Abstract
Background
Bacterial pectinase is an enzyme that could be employed in numerous sectors to break down pectin polysaccharide compounds. The goal of this study is to find pectinase-producing bacteria in avocado peel waste and see if the pectinase enzyme produced can be used to make fruit juice clarification.
Results
The researchers isolated four different bacterial strains from avocado peel waste samples. The potential two bacterial isolates that were identified as being Serratia marcescens and Lysinibacillus macrolides. Finally, the analysis of pectinase production and its application in fruit juice clarification were performed using one of the bacterial strains of Serratia marcescens. The clear apple, lemon, and mango juices were further processed to assess each juice's properties. The highest antioxidant activity was recorded in lemon juice samples. The lemon juice showed the highest total titratable acidity and total phenol content. Apple juices contained the highest total soluble solids, reducing sugar content, and viscosity and the mango juices have the maximum pH value recorded.
Conclusions
The pectinase isolated from the bacterium Serratia marcescens could clear fruit juices. This pectinase needs to be studied more to make sure it works better in the fruit industry and other businesses.
Similar content being viewed by others
Introduction
Enzymes are biological catalysts that help chemical reactions occur under various physicochemical conditions. All enzymes are protein in nature, but each has a unique performance function [1,2,3]. Enzymes were first identified in the mid-nineteenth century and the first to recognize the technical potential of cultivated enzymes and commercialize primarily using fungal enzymes, but 20 years later, Boidin and Affront in France pioneered the synthesis of bacterial enzymes [4, 5].
Today, industrial enzyme technology relies on microbial sources such as bacteria and yeasts. These microorganisms are essential in the production of pectinase enzymes which find applications in biotechnological processes that use pectin as a carbon source [6, 7]. Pectin is a component of the plant's cell wall and middle lamella, and a very thin extracellular layer that connects the young cells [8, 9].
The pectin substances are complex colloidal acid polysaccharides with a long galacturonic acid pillar chain and glycoside bonds. Seven polysaccharides and 17 monosaccharides, such as d-Glucuronic acid, l-Fucose, d-Glucose, d-Mannose, and d-Xylose, are present in these chains of pectin compounds [6, 10, 11]. Pectic acid, pectinic acid, pectin, and protopectin are the four types of pectin substances used as substrates in pectinase processing [12, 13]. The solubility of pectic substances in water was one of the most relevant criteria used to identify those [14, 15].
Only 25% of the Microbial pectinase enzyme was used in the food and industrial sectors around the world, even though the market kept growing [16, 17]. Pectinase finds applications in the extraction of fruit juice, clarification of juice, refining of vegetable fibers, degumming of natural fibers, and wastewater treatment [18, 19]. It also speeds up tea fermentation and eliminates the foam-forming property of instant tea powder by destroying the pectin present in tea powder. Even though they aren't just used to make coffee, they're also used to remove the mucilaginous layer from coffee beans [15, 20].
Fungal organisms produce the vast majority of pectinase used in the industrial environment. The enzyme is used mostly for the degradation of pectic compounds in a variety of industrial sectors because of which their demand has increased in recent years [11, 21]. To address this demand for pectinase, a bacterial source of pectinase could be used. Pectinases break down pectin, causing a decrease in viscosity and the formation of clusters, making centrifugation or filtration easier. As a result, the juice has a more transparent appearance and a more intense taste and color [22, 23]. The efficiency of Pectinolytic enzymes in fruit juice clarity is, however, reliant on the amount of pectin and pectinases available in the substrate, resulting in better juice extraction and clarification [14, 24]. The major sources of microorganisms used to produce pectinase are pectin-containing fruits and their peels, such as orange pulp, avocado peel, potatoes, tomatoes, sugar beet pulp, peaches, strawberries, lemon carrots, and banana peels. A bacterium has not been found in avocados, but pectic and pectin compounds have been found in a lot of research on avocados [7, 12].
Pectinases are used in acidic and alkaline environments and are especially useful in the food and textile industries [25, 26]. Pectinases and their uses are being studied in global research sectors to get optimal fastened activity with enzymes. Pectinase has a wide range of applications, which has increased global demand. The uses of these enzymes are depicted in Fig. 1. There is a lot of value in the pectinase enzyme because it makes fruit juice clear. The main goals of this research were to find and study pectinase-producing bacteria from avocado peel wastes (Serratia marcescens), and improve the clarity of the fruit juice.
Results
Isolation of bacteria from avocado peel
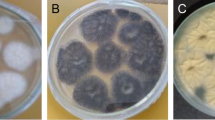
It was used to isolate bacteria from avocado peel waste using serial dilution, pour plating, and streak isolation methods. They were sub-cultured into a new growth medium to obtain a pure isolate. The four pure isolates were obtained after extensive isolation techniques. The pure bacterial isolates were labeled as white colony (W3, W32) red colony (R5), and yellow colony Y31 to make it easier to distinguish between them [Fig. 2].
Primary and secondary screening
There was a strong (hydrolysis) zone among all four bacterial isolates, indicating the existence of pectinase activities. The diameter of each hydrolysis zone was calculated to determine the potential bacterial isolate [Fig. 3]. Isolate R5 measured the largest diameter around the colony at 20.54 ± 1.32 mm [Table 1]. The activities of crude pectinase were measured in the secondary screening. The selected isolates from the primary screening method were subjected to fermentation in a suitable medium, and their behaviors were assessed to be further screened. A liquid sample (0.4 ml) was taken to assess pectinase activation by using phosphate buffer after 24 h of incubation in the production media. Isolate R5 had the highest pectinase activity of 5.410 ± 14 mol/ml/min, isolate W3 had the second highest at 3.090 ± 17 mol/ml/min, and isolate Y31 had the lowest at 2.490 ± 23 mol/ml/min [Table 2].
Morphological and biochemical identification of bacterial isolates
Using Bergey's Manual of Determinative Bacteriology [27] isolates R5 and Y31 were discovered to be Gram-negative bacteria, whereas isolate W3 was confirmed to be Gram-positive bacteria. The isolates were tentatively described as Bacillus species, Serratia species, and Erwinia species [Table 3] using ABIS-online software. We chose gram-positive bacteria from W3 and gram-negative bacteria from R5 for further research.
Molecular identification of isolates
The 16S rRNA gene was amplified by polymerase chain reaction (PCR) using genomic DNA from selected bacterial isolates (R5 and W3) as templates. The genomic DNA and PCR amplification products were analyzed using agarose gel electrophoresis compared with DNA marker isolate R5, and W3, as shown in Fig. 4. To obtain the right sequences, the PCR products were filtered and sequenced. Each isolate's sequences were uploaded to the NCBI database and compared to previously published sequences. The closest neighbors of the isolates R5 (MN932109.1) Serratia marcescens and W3 (MN932110.1) Lysinibacillus macrolides were queried using NCBI BLAST (HTTP:// www.ncbi.nlm.nih.gov/Blast). The nucleotide Serratia marcescens strain 16S ribosomal RNA gene sequences from R5 quest (mBLAST, NCBI) showed 99% homology (Fig. 5). The sequences of the W3 search (mBLAST, NCBI) showed 86% similarity to the nucleotide of Lysinibacillus sp., and 85% similarity to the nucleotide of Lysinibacillus macrolides in the phylogenetic tree (Fig. 6).
Production of pectinase and its optimization
The development of a broad hydrolysis zone on pectin agar plates was used to characterize the screened Pectinolytic bacterial isolates. Fermentation and quantitative screening of isolate R5-Serratia marcescens were performed, measured by pectinase activity. After 48 h of fermentation, the maximum pectinase activity was 5.41 ± 0.14 µmol/ml/min [Table 2].
Effect of fermentation time on pectinase production
To get the most pectinase production from isolate R5-Serratia marcescens, we selected five incubation times (hours) that were used. Pectinase activity increased gradually over 24 h of incubation until optimum pectinase activity was achieved. After the optimum incubation period, the pectinase activities began to decrease. In this analysis, the optimum fermentation period for pectinase development was found to be 72 h, and the highest pectinase activities were found to be 6.86 ± 0.32 µmol/ml/min of released glucose [Table 4] when pectin was used as a substrate.
Effect of pH on pectinase production
The effect of different pH of fermentation medium on pectinase production by bacterial isolate R5-Serratia marcescens was studied. The pectinase production could be affected due to the variations in pH medium. The pectinase activity increased when we reached the optimum pH and decreased after the optimum pH value. The production medium, which was adjusted to pH 8 produced maximum pectinase activity (8.84 ± 0.34 µmol/ml/min), followed by pH 7 (6.66 ± 0.73 µmol/ml/min) [Table 5].
Effect of temperature on pectinase production
The effects of temperature on pectinase production by bacterial isolate R5-Serratia marcescens indicated that maximum pectinase activity was obtained at 35 °C. As a result, pectinase activity increased as temperature increased and slightly decreased after crossing the optimal temperature of 35 °C. However, pectinase activity was not completely lost even if the temperature increased to 50 °C. Although the maximum pectinase activity was 7.76 ± 56 µmol/ml/min of glucose released at 35 °C, pectinase activities above the optimal temperature were frequently higher than those below the optimal temperature [Table 6].
Effect of substrate concentration on pectinase production
The effect of substrate concentration on pectinase production by bacterial isolate R5-Serratia marcescens showed antagonistic effects after 1% of pectin (8.91 ± 0.23 µmol/ml/min). As a result, observed in Table 7, pectinase activity increased with increased substrate concentration (pectin) up to the optimal concentration and decreased after the optimal substrate concentration.
Purification of pectinase and determining protein concentration
Pectinase precipitation is the preferred concentration method and an ideal step in the purification process. One of the most well-known and widely used methods of purifying and concentrating pectinase, especially at the laboratory scale, is salting-out proteins, particularly ammonium sulfate. Pectinase was isolated from isolated R5-Serratia marcescens and subjected to various saturation levels of ammonium sulfate varying from 30–90%. The pectinase activities and protein content partially purified by ammonium sulfate are presented in Table 8.
Application of pectinases in fruit juice clarification and yield
This study tried to determine the effect of pectinase on the volume of juice, juice yield, and juice clarity in terms of transmittance by using apple, lemon, and mango fruits. The experiments were carried out with crude pectinase, partially purified, and water as control. According to the results shown in Table 9, the volume of lemon juice was enhanced by three folds from control to crude pectinase (10.0 -13.0 ml) and two folds increment from purified pectinase (13.0 -14.5 ml), and 86.67% of yield. Using mango juice, the same experiment was carried out. The amount of mango juice increased twofold from control to crude pectinase enzyme (8.0–10.0 ml) after 1 h of pectinase and water treatment and increased almost threefold from crude pectinase to purified pectinase (10.0 -13.5 ml), and 66.67% of yield.
The same experiment was performed to express the yield of juice in terms of percentages. As the above table indicates, the rate of juice yield was improved, starting from control to purified pectinase (Table 9). The yield variation of apple juice between control and crude pectinase was 20%. Similarly, the 20% of apple juice yield variation was recorded between crude pectinase and purified pectinase. Therefore, apple fruit showed consistent variation from control to crude and crude to purified pectinase. The yield of lemon juice variation between control and crude pectinase was 20%, but the divergence between crude pectinase and purified pectinase was recorded as 10%. As a result, there were no consistent differences in lemon juice yield from control to pure pectinase. In mango juice, there was a 13.34% difference in yield from control to crude pectinase and a 23.33% difference in juice output between crude pectinase and purified pectinase.
Effect of pectinase on juice clarification
The transmittances of the clarified juice determined the effect of pectinase on juice clarity in terms of percentage (Figs. 7 and 8). As shown three different fruits [apple, lemon, and mango] were subjected to crude and purified pectinase by taking water as control, the maximum clarity of juice was obtained was 95.82% of transmittance for lemon fruit. When compared to the result achieved with crude pectinase, which was 78.99% of juice clarity transmission, the transmittance of lemon juice treated with purified pectinase was 16.83% higher.
Untreated and treated juice properties
The viscosity, pH and acidity, total soluble solids (TSS), total titratable acidity (TTA), reducing sugar content, pectin presence test, Total phenolic content (TPC), and Antioxidant activity of three different fruits [apple, lemon, and mango juice] were evaluated, and the results were compared to the raw sample (untreated juice is a control), as shown in Tables 10 and 11. The pH of each fruit juice sample decreased with pectinase, and the total titrable acidity (TTA) increased after the juice was clarified by pectinase, as shown in Table 10. These results were probably related to pectin degradation and liberation of galacturonic acids after pectinase treatment. The reducing sugar content of each fruit juice sample increased after treatment by pectinase (Table 10), which is probably related to the liberation of reducing sugar after the degradation of pectin compounds. Because pectinase degrades polysaccharides (pectin), the viscosity of each fruit juice sample was dramatically reduced after treatment with pectinase, as shown in Table 10. It reduced the presence of cohesive network structure in fruit juice samples. The result of the total phenol content of each fruit juice sample also showed a decreasing trend after the treatment of the juice with pectinase, as shown in Table 11. The hydrolysis of pectin led to the decomposition of phenolic compounds and decreased their content in fluids.
Discussion
The hydrolysis of isolate R5 in primary screening is almost identical to that of previous studies [2, 28, 29], which obtained 22.8 mm and 20.5 mm, respectively. This may be due to the ability of bacteria to break down pectin. The hydrolysis zone of isolate (W3), in particular, was linked to the last tomato isolate [6, 23, 30]. Similarly, the results of each isolate's secondary screening method are more similar to the previous investigation of decomposing orange peels; this resulted in the sources of the samples mentioned by [22, 31]. Pectinase-producing bacteria were isolated from coffee pulp [32], and the results of the secondary screening method were lower than isolate R5.
Biologists have used a series of biochemical tests to distinguish closely related bacteria in the detection of bacteria [27]. Bergey's Manual of Determinative Bacteriology, based on their physical and biochemical properties, the ABIS-online software was used to detect Pectinolytic bacteria [27, 29]. In molecular identification, the neighbor-joining method was used to build the phylogenic tree based on the 16S rRNA gene sequences of isolates R5 and W3 and associated nucleotide sequences [2, 22, 33].
Different researchers investigated the molecular characterization of pectinase production with Serratia species. Serratia rubidaea (E9.HM585373) was isolated from tomato fruits and characterized using the 16S rRNA method. As reported by Abd-alla et al., [6] these bacterial species were found to be producing polygalacturonases at a temperature of 40 °C. Serratia rubidaea and Serratia marcescens are different from each other in species labels, but both are used to produce pectinase, even though their isolate sources are different. About 20 enzyme-producing bacterial strains were isolated from municipal solid waste using the 16S rRNA method. Among those bacterial strains, most of the strains were Bacillus species. Sarreen et al., [34] discovered that only two bacterial strains, Serratia marcescens (MH194203) and Lysinibacillus species (MH194187), were associated with this discovery. Serratia oryzaestrain S32 (SOZ00000000.1) was also found in lake water. This showed that bacterial strains could make pectinase, which was found by Huguevieux et al., [35].
The pectinase, which was produced in just 24 h of incubation, was two times more potent than the 2.43 U/ml pectinase obtained by Bacillus sonorensis in the same incubation period [11, 36, 37]. The enzyme activity results obtained after 72 h were similar to those obtained by Jayani et al., [38] who obtained 7.88 U/ml of pectinase activity after 72 h of fermentation time using Bacillus sphaericus. This study finding shows that this bacterial isolate, R5-Serratia marcescens, requires an alkaline condition in pectinase production processes [24, 39]. According to the investigations by Mohandas et al., [36] the highest pectinase activity, 2.43 U/ml, was recorded at alkaline pH 8 by Bacillus sonorensis. According to Sohail and Latif [40], the optimal poly galacturonase production of Bacillus mojavensis was at pH 8.0. Streptomyces species require a slightly alkaline condition of pH 8.5 for maximum pectinase production.
Temperature is one of the essential parameters essential for the success of pectinase production. According to Kothari and Baig [41], the maximum polygalacturonase activities were produced at a temperature of 35 °C by Erwiniacarotovora. In the same bacterial species (Erwinia spp), the highest pectinase (polygalacturonase and pectin lysate) activity was recorded at a temperature of 35 °C [5, 42]. Other recent investigations indicated that the highest amount of pectinase produced by Entero bactertabaci NR1466677 was at the optimal temperature of 35 °C [9, 30].
In this study, various concentrations of pectin substrate were used as a carbon source for bacterial growth and were subjected to a fermentation medium to produce pectinase. The pectinase activity data in this study appear to be comparable to the work of Darah et al., [21]. They obtained maximum polygalacturonase activity at 1% of the pectin concentration for Entero bacteraerogenes. Some bacterial species, such as Entero bactertabaci NR14667, could produce the highest pectinase activity at 0.3% of pectin concentration, as reported by Obafemi et al., [30]. More recently, maximum pectinase activity at 2% of citrus pectin concentration was also recorded for Chryseo bacterium indologenes strain SD [21, 43].
The unique activities of pectinase increased from crude to dialyzed pectinase. With 10 mg of protein concentration, the maximal activity was 47.32 U/mg, suggesting that the protein molecules separated by ammonium mainly contained the enzyme pectinase and that the proportion of protein other than pectinase was higher in the crude form of the enzyme [11, 31, 44]. Purification steps also resulted in the removal of interfering materials found in the crude cell-free sample, allowing for increased enzyme activity [13, 45]. From the application of pectinase, the volume of juice treated with both crude and purified pectinase varied with the type of fruit used in the process. As the results indicate, the highest juice volume was obtained from pectinase-treated lemon fruit, which might be due to the presence of solubility of pectin in lemon fruit [46, 47].
Similarly, the volume of juice variation from fruit to fruit that was treated with pectinase was investigated by using different fruits such as strawberry juice (7.0 – 10.0 ml), grape juice (15.0 -21.5 ml), apple juice (12.0—18.5 ml), peach apple juice (12.0—10.5 ml), chary apple juice (8.0 – 10.0 ml) and orange juice (9.0 – 10.0 ml) [29, 48]. The result of juice clarity treated with crude and purified pectinase was 1.99% and 18.82% more excellent than the previous report by Maktouf et al., [49], which was 77% of transmittance for pectinase using lemon fruit as the raw material for juice clarification. The result of mango juice transmittance obtained in this current experiment affirms the report made by Kumar et al., [50], who received 92.5% of transmittance after 150 min of incubation time. The effect of pectinase on juice clarification was also studied in apple juice. According to Yuan et al., [51], the clarity of apple juice treated with pectinase increased by 71.8% of transmittance. In their experiment, the clarity of apple juice rose to 84% transmittance.
Table 10 indicates that the total soluble solids decreased in each fruit juice sample after clarification. This can be due to the disintegration of solid compounds after the destruction network formed by pectinase [51, 52]. This was similar to the report of de Oliveira et al., [14], for apple juice clarification by pectinase. Table 10 shows that pectin was present in all untreated fruit juice samples, but pectin was not found in treated fruit juice samples by pectinase. A similar finding was reported by Hosseini et al., [53], for pomegranate juice clarification by free pectinase.
This result was similar to that of [37, 52], which was done on the clarification of apple juice by pectinase. In determining the antioxidant activity of each fruit juice sample, ABTS radical scavenging activities (ABTS-RSA) and DPPH radical scavenging activities (DPPH-RSA) of each fruit juice were evaluated, and the results were compared with the raw sample. As shown in Table 11, pectinase activities can change the phenolic compound profiles and the decomposition of another antioxidant by pectin hydrolysis [35, 52, 53].
Conclusion
The fruits contain a high amount of pectin; the extraction of fruit juice has historically resulted in a cloudy, unappealing color and high viscosity. Researchers studied avocado wastes that were found to contain four distinct bacterial strains. One strain was classified as a Serratia marcescens based on morphological and biochemical characteristics. It's important to do more research on this enzyme to make sure and improve the efficiency of the bacteria for use in the fruit industry.
Materials and methods
The major laboratory instruments used in this study were gel documentation, balance, centrifuge, Spectrophotometer, autoclave, refrigerator, microscope, water bath, water bath with shaker, and incubator.
Sample collection
The avocado peel wastes were collected from the juice processing site of ECOPIA PLC, a private limited company, in Addis Ababa, Ethiopia. The samples were transferred into sterilized plastic bags and brought to the microbiology laboratory at AASTU. The sample containing bags were closed and stored in a 4℃ refrigerator until the analysis time [43].
Serial dilutions
Homogenized one gram (1 g) of the avocado peel wastes sample was suspended in 9 ml of sterilized distilled water and was properly mixed. The mixture of 1 g avocado peel waste sample and 9 ml of distilled water were serially diluted from 10–1 to 10–6 in test tubes [54]. Serial dilution and spread plate methods were the techniques used to isolate the target to pectinase enzyme-producing bacteria using Nutrient agar media [55].
Isolation and purification
The growth of bacterial colonies was observed after 24 h of incubation time. The next task was the purification and preservation of the culture. The individual colonies with similar character and size were isolated from culture plates and transferred to new agar plates to obtain pure colonies by the repeated streaking method. The purity of the test isolate was assessed using colony morphology and microscopy; pure colonies of bacteria were preserved with 20% of glycerol and stored at -80 °C for further study [56].
Primary screening of pectinase producing bacteria isolates
All pure colonies from overnight cultured bacteria [freshly activated plates] were transferred to new pectin agar media and incubated at 30 °C for 48 h. At the end of incubation, 0.3% of Congo red solution was flooded onto the Petri dishes and left for 10 min. This solution formed a clear zone around the colonies, which indicates that bacterial isolates can produce pectinase and the diameter of clear zones is proportional to the bacteria’s relative pectinase production capacity [16].
Secondary screening of pectinase producing bacteria isolates
The bacterial isolates showing maximum clear zone on primary screening media were considered the highest pectinase producer [54]. Those bacterial isolates with a higher clear area were subjected to submerged fermentation for pectinase production using the same medium as primary screening but without agar. The freshly cultured (stationary phase bacterial) isolates 0.2 ml in yeast extract broth were inoculated on 100 ml of sterilized production media (the media was fixed for 15 min at 121 °C) in 250 ml of the flask and incubated at 30 °C on a rotary shaker at 125 rpm for 48 h. At the end of incubation time, the media was transferred to a centrifuge tube and centrifuged at 10,000 rpm for 10 min. The supernatant was used as crude enzymes to evaluate the efficiency of bacterial isolates on the production of pectinase activities by using sodium acetate buffer pH 6.8 [1, 45].
Determination of pectinase activity
Pectinase activity was determined by measuring the amount of released reducing sugar under assay conditions or by enzymatic degradation of pectin as described Nelson-Somogyi methods. The procedure was started by mixing 1 ml of substrate solution prepared by Phosphate buffer (pH 7) and 0.4 ml of specific supernatant enzyme in test tubes. Then, the mixed solution was incubated at 40 °C in the water bath for 40 min. After adding 0.3 ml of Somogyi copper reagent and mixture of the test tubes was set in a boiling water bath for 10 min. After incubation, the tubes were cooled to room temperature, and 0.3 ml of the Nelson arseno molybdate reagent was added. The solution was cooled to room temperature and measured at 540 nm after centrifuging at 10,000 rpm for 10 min by taking supernatant as the enzyme. The amount of released glucose per milliliter per minute was calculated using D-glucose (10–100 micromoles) from the standard curves. One unit of pectinase activities was defined as the amount of glucose released in the term of μmol of reducing sugar per ml per minute under Standard assay conditions [43, 57]. The pectinase activity was calculated using the following formula [58].
where RG is released glucose obtained from D-glucose standard curve.
TVA is the total volume of assay.
T is the Incubation time.
VEA is the volume of enzyme used to assay.
Morphological and biochemical tests for the identification of bacterial isolates
Single colonies grown on pectin agar media were smeared and examined under the microscope for morphological conformity using gram staining. The following biochemical tests were performed to identify bacterial isolates: carbohydrate fermentation tests, indole test, methyl red (MR) test, Vegas-Proskauer (VP) test, Citrate utilization test, hydrogen sulfide generation test, catalase test, and urease test [59].
Molecular identification and PCR amplification of screened isolates
Molecular approaches were used to identify the prospective isolates that were examined and selected utilizing the primary and secondary screening procedures (R5 and W3). The genomic DNA of the isolates was extracted using the Bacterial Genomic DNA Extraction Kit, which was modified slightly from the manufacturer's procedure (QIAGEN, QIAamp DNA Mini Kit). The amplification process took 2:35 total time. On a 0.8% agarose gel dyed with a DNA-safe stain, the PCR products were seen. Finally, the PCR products were sequenced, and the obtained sequence data were analyzed using the basic local alignment search tool (BLAST) software (http://www.ncbi.nlm.nih.gov/blast) against the 16S ribosomal RNA sequence database, with the mega X software (http://www.ncbi.nlm.nih.gov/mahalik) to generate the phylogenetic tree from the national center for biotechnology [44].
Production of crude pectinase by submerged fermentation
The pectinase production by submerged fermentation was conducted as described by Kumar A and Sharma R [50]. The bacterial isolate identified as a potential candidate was selected to produce this crude enzyme. About 2 ml of the three hours bacterial cultures (log phase bacteria) were inoculated to a pre-sterilized fermentation medium to maintain pH 8 [43] and incubated at 30 °C using a rotary shaker at 125 rpm for 48 h. At the closed fermentation time, the production medium was centrifuged at 10,000 rpm for 10 min. The clear supernatant was used for pectinases activities [47].
Optimization of pectinase production
The production of pectinase was optimized by using four parameters namely, fermentation time, temperature, pH, and substrate concentration. The relative activity of each parameter was calculated as the percentage by using the following formula:-
where As = the activities of sample in µmol/ml
MS = the maximum activities of the sample in µmol/ml.
Optimization of fermentation time on pectinase production
The production media was prepared at constant pH 7 and 1% substrate concentration to examine the influences of fermentation time on enzyme production by bacterial isolate. A Single colony of R5 isolate was inoculated in 15 ml of yeast extract pectin broth and incubated at a temperature of 30 °C overnight. The production media was sterilized at 121 °C for 15 min. This fixed production media was inoculated with 2 ml of overnight culture bacteria and incubated at a temperature of 30 °C using a rotary shaker with 125 rpm for 24 to 120 h. The activities were assayed in 24 h intervals [60].
Optimization of pH on pectinase production
To investigate the influences of pH variation on enzyme production by bacterial isolate, the pH level of production media was adjusted from a pH 5 to pH 10 using 0.1 M sodium acetate and 0.1 M Sodium hydroxide [4] with a few modifications. The production media was sterilized at 121 °C for 15 min and inoculation with 2 ml of overnight bacterial cultured bacteria. This inoculated media was incubated at 30 °C using a rotary shaker with 125 rpm for 72 h, after which the enzyme activities were assayed.
Optimization of temperature on pectinase production
Temperatures for enzyme production were maintained at the following temperatures to explore the effects of temperature change on enzyme production using bacterial isolate: 25, 30, 35, 40, 45, and 50 °C [61]. The production media (pH 8) was inoculated with 2 ml of overnight cultured bacteria and incubated using a rotary shaker at 125 rpm for 72 h. After the end of the fermentation times, the enzyme activities were assayed.
Optimization of substrate concentration on pectinase production
To study the influences of substrate concentration on enzyme production using the bacterial isolate, the enzyme was produced by various concentrations of substrates (pectin). The concentrations of substrate maintained for enzyme production were 0.25%, 0.5%, 0.75%, 1.0%, 1.25%, and 1.5% [55]. The sterilized production media (pH 8) was inoculated with 2 ml of overnight cultured bacteria and incubated at 30 °C using a rotary shaker at 125 rpm for 72 h. After the end of the fermentation time, the enzyme activities were assayed.
Purification of pectinase by ammonium sulfate precipitation
The crude enzyme was partially purified using ammonium sulfate precipitation methods described by Ramalingam et al., [62]. To avoid the denaturation of enzymes, all purification steps were carried out in a cold environment, utilizing an ice bath and temperatures of 40 °C. About 150 ml of the crude enzyme was precipitated by the addition of four different saturation levels of ammonium sulfate: 30%, 50%, 70%, and 90%. They then dissolved the enzyme proteins that had been frozen in saltwater and dialyzed them with dialysis membranes after the above steps were done [61].
Application of pectinases in fruit juice clarification
The purified pectinase was applied in the fruit juice-making process to test the clarity of the juice. Three different fruits which have the signs of physical damage (lemon, mango, and apple fruits) were bought from a fruit market (Akaki Kality, Addis Ababa) and brought to microbiology laboratories for juice preparation [61].
Juice preparation
Lemon, mango, and apple fruits were washed carefully and chopped into smaller sizes on the side with a sharp knife. Twenty grams (20 g) of each chopped fruit were weighed into separate beakers. Those chopped were treated with crude pectinase, purified pectinase, and untreated samples were kept as controls in which the enzyme was replaced by distilled water. The controls and enzyme-treated samples were incubated in a water bath at 40 °C for 1 h, and the activity was stopped by cooling in an ice bath. After that, each juice was filtered through filter paper before the volume of juice production was measured. After enzymatic treatments, the filtered fruit juice was pasteurized at 60 °C for 20 min. To figure out how much juice was made from each piece of fruit, Rai et al., [20] used the method. The formula is:
Clarification of juice
Using a UV spectrophotometer and the Shet et al., [26] procedure, the clarity of each fruit juice was measured in terms of percentages of transmittances. Around 8 ml of each fruit juice was taken and chilled in a water bath before adding the pectinase enzyme product, after heating at 40 °C to inactivate any natural fruit enzymes or bacteria present. The enzymes (2 mL) were added to 8 mL of fruit juice. After a 4-h incubation period, the samples were heated for 3 min at 40 °C. The juice was centrifuged for 20 min at 3000 rpm, the supernatant was filtered out with filter paper, and the clarity of the juice was calculated by measuring the absorbance at 660 nm with a UV spectrophotometer. Distilled water was used as a blank, and the clarity was expressed in percentages [28].
Untreated and treated juice properties
PH and acidity
The total titrable acidity of the juices was assessed by titration of the juice sample with 0.2 N sodium hydroxide and the pH of cleared and un-clarified fruits (Lemon, Mango, and Apple) juice samples were measured using a pH meter. The results were presented using the phenolphthalein reagent as an indicator and based on g citric acid per 50 ml of each fruit juice [46].
Total soluble solid and reducing sugar content
The total soluble solids (SST) of each fruit (Lemon, Mango, and apple) juice were recorded using a refractometer, and the reducing sugar content of each fruit juices sample was measured by the DNS method [63].
Viscosity
The viscosity of Lemon, Mango, and apple fruits juice samples was evaluated by viscometer at a share rate of 75 rpm and room temperature.
Pectin presence test
The pectin test was performed on Lemon, Mango, and Apple fruit juice samples by combining cold ethanol with each sample and storing it in the refrigerator overnight. The presence of supernatant indicates the presence of pectin in the sample, while the absence of supernatant suggests the absence of pectin [53].
Total phenol content
The Folin-Ciocalteu technique was used to determine each fruit juice sample [23, 25]. The total phenol content of the sample was determined by comparing it to the Gallic acid standard curve, with the result represented in mg gallic acid equivalent per ml juice sample (mg GAE/ml)[49].
Determination of antioxidant activity
The ABTS and DPPH radical scavenging activities of each fruit juice sample were determined using a modified version of the method published by Hosseini et al., [53]. An ABTS solution (6.5 mM) was combined with a potassium persulfate solution (3.5 mM) and stored in darkness for 18 h to make an ABTS solution. After vortexing, the obtained mixtures were kept in darkness for 45 min to complete the antiradical reaction. 0.2 ml of each fruit juice sample was diluted 50 times with distilled water and added to 1.8 ml of the obtained ABTS solution and DPPH ethanolic solution (0.1 mM). After recording the absorbance at 734 nm for ABTS radical scavenging activity (ABTS-RSA) and 517 mn for DPPH radical scavenging activity (DPPH-RSA), these antiradical activities were calculated as follows:
Statistical analysis
Statistical analyses of data were conducted using IBM SPSS statistics 20 and origin 2019. All tests were performed in triplicates, and data were expressed as Mean ± standard deviation.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The genome sequence data for R5 and W3is available in the GenBank repository under the project of Addis Ababa Science and Technology University (Website: https://www.ncbi.nlm.nih.gov/nuccore/MN932109.1 and https://www.ncbi.nlm.nih.gov/nuccore/1796387721).
Abbreviations
- C:
-
Celsius
- g:
-
Gram
- ml:
-
Milliliter
- mg:
-
Milligram
- rpm:
-
Revolutions per minute
- μmol:
-
Micromoles
- μL:
-
Microliters
- nm:
-
Nanomolar
- min:
-
Minutes
- U:
-
Units
- PCR:
-
Polymerase chain reaction
- BLAST:
-
Basic Local Alignment Search Tool
- DNA:
-
Deoxyribonucleic Acid
References
Aaisha GA, Barate DL. Isolation and identification of pectinolytic bacteria from soil samples of Akola region, India. Int J Curr Microbiol Appl Sci. 2016;5(1):514–21.
Masi C, Gemechu G, Tafesse M. Isolation, screening, characterization, and identification of alkaline protease-producing bacteria from leather industry effluent. Annals of Microbiology. 2021;71(1):1–1. https://doi.org/10.1186/s13213-021-01631-x.
Robinson PK. Enzymes: principles and biotechnological applications. Essays Biochem. 2015;15(59):1. https://doi.org/10.1042/bse0590001.
Abdollahzadeh R, Pazhang M, Najavand S, Fallahzadeh-Mamaghani V, Amani-Ghadim AR. Screening of pectinase-producing bacteria from farmlands and optimization of enzyme production from selected strain by RSM. Folia Microbiologica. 2020;65(4):705–19.
Ahmad V, Kamal A, Ahmad K, Khan MS. Protease characteristics of bacteriocin producing Lysinibacillus, isolated from fruits and vegetable waste. Bioinformation. 2014;10(1):13.
Abd-Alla MH, Bashandy SR, Schnell S, Ratering S. Isolation and characterization of Serratia rubidaea from dark brown spots of tomato fruits. Phytoparasitica. 2011;39(2):175–83.
Gemechu G, Masi C, Tafesse M, Kebede G. A review on the bacterial alkaline proteases. J Xidian Univ. 2020;14(11):632–4.
Chapman J, Ismail AE, Dinu CZ. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts. 2018 Jun;8(6):238. https://doi.org/10.3390/catal8060238.
Wilmowicz E, Kućko A, Alché JD, Czeszewska-Rosiak G, Florkiewicz AB, Kapusta M, Karwaszewski J. Remodeling of Cell Wall Components in Root Nodules and Flower Abscission Zone under Drought in Yellow Lupine. Int J Mol Sci. 2022;23(3):1680. https://doi.org/10.3390/ijms23031680.
Nighojkar A, Patidar MK, Nighojkar S. Pectinases: production and applications for fruit juice beverages. Processing and sustainability of beverages. Woodhead Publishing; 2019;2:235–73. https://doi.org/10.1016/B978-0-12-815259-1.00008-2.
Oumer OJ, Abate D. Screening and molecular identification of pectinase producing microbes from coffee pulp. Biomed Res Int. 2018;3:2018. https://doi.org/10.1155/2018/2961767.
Haile S, Ayele A. Pectinase from Microorganisms and Its Industrial Applications. The Scientific World Journal. 2022; 2022.https://doi.org/10.1155/2022/1881305
Korsa G, Masi C, Konwarh R, Tafesse M. Harnessing the potential use of cellulolytic Klebsiella oxytoca (M21WG) and Klebsiella sp. (Z6WG) isolated from the guts of termites (Isoptera). Ann Microbiol. 2022;72(1):1–5. https://doi.org/10.1186/s13213-021-01662-4.
de Oliveira RL, Dias JL, da Silva OS, Porto TS. Immobilization of pectinase from Aspergillus aculeatus in alginate beads and clarification of apple and umbu juices in a packed bed reactor. Food Bioprod Process. 2018 May;1(109):9–18.
Javed R, Nawaz A, Munir M, Hanif M, Mukhtar H, Haq IU, Abdullah R. Extraction, purification and industrial applications of pectinase: A review. J Biotechnol Biores. 2018;1:1–6.
Beg QK, Bhushan B, Kapoor M, Hoondal GS. Production and characterization of thermostable xylanase and pectinase from Streptomyces sp. QG-11–3. J Ind Microbiol Biotechnol. 2000;24(6):396–402.
Raveendran S, Parameswaran B, Ummalyma SB, Abraham A, Mathew AK, Madhavan A, Rebello S, Pandey A. Applications of microbial enzymes in food industry. Food Technol Biotechnol. 2018;56(1):16. https://doi.org/10.17113/ftb.56.01.18.5491
Kashyap DR, Vohra PK, Chopra S, Tewari R. Applications of pectinases in the commercial sector: a review. Biores Technol. 2001;77(3):215–27. https://doi.org/10.1016/S0960-8524(00)00118-8.
Kumar YS, Kumar PV, Reddy OV. Pectinase production from mango peel using Aspergillus foetidus and its application in processing of mango juice. Food Biotechnol. 2012;26(2):107–23. https://doi.org/10.1080/08905436.2012.670830.
Rai P, Majumdar GC, Gupta SD, De S. Effect of various pretreatment methods on permeate flux and quality during ultrafiltration of mosambi juice. J food Eng. 2007;78(2):561–8.
Darah I, Nisha M, Lim SH. Enhancement of polygalacturonase production from Enterobacter aerogenes NBO2 by submerged fermentation. Adv Stud Biol. 2013;5(5):173–89.
Abdullah AL, Sulaiman NM, Aroua MK, Noor MM. Response surface optimization of conditions for clarification of carambola fruit juice using a commercial enzyme. J Food Eng. 2007;81(1):65–71.
Masi C. Immobilization of Pseudomonas Aeruginosa in different matrices for the production of alkaline protease. Int J Recent Technol Eng. 2020;9(1):956–9.
Vaillant F, Millan P, O’Brien G, Dornier M, Decloux M, Reynes M. Crossflow microfiltration of passion fruit juice after partial enzymatic liquefaction. J Food Eng. 1999;42(4):215–24.
Kazemi M, Khodaiyan F, Hosseini SS, Najari Z. An integrated valorization of industrial waste of eggplant: Simultaneous recovery of pectin, phenolics and sequential production of pullulan. Waste Manage. 2019;1(100):101–11.
Shet AR, Desai SV, Achappa S. Pectinolytic enzymes: classification, production, purification and applications. Res J Life Sci Bioinform Pharm Chem Sci. 2018;4:337–48.
Bergey DH, Breed RS, Murray EGD, Hitchens AP. Bergey's Manual of Determinative Bacteriology. A Key for the Identification of Organisms of the Class Schizomycetes. Bergey's Manual of Determinative Bacteriology. A Key for the Identification of Organisms of the Class Schizomycetes., (Edn 5). 1939.
Hitha PK, Girija D. Isolation and screening of native microbial isolates for pectinase activity. Int J Sci Res (Ahmedabad). 2014;3:632–4.
Varghese LK, Rizvi AF, Gupta AK. Isolation, screening and biochemical characterization of pectinolytic microorganism from soil sample of Raipur city. J Biol Chem Res. 2013;30(2):636–43.
Obafemi YD, Ajayi AA, Taiwo OS, Olorunsola SJ, Isibor PO. Isolation of polygalacturonase-producing bacterial strain from tomatoes (Lycopersicon esculentum Mill.). Int J Microbiol. 2019:7505606. https://doi.org/10.1155/2019/7505606.
Siddiqui M, Pande V, Arif M. Production, purification, and characterization of polygalacturonase from Rhizomucor pusillus isolated from decomposting orange peels. Enzyme Res. 2012;2012:138634. https://doi.org/10.1155/2012/138634.
Oumer OJ. Pectinase: substrate, production and their biotechnological applications. Int J Environ Agric Biotechnol. 2017;2(3).
Mahalik S, Mohapatra D, Kumar D. Cellulase production in Lysinibacillus sp isolated from the estuaries of Odisha. Biosci Biotechnol Res Commun. 2018;11:743–53.
Sareen SJ, Thomas S, Sruthy AJ. Molecular characterization of microorganisms in municipal solid waste for production of industrial enzymes and enhanced biodegradation. Biotechnol Res. 2018;4(2):80–7.
Hugouvieux-Cotte-Pattat N, Jacot-des-Combes C, Briolay J. Genomic characterization of a pectinolytic isolate of Serratia oryzae isolated from lake water. J Genomics. 2019;7:64.
Mohandas A, Raveendran S, Parameswaran B, Abraham A, Athira RS, Mathew AK, Pandey A. Production of pectinase from Bacillus sonorensis MPTD1. Food Technol Biotechnol. 2018;56(1):110.
Tabssum F, Ali SS. Screening of pectinase producing gram positive bacteria: isolation and characterization. Punjab Univ J Zool. 2018;33(1):11–5.
Jayani RS, Saxena S, Gupta R. Microbial pectinolytic enzymes: a review. Process Biochem. 2005;40(9):2931–44.
Embaby AM, Masoud AA, Marey HS, Shaban NZ, Ghonaim TM. Raw agro-industrial orange peel waste as a low cost effective inducer for alkaline polygalacturonase production from Bacillus licheniformis SHG10. Springerplus. 2014;3(1):1–3. https://doi.org/10.1186/2193-1801-3-327.
Sohail M, Latif Z. Phylogenetic analysis of polygalacturonase-producing Bacillus and Pseudomonas isolated from plant waste material. Jundishapur J Microbiol. 2016;9(1):e28594. https://doi.org/10.5812/jjm.28594.
Kothari MN, Baig MM. Production and characterization of extracellular polygalacturonase by Erwinia carotovora MTCC 1428. Int J Adv Biotechnol Res. 2013;4(1):981–98.
Sittidilokratna C, Suthirawut S, Chitradon L, Punsuvon V, Vaithanomsat P, Siriacha P. Screening of pectinase producing bacteria and their efficiency in biopulping of paper mulberry bark. Sci Asia. 2007;33(1):131–5 0.2306/scienceasia1513−1874.2007.33.131.
Karabi R, Sujan D, Uddin MK, Rasel B, Hossain MT. Extracellular pectinase from a novel bacterium Chryseobacterium indologenes strain SD and its application in fruit juice clarification. Enzyme Res. 2018;2018:3859752. https://doi.org/10.1155/2018/3859752.
Barman S, Sit N, Badwaik LS, Deka SC. Pectinase production by Aspergillus niger using banana (Musa balbisiana) peel as substrate and its effect on clarification of banana juice. J Food Sci Technol. 2015;52(6):3579–89. https://doi.org/10.1007/s13197-014-1413-8.
Tambekar D, Tambekar S, Rajgire A, Jadhav A, Sawale K. Isolation and characterization of amylase from Lysinibacillus xylanilyticus from alkaline environment. Int J Res Stud Biosci. 2016;4(6):1–4.
Campos PR, Modenes AN, Espinoza-Quinones FR, Trigueros DE, Barros ST, Pereira NC. Improvement on the concentrated grape juice physico-chemical characteristics by an enzymatic treatment and Membrane Separation Processes. An Acad Bras Ciênc. 2016 Mar;88(1):423–36.
Soares MM, Silva RD, Gomes E. Screening of bacterial strains for pectinolytic activity: characterization of the polygalacturonase produced by Bacillus sp. Rev Microbiol. 1999;30:299–303. https://doi.org/10.1590/S0001-37141999000400002.
Demir N, Nadaroglu H, Demir Y, Isik C, Taskin E, Adiguzel A, Gulluce M. Purification and characterization of an alkaline pectin lyase produced by a newly isolated Brevibacillus borstelensis (P35) and its applications in fruit juice and oil extraction. Eur Food Res Technol. 2014;239(1):127–35. https://doi.org/10.1007/s00217-014-2198-8.
Maktouf S, Neifar M, Drira SJ, Baklouti S, Fendri M, Châabouni SE. Lemon juice clarification using fungal pectinolytic enzymes coupled to membrane ultrafiltration. Food Bioprod Process. 2014;92(1):14–9. https://doi.org/10.1016/j.fbp.2013.07.003.
Kumar A, Sharma R. Production of alkaline pectinase by bacteria (Cocci sps.) isolated from decomposing fruit materials. J Phytol. 2012;4(1):1–5.
Yuan P, Meng K, Huang H, Shi P, Luo H, Yang P, Yao B. A novel acidic and low-temperature-active endo-polygalacturonase from Penicillium sp. CGMCC 1669 with potential for application in apple juice clarification. Food Chemistry. 2011;129(4):1369–75.
Diano N, Grimaldi T, Bianco M, Rossi S, Gabrovska K, Yordanova G, Godjevargova T, Grano V, Nicolucci C, Mita L, Bencivenga U. Apple juice clarification by immobilized pectolytic enzymes in packed or fluidized bed reactors. J Agric Food Chem. 2008;56(23):11471–7.
Hosseini SS, Khodaiyan F, Mousavi SM, Azimi SZ. Clarification of the pomegranate juice in a bioreactor packed by pectinase enzymes immobilized on the glass bead activated with polyaldehyde polysaccharides. LWT. 2021;1(137): 110500.
Rehman HU, Siddique NN, Aman A, Nawaz MA, Baloch AH, Qader SA. Morphological and molecular based identification of pectinase producing Bacillus licheniformis from rotten vegetable. Journal of Genetic Engineering and Biotechnology. 2015;13(2):139–44. https://doi.org/10.1016/j.jgeb.2015.07.004.
Jayani RS, Shukla SK, Gupta R. Screening of bacterial strains for polygalacturonase activity: its production by Bacillus sphaericus (MTCC 7542). Enzyme Res. 2010;306785:5. https://doi.org/10.4061/2010/306785.
Savas S, Adiguzel A, Inan K, Ozkan H, Gulluce M, Sahin F. Molecular characterization of thermophilic bacteria isolated from Van City Ercis Town Hasanabdal hot spring. Rom Biotech Lett. 2009;14:4445–54.
Roy K, Dey S, Uddin M, Barua R, Hossain M. Extracellular pectinase from a novel bacterium Chryseobacterium indologenes strain SD and its application in fruit juice clarification. Enzyme Res. 2018;2018.
Khan IG, Barate DL. Effect of various parameters on activity of pectinase enzyme. Int J Adv Res. 2016;4(1):853–62.
Abiola C, Oyetayo VO. Isolation and biochemical characterization of microorganisms associated with the fermentation of Kersting’s groundnut (Macrotylomageocarpum). Res J Microbiol. 2016;11:47–55.
Soares MM, Da Silva R, Carmona EC, Gomes E. Pectinolytic enzyme production by Bacillus species and their potential application on juice extraction. World J Microbiol Biotechnol. 2001;17(1):79–82. https://doi.org/10.1023/A:1016667930174.
Koshy M, De S. Effect of Bacillus tequilensis SALBT crude extract with pectinase activity on demucilation of coffee beans and juice clarification. J Basic Microbiol. 2019;59(12):1185–94.
Ramalingam P, Aswini V, Pradeepa P, Sriram S, Swathi H. Partial purification and characterization of cellulase, pectinase and xylanase from Penicillium chrysogenum. IOSR J Environ Sci Toxicol Food Technol. 2013;5(3):48–59.
Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–8.
Acknowledgements
We thank the University President, Academic Vice President, College Dean, Head of Department, and Lab coordinator of the Department of Biotechnology, College of Biological and Chemical Engineering help us with this research work.
Funding
This research work was supported by the Directorate of Research and Technology Transfer, Addis Ababa Science and Technology University, Addis Ababa, Ethiopia.
Author information
Authors and Affiliations
Contributions
All authors have carried out the avocado peel samples collected from the dumping site of ECOPIA PL randomly from a decayed avocado waste using a pre-sterilized spatula. MT &SH carried out the isolation of potential bacteria. SH & CM carried out the identification of bacteria. All authors are contributed by the final confirmation of potential Pectinase-producing bacteria and manuscript revisions. All authors have approved the final version of the manuscript and agree to be held accountable for the content therein.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
There was no human or animal participation in the research, and no data was collected. With the company's permission, a sample was taken from ECOPIA PLC in Addis Ababa, Ethiopia.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Haile, S., Masi, C. & Tafesse, M. Isolation and characterization of pectinase-producing bacteria (Serratia marcescens) from avocado peel waste for juice clarification. BMC Microbiol 22, 145 (2022). https://doi.org/10.1186/s12866-022-02536-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-022-02536-8