Abstract
Background
Aquatic matrices impacted by sewage may shelter carbapenem-resistant (CR) Gram-negative bacilli (GNB) harboring resistance genes of public health concern. In this study, sewage treatment plants (STPs) servicing well-defined catchment areas were surveyed for the presence of CR-GNB bearing carbapenemase genes (blaKPC or blaNDM).
Results
A total of 325 CR-GNB were recovered from raw (RS) and treated (TS) sewage samples as well as from water body spots upstream (UW) and downstream (DW) from STPs. Klebsiella-Enterobacter (KE) group amounted to 116 isolates (35.7%). CR-KE isolates were recovered from TS, DW (35.7%) and RS samples (44.2%) (p = 0.001); but not from UW samples. KE isolates represented 65.8% of all blaKPC or blaNDM positive strains. The frequency of blaKPC-or-NDM strains was positively associated with the occurrence of district hospitals located near STPs, as well as with the number of hospitalizations and of sewer connections serviced by the STPs. blaKPC-or-NDM strains were recovered from ST samples in 7 out of 14 STPs, including four tertiary-level STPs; and from 6 out of 13 DW spots whose RS samples also had blaKPC-or-NDM strains.
Conclusions
Clinically relevant GNB bearing blaKPC-or-NDM resist sewage treatments and spread into environmental aquatic matrices mainly from STPs impacted by hospital activities.
Similar content being viewed by others
Background
Carbapenems are last-resort antibiotics for treating infections caused by multidrug-resistant (MDR) Gram-negative bacilli (GNB). The spread of carbapenem-resistant (CR) strains has concerned physicians and hospital health managers, becoming an unprecedented threat to public and environmental health [1]. In hospital environments, CR-GNB surveillance was urged as soon as the worldwide emergence of K. pneumoniae strains harboring resistance gene blaKPC was confirmed [2]. blaKPC-positive strains (hereinafter simply referred to as blaKPC strains) are now detected worldwide in a variety of GNB isolates, mainly in Enterobacterales species but also in Pseudomonas aeruginosa and Acinetobacter spp. The high transmissibility of the transposon Tn4401, which bears blaKPC, remains the primary mechanism for the spread of carbapenem resistance among GNB strains [3, 4].
In 2010, the detection of blaNDM(New Delhi Metallo beta lactamase)-positive strains in seepage and water puddle samples exposed the need to broaden the surveillance of carbapenemase genes by incorporating environmental sampling [5]. Studies showed that blaNDM strains were not restricted to nosocomial settings; instead, they widely emerged in the community environment of countries such as India and Pakistan [6]. Concerning Brazil, strains harboring blaKPC or blaNDM (blaKPC-or-NDM) emerged and were firstly detected in nosocomial environments [7,8,9].
Large amounts of antibiotics are discharged in sewer systems due to incomplete metabolism in humans, to the disposal of unused antibiotics and to antibiotic usage in economic activities, such as intensively managed livestock farming [10]. On account of the substantial load of bacteria and antibiotics, hospital effluents are conductive matrices for the exchange of resistance genes between pathogenic and environmental bacteria and, consequently, the selection of resistant strains [11]. Indeed, blaKPC-or-NDM isolates of GNB have been recovered from hospital effluents worldwide [12,13,14,15,16], including Brazil [17, 18]. Additionally, sanitary regulations and sewage treatment processes were not conceived to face the threat of spreading antimicrobial resistance [18, 19]. In many countries, as in Brazil, hospitals are not obliged to treat their effluents before discharging them in the public sewerage [18, 20]. Moreover, even modern disinfection processes applied to sewage treatment allow the escape of CR isolates to the environment. In the United States, CR isolates were recovered from treated effluent in 42% of sewage treatment plants (STPs) using chlorination process, and in 12% of STPs using ultraviolet radiation [21]. Indeed, STPs are selective spots and reservoirs of MDR bacteria which contribute to spreading resistant strains in the environment [18, 20, 21].
The first Brazilian report on blaKPC-positive K. pneumoniae strains isolated from environment dated 2008 [22]. blaKPC isolates were recovered from effluents of a STP servicing a hospital in the city of Rio de Janeiro [22]. Since then, other GNB species carrying carbapenemase genes (including Klebisiella spp., Enterobacter spp., Kluyvera spp., Citrobacter spp., Enterobacter spp. and Serratia spp.) have been recovered from coastal recreational waters [23, 24]. In Rio de Janeiro, a substantial volume of both treated and untreated sewage is continually discharged into Guanabara Bay, which in turn communicates with recreational waters of touristic beaches [23].
In Brazil, the environmental spread of blaKPC-or-NDM isolates is predominantly reported as being a direct consequence of inappropriate sewerage infrastructure allowing the discharge of untreated sewage into the environment. We proposed a study in a different scenario. In the Brazilian capital Brasília, 85% of the sewage produced was collected in a proper sewerage and treated [25] in 14 public STPs, of which 9 carried out tertiary treatment (Table 1). Brasília (with 3 million inhabitants and an area of 5789.16 km2) is organized into administrative regions which differ in number of sewer connections, number of hospitals and economic profile (Table 1). Thus, in Brasília STPs serve communities located in well-defined geographical catchment areas. Additionally, hospitals in the city have reported blaKPC strains since 2010 as well as blaNDM strains since 2013 [7]. This study aimed to characterize the spread of blaKPC-or-NDM-positive GBN strains by way of STPs, taking into account the profile of economic activity in areas serviced by STPs, the burden on the STPs imposed by hospital services, and the level of sewage treatment achieved by STPs.
Results
Carbapenem-resistant (CR) cultures and isolates recovered from sewage and water samples.
CR cultures were produced in different proportions among the analyzed samples (supporting information Table 2). All of the 35 RS samples produced CR cultures, followed by 80% (28/35) of the TS samples, 71% (27/38) of the DW samples and 23% (7/30) of the UW samples. The proportion of CR cultures in TS and DW samples was statistically equal (p = 0.425); however, both proportions were higher than that found among UW samples (p < 0.001) (supporting information Table 2).
A total of 325 CR-GNB isolates were recovered and included intrinsically carbapenem-resistant species, mostly soil saprophyte species of the Pseudomonas putida group (32.6% - 106/325), as well as clinically relevant species, mostly isolates of the Klebsiella-Enterobacter (KE) group (35.7% - 116/325) (Fig. 1). It is worthy of note that mesophilic Aeromonas spp. accounted for 6.4% (21/325) of the CR isolates. Although isolates of P. putida and KE group have been equally represented, their proportions were statistically different when sorted into sample types (Fig. 1 and supporting information Table 2). Isolates of P. putida group were equally recovered (p = 0.588) from RS, TS and DW samples [respectively 27.4% (36/131), 33.6% (31/92) and 32.1% (27/84)]; however, they were recovered at a higher frequency (p = 0.015) from UW samples [66.6% (12/18)] (supporting information Table 2). Differently from P. putida, CR-KE isolates were not recovered from UW samples. Indeed, CR-KE isolates were equally recovered (p = 0.428) from TS and DW samples [respectively 31.5% (28/92) and 38.0% (30/84)], showing a higher recovery frequency (p = 0.001) from RS samples [48.0% (58/131)] (supporting information Table 2).
Taxonomic profile of (A) carbapenem-resistant and (B) blaKPC-or-NDM-positive isolates recovered from sewage and water samples. A Carbapenem-resistant isolates recovered from downstream water and sewage samples form a more diverse set of strains in comparison with the isolates from upstream water samples. Enterobacteria, especially the Klebsiella-Enterobacter group, are overrepresented among carbapenem-resistant isolates recovered from sewage and downstream samples. In contrast, carbapenem-resistant isolates from upstream water samples are mostly represented by intrinsically resistant, saprophyte species. B Strains harboring blaKPC or blaNDM genes (blaKPC-or-NDM) were not recovered from upstream water samples. Klebsiella-Enterobacter group predominates among blaKPC-or-NDM strains recovered from downstream water and sewage samples and is followed by blaKPC-or-NDMAeromonas strains
Detection of carbapenemase genes in CR-GNB isolates
Carbapenemase genes were detected in 124 (39.2%) out of 316 CR-GNB isolates. The genes blaKPC, blaNDM, blaIMP and blaOXA-48 were detected in 27.2% (n = 86), 10.7% (n = 34),1.8% (n = 6) and 1.2% (n = 4) of the tested CR-GNB, respectively. Five strains from RS samples were positive for more than one tested gene. Four isolates were positive for 2 carbapenemase genes (two P. putida strain with genotypes blaKPC + blaNMD and blaKPC + blaOXA-48; one K. pneumoniae strain with blaKPC + blaNMD; and one P. aeruginosa strain with blaKPC + blaIMP). Furthermore, one K. pneumoniae strain from RS samples harbored three carbapenemase genes (blaKPC + blaNMD + blaIMP).
KE isolates were identified in 65.8% (77/117) of the blaKPC-or-NDM strains (p < 0.0001) with the predominance of K. pneumoniae (53.8% - 63/117) (p < 0.0001), followed by E. cloacae (11% - 13/117) and E. aerogenes (3.4% - 4/117) (supporting information Table 2). Isolates of Aeromonas spp. carrying blaKPC (A. hydrophila – n = 8; A. sobria – n = 4; and A. veronii – n = 1) represented 11.1% (13/117) of blaKPC-or-NDM strains (supporting information Table 2). It is interesting to note that two uncommonly reported blaKPC-positive species of Enterobacterales species were identified: Kluyvera ascorbata (99.9% confidence level) and Pantoea agglomerans (93.3% confidence level). Lastly, the transposon Tn4401 was detected in 97.1% (69/71) of the blaKPC strains, including isolates of Klebsiella spp., Enterobacter spp., Aeromonas spp., Citrobacter spp., P. putida group., P. aeruginosa group, P. agglomerans and K. ascorbate (data not shown).
Distribution of bla KPC or bla NDM (bla KPC-or-NDM)-positive GNB across samples
CR-GNB isolates positive for blaKPC-or-NDM were detected at different proportions across analyzed samples (p < 0.001) (supporting information Table 2). blaKPC-or-NDM strains accounted for 50.4% (64/127) of the CR-GNB isolates recovered from RS samples. The proportion of blaKPC-or-NDM strains in TS (26/88–29.5%) and DW (27/83–32.5%) samples was statistically equal (p = 0.742). Conversely, blaKPC-or-NDM strains were not detected in UW samples (supporting information Table 2). With regard to taxonomic groups, Klebsiella-Enterobacter group were predominant among blaKPC-or-NDM strains recovered from RS (70.3% - 45/64), TS (65.3% - 19/27) and DW (70.3% - 17/26) samples (Fig. 1 and supporting information Table 2).
Distribution of bla KPC or bla NDM (bla KPC-or-NDM)-positive GNB across STPs
In Brasília, STPs serve areas showing differences with respect to the volume of sewage produced, the number of sewer connections, the number of hospitals and hospitalizations, and the economic profile (Table 1). blaKPC-or-NDM strains were unevenly recovered among STPs, so that 6 out of 14 STPs (STP-1, STP-3, STP-5, STP-11, STP-12, and STP-13) accounted for 75% (48/64) of the isolation of blaKPC-or-NDM strains recovered from RS samples (Fig. 2).
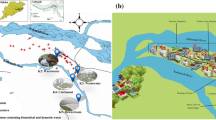
Study setting showing sewage treatment plants (STP) in Brasília, Brazil, and the distribution of blaKPC-or-NDM-positive GNB recovered from raw sewage (RS) and (B) from water bodies downstream (DW) from STPs. The insert at the bottom right displays the geographic position of the city of Brasília (red pin) located on a plateau in the central-western region of Brazil. Sewage treatment plants (blue pins), hospitals (red signals and dots) and intensive management livestock farms (black dots) are indicated. The proportional distribution among the surveyed STPs of 64 blaKPC-or-NDM strains recovered from raw sewage (A) and 27 blaKPC-or-NDM strains recovered from DW samples (B) is shown
Non-parametric statistics showed that the frequency of blaKPC-or-NDM strains increases (showing a positive association) as a function of the total number of hospitals; the number of hospitalizations; the occurrence in STP vicinities (less than 3 km away) of district hospitals; and the number of sewer connections serviced by the STPs (Fig. 3). Additionally, statistics endorsed that the total number of hospitals (mean rank of 73.8 vs. 54.0), the number of district hospitals (mean rank of 72.2 vs. 55.6) and of hospitalizations (mean rank of 71.7 vs. 56.1) ranked higher in the group of blaKPC-or-NDM strains in comparison with blaKPC-or-NDM-negative strains (supporting information Table 3). Otherwise, the frequency of blaKPC-or-NDM strains was statistically lower in STP areas with an increased level of agricultural employment (mean rank 52.7 vs. 67.5) (Fig. 3 and supporting information Table 3).
Frequency analysis of blaKPC-or-NDM isolates recovered from STPs (raw sewage). Frequencies were distributed among categorized groups of STPs considering regional attendance profiles: A volume (L/s) of inflow sewage in the STPs; B sewer connections serviced by STPs; C the total number of hospitals D occurrence of tertiary-level district hospitals within 3 Km from the STPs; E the number of hospitalizations in areas serviced by STPs; F built area (m2) for pig/poultry farming within 3 Km from the STPs; and G participation of agricultural employment in areas serviced by STPs. Levels of significance for the distribution analysis (Fisher’s Exact Test) and for the linear relationship analysis (Cochran-Mantel-Haenszel test) are displayed in each graph
Sewage treatment effect on the containment of bla KPC-or-NDM-positive strains
In order to evaluate the effectiveness of sewage treatment in reducing the spread of CR strains, the frequency of blaKPC-or-NDM strains recovered from RS and TS samples were compared considering the treatment level (secondary or tertiary) accomplished by STPs (Fig. 4). In secondary-level STPs, blaKPC-or-NDM strains were equally detected in RS [40% (16/40)] and TS [46.4% (13/28)] samples (p = 0.627). In contrast, tertiary-level STPs achieved a 33 percentage-point reduction in the frequency of blaKPC-or-NDM strains in TS samples compared to RS samples. In tertiary-level STPs, blaKPC-or-NDM strains were detected in respectively 55.1% (48/87) and 21.6% (13/60) of the strains recovered from RS and TS samples (p < 0.001).
Despite the significant reduction produced by the tertiary-level treatment, blaKPC-or-NDM strains were still recovered from 7 out of 14 analyzed STPs, including four tertiary-level STPs (STP-4, STP-6, STP-10, STP13) and three secondary-level STPs (STP-2, STP-8, STP-12). Most of blaKPC-or-NDM strains recovered from the TS samples belonged to Klebsiella-Enterobacter group (65.3% - 17/26), followed by mesophilic Aeromonas (19.2% - 5/26) (Fig. 1).
bla KPC-or-NDM-positive strains evade sewage treatment, remaining viable in receiving water bodies
Environmental spread of blaKPC-or-NDM-positive strains facilitated by STPs was evaluated comparing the UW and DW samples. DW samples accounted for 23% (27/117) of all of the blaKPC-or-NDM-positive strains. In contrast, blaKPC-or-NDM-positive strains were not isolated from UW samples. blaKPC-or-NDM-positive strains were recovered from 6 out of 13 (46%) DW sites whose RS samples also had blaKPC-or-NDM strains (Fig. 2 and supporting information Fig. 3). Additionally, five of out these six STPs had their TS samples also positive for blaKPC-or-NDM-positive strains. The leakage of blaKPC-or-NDM-positive strains was verified in four tertiary-level STPs (STP-5, STP-6, STP-4/10 and STP-13) and in two secondary-level STPs (STP-2 and STP-12) (Fig. 2 and supporting information Fig. 3).
In relation to the taxonomic groups, Klebsiella-Enterobacter group accounts for 70% (19/27) of blaKPC-or-NDM-positive strains recovered from DW samples, being followed by mesophilic Aeromonas group (14% - 4/27) (Fig. 1 and supporting information Table 2).
Discussion
Wastewater-based epidemiology (WBE) postulates that infectious diseases and drug-related markers, including antibiotic resistance, can be monitored comprehensively through the analysis of population pooled wastewater. Additionally, WBE can provide information on the community and environmental health status as well as on the community exposure to health-threatening issues [26, 27]. Carbapenem-resistant (CR) Gram-negative bacilli (GNB) are classified as critical group for epidemiologic surveillance [1]. CR-GNB isolates recovered from diverse water matrices have been reported to shelter mobile carbapenemase genes of public health concern, such as blaKPC and blaNDM [28]. In this scenario, aquatic matrices have proved to be a conducive environment for the spread of the resistance to carbapenems [28].
Our survey for CR-GNB in STPs settings yielded a collection of 325 isolates. As expected, part of these isolates (124–38.2%) represented species expressing intrinsic resistance to carbapenem (P. putida, S. maltophilia and C. violaceum) which were frequently found in soil and water environments. These saprophytic species are not epidemiologically relevant once they rarely harbor mobile carbapenemase genes and rarely produce human infections. In contrast, 142 (43.6%) CR-GNB isolates represented intrinsically carbapenem susceptible, clinically relevant species which are frequently associated with human infections (Klebsiella spp., Enterobacter spp., P. aeruginosa, Citrobacter spp., Serratia marcescens and Proteae species).
Among 124 CR-GNB isolates harboring carbapenemase genes, 117 (94.3%) strains harbored blaKPC or blaNDM, with blaKPC strains being a majority (86–73.5%) over blaNDM strains (34–29.0%). With regard to blaKPC-associated transposon, Tn4401 was detected in 97% of the blaKPC strains including Enterobacterales species, Aeromonas species, non-fermentative bacilli and soil saprophytes species. Therefore, our data evince the role of Tn4401 as the foremost element for spreading blaKPC in different species of GNB, including environmental strains [3, 4].
Klebsiella spp. and Enterobacter spp. are predominant etiologic agents of nosocomial infections [29,30,31]. Additionally, they account for the most of MDR isolates recovered as commensal colonizers of innate patients [29]. Patrice L Nordmann stated in mid-2010 that there would be many reasons to believe that CR Klebsiella spp. and Enterobacter spp. isolates could spread to community settings as it was extensively described for ESBL producers [32]. Indeed, CR-GNB strains are reported to be responsible for up to 29% of community-acquired infections (CAI) which frequently have Klebsiella spp. and Enterobacter spp. as aetiologic agent [33]. In this study, blaKPC-or-NDM strains belonging to the Klebsiella-Enterobacter group were predominantly recovered from sewage samples (67.8%) and from water samples collected downstream from STPs (55.5%), which reveals the environmental contamination by these strains. The predominance of Klebsiella-Enterobacter group among blaKPC-or-NDM strains from water matrices impacted by sewage discharge has been frequently reported [18, 20, 21, 24].
Aeromonas spp. can carry a diverse set of chromosomal narrow-spectrum β-lactamases including the carbapenem-hydrolyzing enzyme CphA [34]. However, it is assumed that CphA does not often confer in-vitro resistance to carbapenems [35,36,37]. Indeed, in this study 52% of CR Aeromonas strains harbored blaKPC genes. Our data also showed that sewage treatment contributes to the environmental spread of these strains. Most of the blaKPC-positive Aeromonas strains were recovered from TS and DW samples. Other studies have reported the occurrence of blaKPC-positive Aeromonas strains in TS and recreational water samples collected at downstream sites from STPs [21, 23, 38, 39]. Furthermore, Aeromonas strains have been recognized as an emerging cause of human waterborne infections involving recreational activities and ingestion of foods. Due to the occurrence of CphA in Aeromonas spp., third- and fourth-generation cephalosporins are indicated for empirical treatment of Aeromonas infections [36, 40, 41]. Nonetheless, the environmental spread of the blaKPC-positive isolates poses a worrying challenge to the treatment of waterborne infections thought to be produced by Aeromonas spp.
Resistance genes found in human pathogens are increasingly recognized in saprophytic GNB recovered from environmental matrices. Human infections caused by K. ascorbata are sporadically reported but include a variety of clinical presentations such as bacteremia, soft tissue infections, intra-abdominal abscesses, ventilator-associated pneumonia and urinary and biliary tract infections [3, 4, 42]. Differently, P. agglomerans is recognized as one of the most common saprophytic species involved in human infections, frequently resistant to cephalosporins [43]. It is of concern that broader resistance profiles have been detected in these saprophytes. Sporadic studies have already reported carbapenem-resistance genes blaNDM and blaVIM in clinical isolates of P. agglomerans [44, 45], as well as blaKPC gene in K. ascorbate [3, 42]. The spread of carbapenem resistance towards unusual Enterobacterales species is an issue of concern from the “one-health” standpoint. These bacteria, although commonly regarded as avirulent, can spread resistance genes among pathogenic and commensal species as well as among patients and in the environment [42].
Some research groups argue that CR-GNB isolates carried by hospital sewage are responsible for the contamination of aquatic matrices [12,13,14, 18, 46]. Differently, the emergence of CR-GNB isolates in aquatic matrices is also thought to occur due to the exposure to a variety of anthropogenic pollutants, including antibiotics and heavy metals [46,47,48]. Additionally, aquatic matrices would provide a conducive environment for the development of antibiotic resistance even when there are no hospitals nearby [28, 47]. Our results pointed out the impact of both the community and hospital settings on the spread of blaKPC-or-NDM strains through sewage. The frequency of blaKPC-or-NDM strains increased as a function of the number of sewer connections, hospitals and hospital admissions, and as a function of the occurrence of district hospitals located near the STPs. Some reports acknowledge that both the hospital settings and the community are important in the dissemination of antibiotic resistance [28].
The use of antibiotics is not restricted to the clinic or hospital settings. Antibiotics are also employed in intensive livestock farms, where antibiotics are used for disease treatment of animals and for animal growth promotion [49]. The usage of antibiotics in food-producing animals selects resistant bacteria and results in the presence of antibiotic residues in farming effluents. Therefore, the environment impacted by livestock farming has been regarded as reservoirs for resistant bacteria [46]. In our study, five STP’s (STP-3, 4, 10, 12 and 14) are located in areas where intensive livestock farms exist within a 3 km radius from them. However, specifically with regard to carbapenem resistance, the occurrence of blaKPC-or-NDM strains was not statistically associated with the presence of intensive livestock farms near STPs. Although Brazilian legislation has not completely banned antimicrobial growth promoters [50], the use β-lactans agents as animal growth promoters is prohibited in Brazil (Ministry of Agriculture, Livestock and Food Supply – Normative Ruling N° 26, July 9, 2009).
Studies worldwide have shown the resilience of CR bacteria subjected a variety of sewage treatments, including tertiary-level treatments followed by final disinfection steps [18, 21, 39, 51, 52]. In the United States, CR bacteria were recovered from 42% of STPs using chlorination for disinfection and from 12% of STPs using ultraviolet radiation [21]. In this study, 11% of the blaKPC-or-NDM strains were recovered from TS samples from STPs which apply diverse setups of tertiary treatments, such as UNITANK (STP-4), high-rate algal pond followed by overland flow (STP-6), high-rate algal pond followed by polishing pond and chemical polishing (STP-10) and activated sludge followed by chemical polishing (STP-13).
Moving beyond hospital settings, sewage and STPs, clinically relevant blaKPC-or-NDM CR-GNB strains have been isolated from recreational waters in other Brazilian cities such as Rio de Janeiro [23, 24, 38]. In this paper, strains of Klebsiella spp. Enterobacter spp. Aeromonas spp. and Citrobacter spp., all positive for blaKPC-or-NDM (n = 25), were recovered from 6 out of 14 sites downstream from the STPs. Among those, 8 strains were recovered from superficial waters of Lake Paranoá, downstream from STP-5 and STP-13 (Fig. 2). In Brasília, a variety of spots for recreational activities, including bathing, fishing, and sailing, are located along the lakeside of Paranoá Lake. Health implications of recreational exposure to CR-GNB remain uncertain and are scarcely explored. However, the ingestion of water during recreational activities is recognized as an exposure route for asymptomatic colonization of humans [53].
Despite the variety of blaKPC-or-NDM GNB recovered, we recognize some limitations in our study. The culture-dependent approach is prone to overlook part of the environmental microbiota, notably fastidious and underrepresented species. Therefore, our results possibly uncover only a fraction of the CR isolates carried by sewage.
Conclusions
Clinically relevant CR-GNB species (including Klebsiella spp., Enterobacter spp. and Aeromonas spp.) bearing resistance genes of public health concern (blaKPC or blaNDM) spread through sewerage and frequently prove to be resistant to sewage treatments, therefore remaining viable in the receiving water bodies. The presence of blaKPC-or-NDM strains in sewage is statistically correlated with variables linked to the community (number of sewer connections and occurrence of nearby hospitals) and to hospital settings (number of hospitalizations). Aquatic matrices, mainly those impacted by sewage, should be subjected to surveillance of difficult-to-treat antibiotic-resistant bacteria as a strategic measure against antibiotic resistance.
Methods
Sample collection
Three rounds of sewage and receiving water body sampling were carried out in April, July and August 2017 covering 14 sewage treatment plants (STPs), all located in Brasília. The profile of the regions serviced by the STPs as well as the burden on those plants represented by hospital and farming activities were displayed in Table 1. A total of 138 samples were collected, including sewage samples (n = 70) and water samples (n = 68). Sewage samples were collected in STPs directly from sewage inlet pipe [Raw Sewage (RS) - n = 35] and directly from treated sewage outlet pipe [Treated Sewage (TS) - n = 35]. Water samples were collected in receiving water bodies on spots located 50 m upstream [Upstream Water (UW) – n = 30] and 50 m downstream [Downstream Water (DW) – n = 38] from the point where sewage outlet pipe discharged the treated sewage. With regard to STP-13 and STP-5, the water body receiving the treated sewage is Lake Paranoá (Fig. 2). Given the absence of appreciable water streams on the lakeside, the water samples collected from the lake were all considered downstream water samples. STP-4 and STP-10 have contiguous treatment plants occupying an area of 1.1 km2 and discharge their effluents in adjacent spots located at the same water body (Melchior River). In order to evaluate the role of STP-4 and STP-10 in spreading antibiotic resistance into receiving water body, their results were then pooled. Samples were collected in sterile conical tubes (50 mL) during the mornings, preserved at room temperature in insulated boxes, and sent for culture at the Central Laboratory for Public Health (LACEN-DF) in the same work shift.
Selective culture for carbapenem-resistant (CR) gram-negative bacilli (GNB)
Five hundred microliters of each sample were cultured in Luria-Bertani broth supplemented with vancomycin [7.5 mg/L] and ertapenem [2.5 mg/L] at 36.5 °C for 24 h. Positive CR cultures were streaked on chromogenic and differential agar (ChromID® ESBL – bioMérieux) and incubated at 36.5 °C for 24 h for isolation and presumptive identification of Gram-negative bacilli (GNB). Three colonies of each presumptive bacterial group were isolated per sample limited to a maximum of 10 colonies per sample. Colonies were preserved into a semisolid nutrient medium (0.8% agar) stored in hermetically sealed tubes (3 mL) at room temperature and were kept safe from direct light exposure.
Species identification
The bacterial isolates were recovered and grown on Mueller Hinton agar for 24 h at 37 °C for obtaining isolated colonies. The identification was accomplished using Vitek MS system (Matrix-assisted laser desorption ionization time of flight mass spectrometry - MALDI-TOF MS system - BioMerieux) in accordance with the manufacturer’s instructions. Escherichia coli strain ATCC™ (American Type Culture Collection) 8739 was used as a positive control. The Myla® database was accessed for the identification of bacterial isolates. A confidence level greater than 80% was adopted for genus assignment and greater than 90% for species.
Resistance genotyping
Carbapenemase genes were detected by standard polymerase chain reactions (PCR). Supernatants derived from bacterial suspensions in deionized water and treated by boiling (100 °C for 15 min) were used as the source of DNA template. Primers were used to detect multiple alleles of carbapenemase genes (blaKPC, blaNDM, blaIMP, blaVIM and blaOXA-48) (supporting information Table 1 and supporting information Fig. 1) [7]. Multiplex PCR was applied to detect alleles of the gene blaVIM. Additionally, primers (supporting information Table 1) were designed to specifically detect blaKPC-harboring Tn4401 (based on the GenBank sequence CP039969.1) (supporting information Fig. 2). The forward primer (position: 83980..83999) targets the Tn4401 transposase gene ISKpn6, while the reverse primer (position: 84630..84614) recognizes the blaKPC locus located 250-bp downstream from ISkpn6.
Statistical analysis
Statistical analyses were performed using the IBM® SPSS® Statistics software (version 20). Non-parametric analyses were carried out with Fisher’s Exact Test (2-sided). Linear-by-linear associations of ordered categories were assessed with the Mantel-Haenszel test. With regard to continuous variables, non-parametric Mann-Whitney U-tests were performed to compare differences between independent groups. Results with p ≤ 0.05 were considered to be statistically significant.
Availability of data and materials
The datasets generated and analyzed during the current study are available in the Figshare repository, https://figshare.com/s/67b41b9021059b41ee6d.
Abbreviations
- CR:
-
Carbapenem-resistant
- MDR:
-
Multidrug-resistant
- GNB:
-
Gram-negative bacilli
- bla:
-
β-lactamase
- STP:
-
Sewage treatment plant
- RS:
-
Raw sewage
- TS:
-
Treated sewage
- UW:
-
Upstream water
- DW:
-
Downstream water
References
Talebi Bezmin Abadi A, Rizvanov AA, Haertlé T, Blatt NL. World Health Organization report: current crisis of antibiotic resistance. BioNanoScience. 2019;9:778–88. https://doi.org/10.1007/s12668-019-00658-4.
Lee C-R, Lee JH, Park KS, Kim YB, Jeong BC, Lee SH. Global dissemination of Carbapenemase-producing Klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol. 2016;7:895. https://doi.org/10.3389/fmicb.2016.00895.
Wang L, Jing Y, Lai K, An J, Yang J. A case of biliary tract infection caused by KPC-2-producing Kluyvera ascorbata. Case Rep Infect Dis. 2018;2018:1–2.
Lee J, Hwang J-H, Jo DS, Lee HS, Hwang J-H. Kluyvera ascorbata as a pathogen in adults and children: clinical features and antibiotic susceptibilities in a single center study. Jpn J Infect Dis. 2019;72:142–8. https://doi.org/10.7883/yoken.JJID.2018.375.
Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011;11:355–62.
Johnson AP, Woodford N. Global spread of antibiotic resistance: the example of New Delhi metallo-β-lactamase (NDM)-mediated carbapenem resistance. J Med Microbiol. 2013;62:499–513. https://doi.org/10.1099/jmm.0.052555-0.
Faria-Junior C, Rodrigues LD, Carvalho JO, Franco OL, Pereira AL. NDM-Producing enterobacteriaceae strains among hospitals in Brasília, Brazil. J Microbiol Exp. 2016;3. https://doi.org/10.15406/jmen.2016.03.00083.
AP C-A, Pereira PS, Albano RM, Berião GC, TPG C, Timm LN, et al. Isolation of NDM-producing Providencia rettgeri in Brazil. J Antimicrob Chemother. 2013;68:2956–7. https://doi.org/10.1093/jac/dkt298.
Monteiro J, Santos AF, Asensi MD, Peirano G, Gales AC. First report of KPC-2-producing Klebsiella pneumoniae strains in Brazil. Antimicrob Agents Chemother. 2009;53:333–4. https://doi.org/10.1128/AAC.00736-08.
Nagulapally SR, Ahmad A, Henry A, Marchin GL, Zurek L, Bhandari A. Occurrence of ciprofloxacin-, trimethoprim-sulfamethoxazole-, and vancomycin-resistant Bacteria in a municipal wastewater treatment plant. Water Environ Res. 2009;81:82–90. https://doi.org/10.2175/106143008x304596.
Taylor NGH, Verner-Jeffreys DW, Baker-Austin C. Aquatic systems: maintaining, mixing and mobilising antimicrobial resistance? Trends Ecol Evol. 2011;26:278–84.
Cahill N, O’Connor L, Mahon B, Varley Á, McGrath E, Ryan P, et al. Hospital effluent: a reservoir for carbapenemase-producing Enterobacterales? Sci Total Environ. 2019;672:618–24.
Koh TH, Ko K, Jureen R, Deepak RN, Tee NWS, Tan TY, et al. High counts of carbapenemase-producing enterobacteriaceae in hospital sewage. Infect Control Hosp Epidemiol. 2015;36:619–21. https://doi.org/10.1017/ice.2015.44.
Lamba M, Graham DW, Ahammad SZ. Hospital wastewater releases of Carbapenem-resistance pathogens and genes in urban India. Environ Sci Technol. 2017;51:13906–12. https://doi.org/10.1021/acs.est.7b03380.
Marathe NP, Berglund F, Razavi M, Pal C, Dröge J, Samant S, et al. Sewage effluent from an Indian hospital harbors novel carbapenemases and integron-borne antibiotic resistance genes. Microbiome. 2019;7:97. https://doi.org/10.1186/s40168-019-0710-x.
Subirats J, Royo E, Balcázar JL, Borrego CM. Real-time PCR assays for the detection and quantification of carbapenemase genes (Bla KPC, Bla NDM, and Bla OXA-48) in environmental samples. Environ Sci Pollut Res. 2017;24:6710–4. https://doi.org/10.1007/s11356-017-8426-6.
Miranda CC, de Filippis I, Pinto LH, Coelho-Souza T, Bianco K, Cacci LC, et al. Genotypic characteristics of multidrug-resistant Pseudomonas aeruginosa from hospital wastewater treatment plant in Rio de Janeiro. Brazil J Appl Microbiol. 2015;118:1276–86. https://doi.org/10.1111/jam.12792.
Picão RC, Cardoso JP, Campana EH, Nicoletti AG, Petrolini FVB, Assis DM, et al. The route of antimicrobial resistance from the hospital effluent to the environment: focus on the occurrence of KPC-producing Aeromonas spp. and Enterobacteriaceae in sewage. Diagn Microbiol Infect Dis. 2013;76:80–5.
Carlos de Lima Rocha A, Cynamon Kligerman D, Lopes da Mota Oliveira J. Overview of research on the treatment and reuse of effluents from the antibiotics industry Panorama da pesquisa sobre tratamento e reúso de efluentes da indústria de antibióticos. Saúde em Debate. 2019;3:165–80. https://doi.org/10.1590/0103-11042019S312.
Zagui GS, de Andrade LN, Moreira NC, Silva TV, Machado GP, da Costa Darini AL, et al. Gram-negative bacteria carrying β-lactamase encoding genes in hospital and urban wastewater in Brazil. Environ Monit Assess. 2020;192:1–11. https://doi.org/10.1007/s10661-020-08319-w.
Mathys DA, Mollenkopf DF, Feicht SM, Adams RJ, Albers AL, Stuever DM, et al. Carbapenemase-producing Enterobacteriaceae and Aeromonas spp present in wastewater treatment plant effluent and nearby surface waters in the US. Plos One. 2019;14:e0218650. https://doi.org/10.1371/journal.pone.0218650.
Chagas TPG, Seki LM, da Silva DM, Asensi MD. Occurrence of KPC-2-producing Klebsiella pneumoniae strains in hospital wastewater. J Hosp Infect. 2011;77:281.
Montezzi LF, Campana EH, Corrêa LL, Justo LH, Paschoal RP, Da Silva ILVD, et al. Occurrence of carbapenemase-producing bacteria in coastal recreational waters. Int J Antimicrob Agents. 2015;45:174–7.
Paschoal RP, Campana EH, Corrêa LL, Montezzi LF, Barrueto LRL, Da Silva IR, et al. Concentration and variety of carbapenemase producers in recreational coastal waters showing distinct levels of pollution. Antimicrob Agents Chemother. 2017;61. https://doi.org/10.1128/AAC.01963-17.
Brazilian Ministry of Regional Development. National System for Information on Sanitation - Assessment of Water and Sewage Services. 2018 [WWW document]. URL http://www.snis.gov.br/downloads/diagnosticos/ae/2018/Diagnostico_AE2018.pdf. http://www.snis.gov.br/downloads/diagnosticos/ae/2018/Diagnostico_AE2018.pdf. Accessed 25 Nov 2020.
Mao K, Zhang K, Du W, Ali W, Feng X, Zhang H. The potential of wastewater-based epidemiology as surveillance and early warning of infectious disease outbreaks. Curr Opin Environ Sci Health. 2020;17:1–7.
Sims N, Kasprzyk-Hordern B. Future perspectives of wastewater-based epidemiology: monitoring infectious disease spread and resistance to the community level. Environ Int. 2020;139:105689.
Adegoke AA, Fatunla OK, Okoh AI. Critical threat associated with carbapenem-resistant gram-negative bacteria: prioritizing water matrices in addressing total antibiotic resistance. Ann Microbiol. 2020;70:43. https://doi.org/10.1186/s13213-020-01579-4.
Baier C, Pirr S, Ziesing S, Ebadi E, Hansen G, Bohnhorst B, et al. Prospective surveillance of bacterial colonization and primary sepsis: findings of a tertiary neonatal intensive and intermediate care unit. J Hosp Infect. 2019;102:325–31. https://doi.org/10.1016/j.jhin.2019.01.021.
Tian L, Sun Z, Zhang Z. Antimicrobial resistance of pathogens causing nosocomial bloodstream infection in Hubei Province, China, from 2014 to 2016: a multicenter retrospective study. BMC Public Health. 2018;18:1121. https://doi.org/10.1186/s12889-018-6013-5.
Turner PJ. MYSTIC Europe 2007: activity of meropenem and other broad-spectrum agents against nosocomial isolates. Diagn Microbiol Infect Dis. 2009;63:217–22. https://doi.org/10.1016/J.DIAGMICROBIO.2008.11.004.
Nordmann P, Cornaglia G. Carbapenemase-producing Enterobacteriaceae: a call for action! Clin Microbiol Infect. 2012;18:411–2.
Kelly AM, Mathema B, Larson EL. Carbapenem-resistant Enterobacteriaceae in the community: a scoping review. Int J Antimicrob Agents. 2017;50:127–34. https://doi.org/10.1016/J.IJANTIMICAG.2017.03.012.
Sinclair HA, Heney C, Sidjabat HE, George NM, Bergh H, Anuj SN, et al. Genotypic and phenotypic identification of Aeromonas species and CphA-mediated carbapenem resistance in Queensland, Australia. Diagn Microbiol Infect Dis. 2016;85:98–101.
Queenan AM, Bush K. Carbapenemases: the versatile β-lactamases. Clin Microbiol Rev. 2007;20:440–58. https://doi.org/10.1128/CMR.00001-07.
Rosso F, Cedano JA, Parra-Lara LG, Sanz AM, Toala A, Velez JF, et al. Emerging carbapenem-resistant Aeromonas spp. infections in Cali, Colombia. Brazilian J Infect Dis. 2019;23:336–42. https://doi.org/10.1016/j.bjid.2019.08.005.
Hayes MV, Thomson CJ, Amyes SGB. The “hidden” carbapenemase of Aeromonas hydrophila. J Antimicrob Chemother. 1996;37:33–44. https://doi.org/10.1093/jac/37.1.33.
Araujo CFM, Silva DM, Carneiro MT, Ribeiro S, Fontana-Maurell M, Alvarez P, et al. Detection of carbapenemase genes in aquatic environments in Rio de Janeiro. Brazil Antimicrob Agents Chemother. 2016;60:4380–3. https://doi.org/10.1128/AAC.02753-15.
Rodríguez EA, Garzón LM, Gómez ID, Jiménez JN. Multidrug resistance and diversity of resistance profiles in carbapenem-resistant gram-negative bacilli throughout a wastewater treatment plant in Colombia. J Glob Antimicrob Resist. 2020;22:358–66.
Hiransuthikul N, Tantisiriwat W, Lertutsahakul K, Vibhagool A, Boonma P. Skin and soft-tissue infections among tsunami survivors in southern Thailand. Clin Infect Dis. 2005;41:e93–6. https://doi.org/10.1086/497372.
Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010;23:35–73. https://doi.org/10.1128/CMR.00039-09.
Geffen Y, Adler A, Paikin S, Khabra E, Gorenshtein S, Aronov R, et al. Detection of the plasmid-mediated KPC-2 carbapenem-hydrolysing enzyme in three unusual species of the Enterobacteriaceae family in Israel. J Antimicrob Chemother. 2013;68:719–20. https://doi.org/10.1093/jac/dks443.
Raphael E, Riley LW. Infections caused by antimicrobial drug-resistant saprophytic Gram-negative bacteria in the environment. Front Med. 2017;4 OCT:183. https://doi.org/10.3389/fmed.2017.00183.
Arbizú-Medina O, García-Rosales K, Cerda-Aragón H, Martínez-García W, Pérez-Martínez A, Lanzas-Baca Y, et al. New Delhi Metallo-β-lactamase in Enterobacteriaceae species isolated from hospitalized patients, Managua Nicaragua work done in the molecular biology laboratory MA. 2018.
Okoche D, Asiimwe BB, Katabazi FA, Kato L, Najjuka CF. Prevalence and characterization of Carbapenem-resistant Enterobacteriaceae isolated from Mulago National Referral Hospital, Uganda. Plos One. 2015;10:e0135745. https://doi.org/10.1371/journal.pone.0135745.
Kraemer SA, Ramachandran A, Perron GG. Antibiotic pollution in the environment: from microbial ecology to public policy. Microorganisms. 2019;7:180. https://doi.org/10.3390/microorganisms7060180.
Khan GA, Berglund B, Khan KM, Lindgren P-E, Fick J. Occurrence and abundance of antibiotics and resistance genes in rivers, canal and near drug formulation facilities – a study in Pakistan. Plos One. 2013;8:e62712. https://doi.org/10.1371/journal.pone.0062712.
Xu Y, Wang X, Tan L, Mao D, Luo Y. Metal impacts on the persistence and proliferation of β-lactam resistance genes in Xiangjiang river. China Environ Sci Pollut Res. 2019;26:25208–17. https://doi.org/10.1007/s11356-019-05698-7.
Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci U S A. 2015;112:5649–54. https://doi.org/10.1073/pnas.1503141112.
Maron DF, Smith TJS, Nachman KE. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Global Health. 2013;9:48. https://doi.org/10.1186/1744-8603-9-48.
Zhang L, Ma X, Luo L, Hu N, Duan J, Tang Z, et al. The prevalence and characterization of extended-Spectrum β-lactamase- and Carbapenemase-producing Bacteria from hospital sewage, treated effluents and receiving rivers. Int J Environ Res Public Health. 2020;17:1183. https://doi.org/10.3390/ijerph17041183.
Yang F, Mao D, Zhou H, Luo Y. Prevalence and fate of Carbapenemase genes in a wastewater treatment plant in Northern China. Plos One. 2016;11:e0156383. https://doi.org/10.1371/journal.pone.0156383.
Leonard AFC, Zhang L, Balfour AJ, Garside R, Gaze WH. Human recreational exposure to antibiotic resistant bacteria in coastal bathing waters. Environ Int. 2015;82:92–100.
Acknowledgements
Not Applicable.
Funding
This work was supported by the Fundação de Apoio à Pesquisa do Distrito Federal (FAP-DF) with the grant No 193.000.713/2016.
Author information
Authors and Affiliations
Contributions
ALP wrote the manuscript. ALP and CFJ conceived the study and designed the experiments. ALP, WNA, RH and TACL were responsible for concepts, vision, and direction of the study. ALP and PMO performed genotyping experiments and analyzed the data. CFJ, PMO, EGA, GRCCL carried out the isolation and identification of the strains. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pereira, A.L., de Oliveira, P.M., Faria-Junior, C. et al. Environmental spreading of clinically relevant carbapenem-resistant gram-negative bacilli: the occurrence of blaKPC-or-NDM strains relates to local hospital activities. BMC Microbiol 22, 6 (2022). https://doi.org/10.1186/s12866-021-02400-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-021-02400-1