspension was adjusted to 1 × 102, 1 × 103, 1 × 104, 1 × 105, 1 × 106, 1 × 107, 1 × 108, 1 × 109, or 1 × 1010 CFU/mL in normal saline (0.65%). The suspension was injected into duplicate groups of five fish. The 14-day mortality rates were recorded, and A. hydrophila was re-isolated from the dead moribund fish and confirmed via PCR. The clinical signs were recorded and photographed.
Manufacturing and impact of titanium dioxide on bacterial cells
Titanium (IV) isopropoxide (TIP) (C12H28O4Ti) (purity: 97%), 2-propanol [(CH3)2CHOH] (purity: 99%), and hydrochloric acid (HCL) (concentration: 98%) were purchased from Sigma-Aldrich. A mixture of TIP and 2-propanol with a ratio of 1:4 was stirred using a magnetic stirrer at 200 rpm for 1 h at room temperature. Another mixture of deionized water and 2-propanol with a ratio of 1:1 was added to the first one drop by drop. Then, the mixture was stirred as above. The pH value of the solution was adjusted at pH = 3, 3.5, or 4 using HCL then final solution was stirred as above. The final mixture was placed in a water bath at 80 °C to evaporate any liquid in the mixture. The resulting white powder TiO2 was hydrogenated for at different temperatures (300 °C, 400 °C, 500 °C, and 600 °C) using (tubular furnace). Three different nanosized particles of TiO2 anatase 13, 32, and 123 nm were formulated. Then, the shapes were photographed via a JEOL expository scanning electron microscope (SEM) device [56].
The time-dependent antibacterial activity of TiO2 NPs was evaluated as follows: Briefly, 50 µl of 24-h old A. hydrophila inoculum (corresponding to a concentration of the calculated LD50 of the MDR strains) was exposed to a dilution of the three sized-TiO2 NPs 20 µg/mL for 1, 12, and 24 h, according to previous studies [30, 33, 57]. The culture grew in triplicate on standard count plate (Sigma-Addrich) at 37 °C for 24 h. Meanwhile, the tryptic soya broth alone was considered as the negative control. The bacteria counts were calculated by the number of growing colonies in the culture media.
Biosafety procedure
This study followed the biosafety measures on the pathogen safety data sheets (Infectious substances–A. hydrophila, Pathogen Regulation Directorate [58].
Results
Genetic identification of Aeromonas spp.
The gyrB gene was successfully amplified from all 18 isolates of Aeromonas spp. (kdy 10,620–kdy 10,637). The multiple alignments of the gyrB gene sequences confirmed that all 18 strains belonged to the genus Aeromonas. The accession numbers obtained from the sequencing of the gyrB genes ranged from ON745861 to ON745878. The BLAST analysis of these sequences showed a 99.91%–97.06% similarity to A. hydrophila (AB436660T, AB436661T, AY987520T, CP000462T, AJ868394T, OL321923, OL321922, and AB473068). Consequently, the current Aeromonas strains were genetically identified as A. hydrophila. The intraspecies similarity of A. hydrophila isolates was 99.81%–100% with 1–2-bp nucleotide differences.
By contrast, the neighbor-joining phylogenetic analysis showed two large clades. The first lineage was further divided into two subclades. The first one clustered the current 18 strains of A. hydrophila with other A. hydrophila isolates recovered from GenBank with a bootstrap value of 97% and separated from the second subclade. Then, the second subclade clustered A. veronii isolates with A. sobria isolates to form one subclade with a high bootstrap value at 99%. Further, it included A. caviae isolates with a high bootstrap value of 99% (Fig. 1). Pseudomonas fluorescens (KX640817) was used as an outgroup isolate.
Detection of MDR and ARG in the isolated strains
In Table 2, the MDR index was between 0.27 and 0.45 in eight A. hydrophila strains (ON745861, ON745862, ON745863, ON745866, ON745867, ON745876, ON745877, and ON745878). Meanwhile, it was 0.18 in 10 strains.
The antibiotic resistance genes sul1 and tetA were found in 100% (18/18) of the isolates, qnrs in 44.4% (8/18), and ermB in 27.78% (5/18).
Phenotypic antibiotic resistance
Table 3 shows the bacterial strains (kdy 10,620–kdy 10,637), all bacterial strains were resistant to tetracycline, trimethoprim & sulfamethoxazole. Meanwhile, 83.3% of bacterial strains were sensitive to ciprofloxacin and florfenicol and 72.23% to erythromycin. In addition, 33.33%, 27.77%, and 38.9% of isolates were less sensitive to gentamycin, amoxicillin, and ampicillin, respectively.
Virulence genes and biochemical identification of Aeromonas spp.
As shown in Table 4, the virulence genes heat-stable cytotonic enterotoxin (ast), cytotoxic enterotoxin (act, alt), hemolysin (hly), serine protease (ser), and extracellular lipase (lip) were detected in 18 isolates at different percentages (83.3% [15/18], 100% [18/18], 44.4% [8/18], 100% [18/18], 50% [9/18], and 50% [9/18], respectively) in bacterial strains kdy 10,620–10,638.
Bacterial isolates possess different biochemical characters under identification numbers 107126, 7,456,754, 7,467,754, and 7,576,755 using API20E. Also, biofilm production, hemolysin activity, and proteolytic activity were detected in the bacterial isolates with percentages of 61.11% (11/18), 100% (18/18), and 72.22% (13/18), respectively.
Bacterial pathogenesis
In Fig. 2, the fish presented with the following clinical signs: opaque, slightly protruded eye, dentated dorsal and tail fin, hemorrhagic body surface, and sloughed scales. The post mortem signs were dark brown liver, distended gall bladder, splenomegaly, and empty intestinal tract.
(1) Arrows–A: Opaque, slightly protruded eye. B: Dentated dorsal fin. C: Dentated tail fin. (2) Arrows–A: Opaque eye. B: Extensive dentated dorsal and tail fin. D: Empty abdomen with hemorrhagic body surface and sloughed scales. (3) Arrows–A: Dark brown liver. B: Distended gall bladder. C: Splenomegaly. D: Empty intestinal tract. E: Slightly opaque eye
As depicted in Table 5, the LD50s of ON745861, ON745862, ON745863, ON745866, ON745867, ON745876, ON745877, and ON745878 were 1.71 × 105, 1.93 × 105, 1.00 × 105, 2.37 × 106, 3.02 × 106, 1.43 × 106, 2.62 × 104, and 1.92 × 105 CFU/mL, respectively.
Antibacterial effect of TiO2 NPs
Figure 3 shows the characteristics of TiO2 NPs. The average sizes of anatase crystalline were 13, 32, and 123 nm. The TiO2 NPs (anatase) was nano powder, and its purity was 97.45%. To confirm the TiO2 NPs purity, size and shape were evaluated using SEM, as shown in the Supporting Information.
The antibacterial properties of three nanosized TiO2 (at a concentration of 20 µg/mL) were evaluated against the highly pathogenic A. hydrophila for 1, 12, and 24 h (Table 5). Regardless of bacterial strain and exposure time, the small-size TiO2 NPs had a higher bactericidal activity (13 > 32 > 123 nm) than the larger sizes. After 1 h of exposure to TiO2 NPs measuring 13 nm, the bacterial count ranged from 10% to 57.3% from the initial count (LD50). Meanwhile, TiO2 NPs measuring 32 nm had decreased bacterial count at 15.59%–61.18%. Finally, the bacterial count was between 42% and 68.35% after exposure to TiO2 NPs measuring 123 nm. After 24 h, there was no bacterial growth. Regardless of TiO2 NPs size and exposure time, the bacterial count varied in different bacterial isolates.
Discussion
Aeromonas hydrophila was isolated from moribund broodstock Nile tilapia in different six hatcheries. They were recognized phenotypically and genotypically, and the sequence of gyrB gene revealed the presence of 18 Aeromonas hydrophila strains. (kdy 10,620–kdy 10,637). During disease outbreaks, the accurate identification of the causal pathogens is a challenge to fish pathologists [59]. Similarly, some researchers found that Aeromonas strains could be identified by sequencing gyrB, 16 s rRNA, and rpoD genes [60].
In this study, all Aeromonas strains were resistant to tetracycline, trimethoprim & sulfamethoxazole. Meanwhile, 83.3% of the strains were sensitive to ciprofloxacin and florfenicol and 72.23% to erythromycin. Furthermore, 33.33%, 27.77%, and 38.9% of the strains were less sensitive to gentamycin, amoxicillin, and ampicillin, respectively. Similarly, opportunistic pathogenic bacteria can resist a wide range of antibiotics causing bacterial infections, particularly in immune-compromised individuals [61]. Accordingly, aeromonads were sensitive to fluoroquinolones, and ciprofloxacin could be the treatment of choice [2, 62]. Similarly, aeromonads collected from diseased fish were resistant to ampicillin, amoxicillin, and erythromycin [63]. On the contrary, only 15% of Aeromonas spp. were resistant to tetracycline [64]]. Furthermore, 7.7%, 17.9%, 25.6%, 43.6%, and 47.7% of Aeromonas spp. were resistant to doxycycline, trimethoprim/sulfamethoxazole, chloramphenicol, colistin, and erythromycin [63]. These differences in percentages of resistant bacteria could be due to the date of conducting the survey, the veterinary choice for the antibiotic, and the history of antibiotic abuse in the fish farm.
The MDR indices were high, ranging from 0.27 to 0.45, in eight A. hydrophila strains (kdy 10,620, kdy 10,621, kdy 10,622, kdy 10,625, kdy 10,626, kdy 10,635, kdy 10,636, and kdy 10,637). Meanwhile, the index was 0.18 in the remaining isolates in this study. Similarly, Liu et al. [65] found that the MDR in A. hydrophila pathogenic strains ranged from 0.2 to 0.6. The MAR index of A. hydrophila (87.2%) was > 0.2. Thus, these strains were obtained from a high-risk source of infection [54]. Accordingly, the MDR was 0.387 regardless of the sources of Aeromonas strains, which were cultured freshwater animals in China [66]. Meanwhile, the MDR was 0.263 and 0.287 in Aeromonads isolated from human patients [67, 68].
In this investigation, ARG sul1 and tetA were found in all A. hydrophila isolates, while ARG qnrs and ermB were in 44.4% and 27.78% of the isolates, respectively. Accordingly, 26.3% of pathogenic bacteria detected in Mediterranean fish farms carried at least one of the tetracycline RGs [69] and tetracycline RGs accounted for more than half of the total ARGs isolated from infected bacteria in fish and ducks [70]. In addition, sulphonamide RGs (sulII and sulIII) were detected in 56.14% of bacterial isolates in fish tanks [71]. Moreover, quinolone RGs (qnrs) were discovered in Aeromonas spp. isolated from the Seine River in Paris [72]. Accordingly, Jang et al. [73] recorded a low incidence of ermC-encoding erythromycin-resistant pathogens isolated from the effluent of coastal aquaculture in Jeju Island, South Korea. Similarly, Deng et al. [66] found that at least 18.86% of Aeromonas strain isolates from cultured freshwater animals were resistant to trimethoprim/sulfamethoxazole and that 18.9% and 4.7% of them also carried RG for sul1 and qnrs. Similarly, to our findings, Shuang et al. [74] revealed that sul1 and qnrs were present in Aeromonas strains at rates of 12.1% and 22.8%, respectively, and that these bacteria were trimethoprim/sulfamethoxazole-resistant.
In this study, the virulence genes were detected in the eighteenth A. hydrophila strains (kdy 10,620–10,638) with various percentages of ast (83.3%), act (100%), alt (44.4%), hly (100%), ser (50%), and lip (50%). Similarly, approximately 50% of A. hydrophila had 50% of the virulence genes tested (namely, aer, ahp, alt, hly, lip, fla, ela, and/or act) meanwhile, none were found in 2% [64]. Based on our findings, although with different percentages recovered, the virulence genes were found in 41% of A. hydrophila isolates, including aer (33.33%), lip (23.1%), hlyA (5.13%), and ast (2.56%) [63]. Accordingly, the lip gene was detected in 50% of A. hydrophila, and it could alter the cell membrane structure of fish tissues and, thus, could manifest bacterial pathogenicity [21].
In this study, A. hydrophila had more than one virulence gene, and the most common virulence gene patterns were ast, act, and hly. Similarly, the virulence gene ast was the most isolated among 94 Aeromonas isolates, and additional virulence genes, such as alt, ast, act, aer ser, fla, and hly, were also discovered [74, 75]. Moreover, the most prominent virulence gene pattern was aer/hly/fla, with a prevalence rate of 12.6% [76]. In addition, an association was discovered between the lip and aer genes and the hlyA and ast genes in A. hydrophila isolated from Mugil cephalus indicating a potential synergy among these genes during infection [62]. Certainly, the virulence genes could share genomic locations on mobile genetic elements [77]. Different findings, El-Bahar et al. [78] claimed that hlyA gene, which destroys red blood cells and causes anemia, was detected in 10% of A. hydrophila strains. Furthermore, it was not detected among Aeromonas strains from freshwater lakes in Malaysia [79]. Moreover, only 5% of A. hydrophila isolates harbored the ast gene, which can promote gut vascular permeability and intestinal mucosal detachment [80]. Previous findings could explain the occurrence of Aeromoniasis as virulence factors, either alone or in combination, may allow Aeromonas spp. to invade host cells, thereby overlapping the immunological response and causing diseases [81, 82].
The clinical signs in experimental O. niloticus infected with A. hydrophila are similar to those of septicemic bacteria (opaque, slightly protruded eye, dentated dorsal, and tail fin, hemorrhages on the body surface, and sloughed scales). Meanwhile, the post-mortem signs were dark brown liver, distended gall bladder, splenomegaly, and empty intestinal tract. Similarly, Aeromonas infections resulted in exophthalmia, hemorrhage, ulceration, fin rot, lethargy, loss of scale, skin discoloration, and hemorrhagic/necrotized internal organs in different types of fish [83, 84]. The observed clinical signs are attributed to bacterial toxins such as aerolysin and cytotoxic enterotoxin, which can cause damage to body tissues and erythrocyte membranes resulting in hemorrhagic scales; fin and tail atrophy of broodstock Nile tilapia. In this investigation, 61.11% of A. hydrophila could produce a biofilm. In accordance, most bacterial species in the aquatic system could form biofilm [85, 86], which increased virulence and resistance that may potentially decrease the LD50 by increasing the viable bacterial cells, impervious to ordinary antimicrobial agents and disinfectants, thereby becoming a repository for the spread of pathogenic bacteria [87]. Multiple virulence genes may manage pathogenic A. hydrophila to combat normal commensal bacteria [88], and they have synergistic effects on pathogenicity [89] and act in combination with other virulence factors, such as biofilm production, hemolysin activity, and proteolytic activity, which could act synergistically to cause clinical diseases [80].
The antibacterial activity of several metal oxide nanoparticles, such as TiO2, is effective against both gram-negative and gram-positive bacteria [90, 91]. Cell death has been recorded because of nanomaterials aggregation within the bacterial membrane [28]. Based on previous findings on MDR and virulence genes, TiO2 NPs were considered in the treatment of the isolated A. hydrophila, three TiO2 NPs measuring 13, 32, and 123 nm were assessed against the highly pathogenic A. hydrophila (gram-negative) in time factor (1, 12, and 24 h).
The growth of A. hydrophila was suppressed more effectively and swiftly with small-size TiO2 NPs (13 nm) than with large-size ones. Similar to our findings on different bacterial spp., Ngoepe et al. [92] reported that TiO2 with a size of 6–10 nm had a high antibacterial activity at a concentration of 0.05 mg/mL, which was selectively active against gram-negative E. coli strain. Similarly, smaller-size NPs had more significant toxicity than larger ones when administered at the same concentration [93, 94]. These findings are attributed to the fact that smaller-sized NPs not only had a larger surface area but could also cross cell membrane barriers and accumulate inside the bacterial cells [29, 95]. Similarly, Sherif et al. [96] showed that TiO2 NPs (anatase crystal) could affect the microbiota in Nile tilapia, thereby reducing the number of beneficial bacteria. However, Sherif et al. [97] found that nano selenium could inhibit the recurrence of A. hydrophila infection. By contrast, Garcidueñas-Piña et al. [98], TiO2-Cu2 + nanoparticles did not exhibit bactericidal action against E. coli even at the highest concentration (10 mg/mL).
After 1 h of exposure to TiO2 NPs, small-size NPs (13 nm) were more effective than large-size ones (32 and 123 nm). In addition, after 24 h, the growth of A. hydrophila was suppressed of regardless the particle size. In previous studies, on exposure to graphene oxide NPs, a positive correlation was observed between cell death and exposure time [99] and concentration [34]. That is, if the cell culture exposed to nanoparticles was longer, the cell death was higher [99]. Accordingly, Rincon & Pulgarin [100] confirmed that a longer exposure duration is required for bacterial inactivation if the initial concentration of bacteria is higher. Whereas, other studies showed that TiO2 needs more time to exert its action, as the percentage of cell death was 75% after 96 h of incubation [57].
Conclusion
Mortality in Nile tilapia broodstock is attributed to stress conditions. Hence, these animals were vulnerable to 18 isolates of A. hydrophila harboring different patterns of virulence genes as the heat-stable cytotonic enterotoxin (ast), cytotoxic enterotoxin (act), and hly genes were the most prevalent. The MAR index ranged from 0.27 to 0.45 in eight A. hydrophila strains. Meanwhile, it was 0.18 in the other strains. The resistant genes sul1 and tetA were found in 100% of the bacterial isolates, while qnrs and ermB were present in 44.4% and 27.78%. Furthermore, TiO2 NPs had bactericidal activity, thereby resulting in a considerable reduction in bacterial load, it was noticed that the lower the nanosize the lower the bacterial count as the lowest bacterial count (10% to 57.3%) after 1 h of the exposure to TiO2 NPs measuring 13 nm.
Availability of data and materials
Data are available on request from the corresponding author.
References
FAO, Food and Agriculture Organization of the United Nations The state of world fisheries and aquaculture 2020: Sustainability in action. Rome: Food and Agriculture Organization of the United Nations; 2020. p. 1–244.
Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010;23(1):35–73. https://doi.org/10.1128/cmr.00039-091.
Ottaviani, D, Parlani C, Citterio B, Masini L, Leoni F, Canonico C, ... Pianetti A. Putative virulence properties of Aeromonas strains isolated from food, environmental and clinical sources in Italy: a comparative study. Int J Food Microbiol. 2011;144(3): 538–545.https://doi.org/10.1016/j.ijfoodmicro.2010.11.020
Abdelsalam M, Elgendy MY, Elfadadny MR, Ali SS, Sherif AH, Abolghait SK. A review of molecular diagnoses of bacterial fish diseases. Aquac Int. 2022. https://doi.org/10.1007/s10499-022-00983-8.
Yu J, Koo BH, Kim DH, Kim DW, Park SW. Aeromonas sobria infection in farmed mud loach (Misgurnus mizolepis) in Korea, a bacteriological survey. Iran J Vet Res. 2015;16(2):194–201.
Dhooghe E, Van Laere K, Eeckhaut T, Leus L, Van Huylenbroeck J. Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult (PCTOC). 2011;104(3):359–73. https://doi.org/10.1007/s11240-010-9786-5.
Sen K, Rodgers M. Distribution of six virulence factors in Aeromonas species isolated from US drinking water utilities: a PCR identification. J Appl Microbiol. 2004;97(5):1077–86. https://doi.org/10.1111/j.1365-2672.2004.02398.x.
Sherif AH, AbuLeila RH. Prevalence of some pathogenic bacteria in caged-Nile Tilapia (Oreochromis Niloticus) and their possible treatment. Jordan J Biol Sci. 2022;15(2):239–47. https://doi.org/10.54319/jjbs/150211.
Hossain MJ, Sun D, McGarey DJ, Wrenn S, Alexander LM, Martino ME, ... Liles MR. An Asian origin of virulent Aeromonas hydrophila responsible for disease epidemics in United States-farmed catfish. MBio. 2014;5(3): e00848-14. https://doi.org/10.1128/mBio.00848-14
Peterman MA, Posadas BC. Direct economic impact of fish diseases on the East Mississippi catfish industry. N Am J Aquac. 2019;81(3):222–9. https://doi.org/10.1002/naaq.10090.
Cornaglia G, Giamarellou H, Rossolini GM. Metallo-β-lactamases: a last frontier for β-lactams? Lancet Infect Dis. 2011;11(5):381–93. https://doi.org/10.1016/s1473-3099(11)70056-1.
Rojas R, Miranda CD, Amaro AM. Pathogenicity of a highly exopolysaccharide-producing Halomonas strain causing epizootics in larval cultures of the Chilean scallop Argopecten purpuratus (Lamarck, 1819). Microb Ecol. 2009;57(1):129–39. https://doi.org/10.1007/s00248-008-9401-z.
Rojas R, Miranda CD, Opazo R, Romero J. Characterization and pathogenicity of Vibrio splendidus strains associated with massive mortalities of commercial hatchery-reared larvae of scallop Argopecten purpuratus (Lamarck, 1819). J Invertebr Pathol. 2015;124:61–9. https://doi.org/10.1016/j.jip.2014.10.009.
Miranda CD, Rojas R, Geisse J, Romero J, González-Rocha G. Scallop larvae hatcheries as source of bacteria carrying genes encoding for non-enzymatic phenicol resistance. Mar Pollut Bull. 2015;95(1):173–82. https://doi.org/10.1016/j.marpolbul.2015.04.026.
Amraei S, Eslami G, Taherpour A, Hashemi A. Relationship between MOX genes and antibiotic resistance in Klebsiella pneumoniae strains in nosocomial infections. Micro Nano Bio Aspects. 2022;1(2):12–7. https://doi.org/10.22034/mnba.2022.155320.
Varela MF, Stephen J, Lekshmi M, Ojha M, Wenzel N, Sanford LM, et al. Bacterial Resistance to Antimicrobial Agents Antibiotics. 2021;10(5):593. https://doi.org/10.3390/antibiotics10050593.
Ishida Y, Ahmed AM, Mahfouz NB, Kimura T, El-khodery SA, Moawad AA, Shimamoto T. Molecular analysis of antimicrobial resistance in gram-negative bacteria isolated from fish farms in Egypt. J Vet Med Sci. 2010;72(6):727–34. https://doi.org/10.1292/jvms.09-0538.
Pridgeon JW, Klesius PH. Major bacterial diseases in aquaculture and their vaccine development. CAB Rev. 2012;7:1–16. https://doi.org/10.1079/PAVSNNR20127048.
Sreedharan K, Philip R, Singh ISB. Virulence potential and antibiotic susceptibility pattern of motile aeromonads associated with freshwater ornamental fish culture systems: a possible threat to public health. Braz J Microbiol. 2012;43(2):754–65. https://doi.org/10.1590/S1517-83822012000200040.
Vivekanandhan G, Savithamani K, Hatha AAM, Lakshmanaperumalsamy P. Antibiotic resistance of Aeromonas hydrophila isolated from marketed fish and prawn of South India. Int J Food Microbiol. 2002;76(1–2):165–8. https://doi.org/10.1016/S0168-1605(02)00009-0.
Nawaz M, Khan SA, Khan AA, Sung K, Tran Q, Kerdahi K. Steele R Detection and characterization of virulence genes and integrons in Aeromonas veronii isolated from catfish. Food microbiol. 2010;27(3):327–31. https://doi.org/10.1016/j.fm.2009.11.007.
Laith AR, Najiah M. Aeromonas hydrophila: antimicrobial susceptibility and histopathology of isolates from diseased catfish, Clarias gariepinus (Burchell). J Aquac Res Dev. 2014;5(2):215.
Figueira V, Vaz-Moreira I, Silva M, Manaia CM. Diversity and antibiotic resistance of Aeromonas spp. in drinking and waste water treatment plants. Water Res. 2011;45(17):5599–611.
Sherif AH, Gouda M, Darwish S, Abdelmohsin A. Prevalence of antibiotic-resistant bacteria in freshwater fish farms. Aquac Res. 2021;52(5):2036–47. https://doi.org/10.1111/are.15052.
Winton JR. Fish health management. Fish hatchery management. 2nd ed. Bethesda: American Fisheries Society; 2001. p. 559–639.
Olafsen JA. Interactions between fish larvae and bacteria in marine aquaculture. Aquaculture. 2001;200(1–2):223–47. https://doi.org/10.1016/S0044-8486(01)00702-5.
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W. Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev. 2000;64(4):655–71.
Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275(1):177–82. https://doi.org/10.1016/j.jcis.2004.02.012.
Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, Memic A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomedicine. 2012;7:6003–9. https://doi.org/10.2147/IJN.S35347.
Sherif AH, Alsokary ET, Esam HA. Assessment of titanium dioxide nanoparticle as treatment of Aeromonas hydrophila infection in Oreochromis niloticus. J Hell Vet Medical Soc. 2019;70(3):1697–706. https://doi.org/10.12681/jhvms.21796.
Liu S, Zeng TH, Hofmann M, Burcombe E, Wei J, Jiang R, Kong J, Chen Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano. 2011;5(9):6971–80. https://doi.org/10.1021/nn202451x.
Raghupathi KR, Koodali RT, Manna AC. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir. 2011;27(7):4020–8. https://doi.org/10.1021/la104825u.
Ravikumar S, Gokulakrishnan R, Selvanathan K, Selvam S. Antibacterial activity of metal oxide nanoparticles against ophthalmic pathogens. Int J Pharm Res Dev. 2011;3(5):122–7.
Gurunathan S, Han JW, Dayem AA, Eppakayala V, Kim JH. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int J Nanomedicine. 2012;7:5901–14. https://doi.org/10.2147/IJN.S37397.
Chakra CH, Rajendar V, Rao KV, Kumar M. Enhanced antimicrobial and anticancer properties of ZnO and TiO2 nanocomposites. 3 Biotech. 2017;7(2):1–8. https://doi.org/10.1007/s13205-017-0731-8.
Senarathna ULNH, Fernando SSN, Gunasekara TDCP, Weerasekera MM, Hewageegana HGSP, Arachchi NDH, ... Jayaweera PM. Enhanced antibacterial activity of TiO2 nanoparticle surface modified with Garcinia zeylanica extract. Chem Cent J. 2017;11(1): 1–8. https://doi.org/10.1186/s13065-017-0236-x
Xing Y, Li X, Zhang L, Xu Q, Che Z, Li W, ... Li K. Effect of TiO2 nanoparticles on the antibacterial and physical properties of polyethylene-based film. Prog Org Coat. 2012;73(2–3): 219-224. https://doi.org/10.1016/j.porgcoat.2011.11.005
Sherif AH, Eldessouki EA, Sabry NM, Ali NG. The protective role of iodine and MS-222 against stress response and bacterial infections during Nile tilapia (Oreochromis niloticus) transportation. Aquac Int. 2022;1–16. https://doi.org/10.1007/s10499-022-00984-7
Schaperclaus W, Kulow H, Shreckenbach KL. Prophylaxis and therapy of fish diseases. Fish Diseases, Vol. I. A. A. Rotterdam: Balkema; 1992.
Madigan MT, Martinko J. Brock Biology of Microorganisms. 11th ed. Prentice Hall. 2005;8:149–52.
Christensen GD, Simpson WA, Bisno AL, Beachey EH. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect immune. 1982;37(1):318–26. https://doi.org/10.1128/iai.37.1.318-326.1982.
Chen JD, Huang SL. Hemolyshin form Edwardsiella tarda Strain ET 16 Isolated from Eel Anguilla japonica Identified as a Hole-forming Toxin. Fish Sci Res. 1996;62(4):538–42. https://doi.org/10.2331/fishsci.62.538.
Arai T, Komatsu S, Komatsu Y. Extracellular protease production of various bacteria and the role of proteases on the pathogenicity of opportunistic pathogens. Keio J Med. 1981;30(1):1–9. https://doi.org/10.2302/kjm.30.1.
Hu M, Wang N, Pan ZH, Lu CP, Liu YJ. Identity and virulence properties of Aeromonas isolates from diseased fish, healthy controls and water environment in China. Lett Appl Microbiol. 2012;55(3):224–33.
Hall TA. Bioedit: A user friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–8.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9.
Randall LP, Cooles SW, Osborn MK, Piddock LJV, Woodward MJ. Antibiotic resistance genes, integrons and multiple antibiotic resistance in thirty-five serotypes of Salmonella enterica isolated from humans and animals in the UK. J Antimicrob Chemother. 2004;53(2):208–16. https://doi.org/10.1093/jac/dkh070.
Rozi R, Rahayu K, Daruti DN. Detection and analysis of hemolysin genes in Aeromonas hydrophila isolated from Gouramy (Osphronemusgouramy) by polymerase chain reaction (PCR). IOP Conf Ser Earth Environ Sci. 2017;137(1):012001.
Kerrn MB, Klemmensen T, Frimodt-Moller N, Espersen F. Susceptibility of Danish Escherichia coli strains isolated from urinary tract infections and bacteraemia, and distribution of sul genes conferring sulphonamide resistance. J Antimicrob Chemother. 2002;50(4):513–6. https://doi.org/10.1093/jac/dkf164.
Mendez B, Tachibana C, Levy SB. Heterogeneity of tetracycline resistance determinants. Plasmid. 1980;3(2):99–108. https://doi.org/10.1016/0147-619X(80)90101-8.
Robicsek A, Strahilevitz J, Sahm DF, Jacoby GA, Hooper DC. Qnr prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob Agents Chemother. 2006;50(8):2872–4. https://doi.org/10.1128/AAC.01647-05.
Malhotra-Kumar S, Lammens C, Piessens J, Goossens H. Multiplex PCR for simultaneous detection of macrolide and tetracycline resistance determinants in Streptococci. Antimicrob Agents Chemother. 2005;49(11):4798–800. https://doi.org/10.1128/AAC.49.11.4798-4800.2005.
Wayne PA. Clinical and Laboratory Standards Institute: Performance standards for antimicrobial susceptibility testing: 20th informational supplement. CLSI document M100-S20. 2010.
Krumperman PH. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl Environ Microbiol. 1983;46(1):165–70. https://doi.org/10.1128/aem.46.1.165-170.1983.
Reed LJ, Muench H. Simple method of estimating fifty percent endpoint. Am J Hyg. 1938;27:493–7.
El-Shafai NM, El-Khouly ME, El-Kemary M, Ramadan MS, Derbalah AS, Masoud MS. Fabrication and characterization of graphene oxide–titanium dioxide nanocomposite for degradation of some toxic insecticides. J Ind Eng Chem. 2019;69:315–23. https://doi.org/10.1016/j.jiec.2018.09.045.
Ahmad NS, Abdullah N, Yasin FM. Toxicity assessment of reduced graphene oxide and titanium dioxide nanomaterials on gram-positive and gram-negative bacteria under normal laboratory lighting condition. Toxicol Rep. 2020;7:693–9. https://doi.org/10.1016/j.toxrep.2020.04.015.
Wynne MK, Hornung MR, Michaud DS, Branch CS, Copes R, Colby WD, et al. The Right Honourable Stephen Harper Prime Minister of Canada pm@ pm. gc. ca The Honourable Leona Aglukkaq Minister of Health, Health Canada.
Rasmussen-Ivey CR, Hossain MJ, Odom SE, Terhune JS, Hemstreet WG, Shoemaker CA, ... Liles MR. Classification of a hypervirulent Aeromonas hydrophila pathotype responsible for epidemic outbreaks in warm-water fishes. Front microbiol. 2016;7: 1615. https://doi.org/10.3389/fmicb.2016.01615
Chen PL, Wu CJ, Tsai PJ, Tang HJ, Chuang YC, Lee NY, ... Ko WC. Virulence diversity among bacteremic Aeromonas isolates: ex vivo, animal, and clinical evidences. PLoS One. 2014;9(11): e111213. https://doi.org/10.1371/journal.pone.0111213
Amraei S, Eslami G, Taherpour A, Hashemi A. The role of ACT and FOX genes in Klebsiella pneumoniae strains isolated from hospitalized patients. Micro Nano Bio Aspects. 2022;1(2):18–25. https://doi.org/10.22034/mnba.2022.155447.
Ramadan H, Ibrahim N, Samir M, Abd El-Moaty A, Gad T. Aeromonas hydrophila from marketed mullet (Mugil cephalus) in Egypt: PCR characterization of β-lactam resistance and virulence genes. J Appl Microbiol. 2018;124(6):1629–37. https://doi.org/10.1111/jam.13734.
Tartor YH, EL-Naenaeey ESY, Abdallah HM, Samir M, Yassen MM, Abdelwahab AM. Virulotyping and genetic diversity of Aeromonas hydrophila isolated from Nile tilapia (Oreochromis niloticus) in aquaculture farms in Egypt. Aquaculture. 2021;541:736781.
Azzam-Sayuti M, Ina-Salwany MY, Zamrisaad M, Yusof MT, Annas S, Najihah MY, Amal MNA. The prevalence, putative virulence genes and antibiotic resistance profiles of Aeromonas spp. isolated from cultured freshwater fishes in peninsular Malaysia. Aquaculture. 2021;540:736719. https://doi.org/10.1016/j.aquaculture.2021.736719.
Liu J, Gao S, Dong Y, Lu C, Liu Y. Isolation and characterization of bacteriophages against virulent Aeromonas hydrophila. BMC microbiol. 2020;0(1):1–13. https://doi.org/10.1186/s12866-020-01811-w.
Deng YT, Wu YL, Tan AP, Huang YP, Jiang L, Xue HJ, Wang WL, Luo L, Zhao F. Analysis of antimicrobial resistance genes in Aeromonas spp. isolated from cultured freshwater animals in China. Vet Microbiol. 2014;20(4):350–6. https://doi.org/10.1089/mdr.2013.0068.
Zhou Y, Yu L, Nan Z, Zhang P, Kan B, Yan D, Su J. Taxonomy, virulence genes and antimicrobial resistance of Aeromonas isolated from extra-intestinal and intestinal infections. BMC Infect Dis. 2019;19(1):1–9. https://doi.org/10.1186/s12879-019-3766-0.
Shuang MENG, Du XL, Wang YL, Qu FT, Xie GL, Zhou HJ, ... Cui ZG. Comparative Study of the Genetic Diversity, Antimicrobial Resistance, and Pathogenicity of Aeromonas Isolates from Clinical Patients and Healthy Individuals. Biomed Environ Sci. 2021;34(6): 454–464. https://doi.org/10.3967/bes2021.062
Rigos G, Troisi GM. Antibacterial agents in Mediterranean finfish farming: A synopsis of drug pharmacokinetics in important euryhaline fish species and possible environmental implications. Rev Fish Biol Fish. 2005;15(1–2):53–73. https://doi.org/10.1007/s11160-005-7850-8.
Huang L, Xu YB, Xu JX, Ling JY, Chen JL, Zhou JL, Zheng L, Du QP. Antibiotic resistance genes (ARGs) in duck and fish production ponds with integrated or non-integrated mode. Chemosphere. 2017;168:1107–14. https://doi.org/10.1016/j.chemosphere.2016.10.096.
He X, Xu Y, Chen J, Ling J, Li Y, Huang L, Zhou X, Zheng L, Xie G. Evolution of corresponding resistance genes in the water of fish tanks with multiple stresses of antibiotics and heavy metals. Water Res. 2017;124:39–48.
Cattoir V, Poirel L, Mazel D, Soussy CJ, Nordmann P. Vibrio splendidus as the source of plasmid-mediated QnrS-like quinolone resistance determinants. Antimicrob Agents Chemother. 2007;51(7):2650–1. https://doi.org/10.1128/AAC.00070-07.
Jang HM, Kim YB, Choi S, Lee Y, Shin SG, Unno T, Kim YM. Prevalence of antibiotic resistance genes from effluent of coastal aquaculture. South Korea Environ Pollut. 2018;233:1049–57. https://doi.org/10.1016/j.envpol.2017.10.006.
Dallal MMS, Fard RMN, Talkhabi MK, Aghaiyan L, Salehipour Z. Prevalence, virulence and antimicrobial resistance patterns of Aeromonas spp. isolated from children with diarrhea. Germs. 2016;6(3):91–6.
Puthucheary SD, Puah SM, Chua KH. Molecular characterization of clinical isolates of Aeromonas species from Malaysia. PloS One. 2012;7(2):e30205. https://doi.org/10.1371/journal.pone.0030205.
Mzula A, Wambura PN, Mdegela RH, Shirima GM. Virulence pattern of circulating aeromonads isolated from farmed Nile tilapia in Tanzania and novel antibiotic free attenuation of Aeromonas hydrophila strain TZR7-2018. Aquac Rep. 2020;17: 100300.
Rasmussen-Ivey CR, Figueras MJ, McGarey D, Liles MR. Virulence factors of Aeromonas hydrophila: in the wake of reclassification. Front microbiol. 2016;7:1337. https://doi.org/10.3389/fmicb.2016.01337.
El-Bahar HM, Ali NG, Aboyadak IM, Khalil SA, Ibrahim MS. Virulence genes contributing to Aeromonas hydrophila pathogenicity in Oreochromis niloticus. Int Microbiol. 2019;22(4):479–90.
Khor WC, Puah SM, Tan JAMA, Puthucheary SD, Chua KH. Phenotypic and genetic diversity of Aeromonas species isolated from fresh water lakes in Malaysia. PLoS One. 2015;10(12):e0145933. https://doi.org/10.1371/journal.pone.0145933.
Oliveira ST, Veneroni-Gouveia G, Costa MM. Molecular characterization of virulence factors in Aeromonas hydrophila obtained from fish. Pesqui Vet Bras. 2012;32:701–6. https://doi.org/10.1590/S0100-736X2012000800004.
Beaz-Hidalgo R, Figueras MJ. Aeromonas spp. whole genomes and virulence factors implicated in fish disease. J Fish Dis. 2013;36(4):371–88. https://doi.org/10.1111/jfd.12025.
Fernández-Bravo A, Figueras MJ. An update on the genus Aeromonas: Taxonomy, epidemiology, and pathogenicity. Microorganisms. 2020;8(1):129. https://doi.org/10.3390/microorganisms8010129.
Austin B, Austin DA. Bacterial fish pathogens: disease of farmed and wild fish, sixth edition. Springer International Publishing. 2016. https://doi.org/10.1007/978-3-319-32674-0.
Basri L, Nor RM, Salleh A, Md Yasin IS, Saad MZ, Abd Rahaman NY, ... Amal MNA. Co-infections of tilapia lake virus, Aeromonas hydrophila and Streptococcus agalactiae in farmed red hybrid tilapia. Animals. 2020;10(11): 2141. https://doi.org/10.3390/ani10112141
Szewzyk U, Szewzyk R, Manz W, Schleifer KH. Microbiological safety of drinking water. Annu Rev Microbiol. 2000;54(1):81–127.
Baig U, Ansari MA, Gondal MA, Akhtar S, Khan FA, Falath WS. Single step production of high-purity copper oxide-titanium dioxide nanocomposites and their effective antibacterial and anti-biofilm activity against drug-resistant bacteria. Mater Sci Eng C. 2020;113:110992. https://doi.org/10.1016/j.msec.2020.110992.
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49(1):711–45.
Hossain MJ, Waldbieser GC, Sun D, Capps NK, Hemstreet WB, Carlisle K, ... Liles MR. Implication of lateral genetic transfer in the emergence of Aeromonas hydrophila isolates of epidemic outbreaks in channel catfish. PLoS One. 2013;8(11): e80943. https://doi.org/10.1371/journal.pone.0080943
Albert MJ, Ansaruzzaman M, Talukder KA, Chopra AK, Kuhn I, Rahman M, ... Mollby R. Prevalence of enterotoxin genes in Aeromonas spp. isolated from children with diarrhea, healthy controls, and the environment. J Clin Microbiol. 2000;38(10): 3785–3790. https://doi.org/10.1128/JCM.38.10.3785-3790.2000
Adams LK, Lyon DY, Alvarez PJ. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006;40(19):3527–32.
De Falco G, Porta A, Petrone AM, Del Gaudio P, El Hassanin A, Commodo M, ... D'Anna A. Antimicrobial activity of flame-synthesized nano-TiO2 coatings. Environ Sci Nano. 2017;4(5): 1095–1107.
Ngoepe NM, Mathipa MM, Hintsho-Mbita NC. Biosynthesis of titanium dioxide nanoparticles for the photodegradation of dyes and removal of bacteria. Optik. 2020;224: 165728.
Hashizume N, Oshima Y, Nakai M, Kobayashi T, Sasaki T, Kawaguchi K, ... Imatanaka N. Categorization of nano-structured titanium dioxide according to physicochemical characteristics and pulmonary toxicity. Toxicol Rep. 2016;3: 490–500. https://doi.org/10.1016/j.toxrep.2016.05.005
Jahan S, Yusoff IB, Alias YB, Bakar AFBA. Reviews of the toxicity behavior of five potential engineered nanomaterials (ENMs) into the aquatic ecosystem. Toxicol Rep. 2017;4:211–20. https://doi.org/10.1016/j.toxrep.2017.04.001.
Xiong D, Fang T, Yu L, Sima X, Zhu W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: acute toxicity, oxidative stress and oxidative damage. Sci Total Environ. 2011;409(8):1444–52. https://doi.org/10.1016/j.scitotenv.2011.01.015.
Sherif AH, El‐Sharawy MES, El‐Samannoudy SI, Adel Seida A, Sabry NM, Eldawoudy M, ... Younis NA. The deleterious impacts of dietary titanium dioxide nanoparticles on the intestinal microbiota, antioxidant enzymes, diseases resistances and immune response of Nile tilapia. Aquac Res. 2021;52(12): 6699–6707. https://doi.org/10.1111/are.15539
Sherif AH, Gouda MY, Zommara MA, Abd El-Rahim AH, Mahrous KF, Salama ASS. Inhibitory effect of nano selenium on the recurrence of Aeromonas hydrophila bacteria in Cyprinus carpio. Egypt J Aquat Biol Fish. 2021;25(3):713–38. https://doi.org/10.21608/EJABF.2021.180901.
Garcidueñas-Piña C, Medina-Ramírez IE, Guzmán P, Rico-Martínez R, Morales-Domínguez JF, Rubio-Franchini I. Evaluation of the antimicrobial activity of nanostructured materials of titanium dioxide doped with silver and/or copper and their effects on Arabidopsis thaliana. Int J Photoenergy. 2016. https://doi.org/10.1155/2016/8060847.
Akhavan O, Ghaderi E. Escherichia coli bacteria reduce graphene oxide to bactericidal graphene in a self-limiting manner. Carbon. 2012;50(5):1853–60. https://doi.org/10.1016/j.carbon.2011.12.035.
Rincon AG, Pulgarin C. Bactericidal action of illuminated TiO2 on pure Escherichia coli and natural bacterial consortia: post-irradiation events in the dark and assessment of the effective disinfection time. Appl Catal B Environmental. 2004;49(2):99–112. https://doi.org/10.1016/j.apcatb.2003.11.013.
Acknowledgements
We are grateful for Egyptian knowledge Bank (EKB) and Science, Technology & Innovation Funding Authority (STDF), Cairo, Egypt for English language editing.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors are equally contributed to this work. All authors analysed and interpreted the data. All authors performed the experimental study and were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The above described methodology was approved by the Ethics Committee at the Animal Health Research Institute and European Union directive 2010/63UE, and all methods were carried out in accordance with relevant guidelines and regulations. This study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). This paper does not contain any studies with human participants by any of the authors. No specific permissions were required for access to the artificial pond in wet laboratory Animal Health Research Institute, Kafrelsheikh, Egypt. The field studies did not involve endangered or protected species.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sherif, A.H., Kassab, A.S. Multidrug-resistant Aeromonas bacteria prevalence in Nile tilapia broodstock. BMC Microbiol 23, 80 (2023). https://doi.org/10.1186/s12866-023-02827-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-023-02827-8