Abstract
Background
This study aimed to determine the frequency of methicillin-resistant Staphylococcus aureus (MRSA), antibiotic resistance patterns, superantigenic toxins profile, and clonality of this pathogen in patients with cancer.
Results
In total, 79 (25.7%) isolates were confirmed as Staphylococcus species, from which 38 (48.1%) isolates were S. aureus, and 29 (76.3%) isolates were confirmed as MRSA. The highest resistance in MRSA strains was seen against ciprofloxacin (86.2%) and erythromycin (82.8%). Teicoplanin, and linezolid were the most effective antibiotics. From all MRSA isolates, 3 strains (10.3%) were resistant to vancomycin with minimum inhibitory concentration values of 128 μg/ml. The prevalence of superantigenic toxins genes was as follows: pvl (10.5%), tsst-1 (36.8%), etA (23.7%), and etB (23.7%). The t14870 spa type with frequency of 39.5% was the most prevalent clone type circulating in the cancer patients.
Conclusions
This study showed the circulating of spa t14870 as the most predominant MRSA clone in cancer patients of southwest Iran. Also, a diverse antibiotic resistance pattern and toxin profiles were seen among MRSA isolates.
Similar content being viewed by others
Background
Staphylococcus aureus is one of the most important pathogens in both hospital- and community- acquired infections with significant patient morbidity and mortality rate worldwide [1]. S. aureus as a potential pathogen causes various infections including superficial and deep skin infections, soft tissue infections, bacteremia, endocarditis, osteomyelitis, pneumonia, toxic- shock syndrome, and staphylococcal scaled skin syndrome [2]. Over the past several decades, following the introduction of antibiotics, emergence and dissemination of methicillin resistant S. aureus (MRSA) remains a challenging public health issue [3]. MRSA strains characterized by the presence of the mecA gene, which is located on the staphylococcal chromosomal cassette mec (SCCmec type I - XI) as a mobile genetic element and encoding low-affinity penicillin binding protein (PBP2-a) have resistance to multiple antibiotics including penicillin and other beta-lactams [1, 4].
Both MRSA and methicillin-sensitive S. aureus (MSSA) strains produce several virulence factors and toxins that have a significant role in the pathogenicity of the diseases and notably that some of them have superantigenic properties [5]. S. aureus superantigens (SAgs) are known as the most potent T cell mitogens. They stimulate large subpopulations of T lymphocytes via cross-linking their T cell receptor with major histocompatibility complex class-II molecules (MHC-II) on antigen presenting cells, resulting in T cell and B cell proliferation and massive cytokine release which may lead to systemic shock [6]. To date, more than 26 different superantigens have been described in the S. aureus species; comprising the toxic shock syndrome toxin (TSST-1), exfoliative toxins (ETs), Panton-Valentine leukocidin (PVL), 11 staphylococcal enterotoxins (SEs), 14 enterotoxin-like proteins (SEls) as well as staphylococcal protein A (spA); that is the only known B cell superantigen produced by S. aureus [6, 7].
The capability of MRSA to cause a broad range of human diseases is based on combination of host factors and bacterial virulence factors [2]. There are several risk factors that cause patients at higher risk for acquisition infections with MRSA including age, length of hospitalization, initial antibiotic treatment, severe underlying disease, surgery and compromised host immunity [2, 6]. Particularly, cancer patients due to impaired cellular immunity, are very prone to serious infections with significant complications particularly with multidrug resistant bacteria (MDRB), and they have multiple predisposing factors that increase the risk of infection; including chemotherapy, radiation therapy, surgery, stem cell transplantation, bone marrow transplantation [8].
Emergence of antimicrobial resistance among MRSA associated with infections in cancer patients is particular concern in recent years. In addition, only limited data are available for the MRSA distribution, antibiotic resistance patterns, and prognosis of these infections in oncological patients [9]. Consequently, newer therapeutic approaches, required to be developed based on local epidemiologic and susceptibility/resistance data for infection prevention, control, and antimicrobial stewardship are important in the overall management of infections in cancer patients [10].
Therefore, this research was conducted for the first time on cancer patients hospitalized in Ahvaz Oncology Center to determine the resistance patterns, and superantigenic toxins profiles (etA, etB, tsst-1, and pvl) in MRSA isolates collected from cancer patients. Also, the profile of Staphylococcus aureus protein A (spa) types was investigated in the isolates.
Methods
Sampling and data collection
This cross-sectional study was performed during 16 months from February 2018 to March 2019. In total, 307 bacterial isolates were obtained from various clinical specimens of hospitalized cancer patients referred to Shahid Baghaei educational hospital, one of the main referral centers for oncologic disorders in Ahvaz, Khuzestan Province, southwest of Iran. These isolates were recovered from blood, wound, tracheal tube secretions, eyes, ears, urine, abscess, and cerebrospinal fluid (CSF). The collected isolates were transferred to the Department of Microbiology of Ahvaz Jundishapur University of Medical Sciences. These isolates were identified by conventional microbiological methods. In brief, bacterial isolates were subcultured on Blood Agar (Merck, Germany) plates and incubated at 37°C for 24 h. Finally, they were identified as staphylococci strains using phenotypic laboratory methods such as colony morphology, Gram stain, and conventional biochemical tests including catalase test, mannitol fermentation, DNase production, and plasma coagulase reaction [11]. The S. aureus ATCC 29213 strain was used as the reference strain. All phenotypically identified staphylococci were stored in Trypticase Soy Broth (TSB; Merck, Germany) containing 20% glycerol and were kept at −70°C for further molecular investigation.
Confirmation of S. aureus and coagulase-negative staphylococci (CoNS) isolates by polymerase chain reaction (PCR)
The 16S rRNA (specifically detecting Staphylococcus species) and nuc (distinguishing S. aureus from CoNS) genes amplification was done by a multiplex-polymerase chain reaction (M-PCR) using sets of designed primers as previously described (Table 1) [12].
Methicillin-resistant S. aureus and methicillin-resistant coagulase-negative staphylococci (MRCoNS) screening
MRSA and MRCoNS isolates were identified with cefoxitin disc (30 μg) on Mueller Hinton Agar (MHA, Merck, Germany) plates in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines [13]. Finally, the resistant isolates were confirmed as MRSA and MRCoNS using PCR for amplification of mecA gene according to previous study (Table 1) [14]. The S. aureus ATCC 33591 and ATCC 25923 were used as the positive and negative controls for the mecA gene, respectively.
Antimicrobial susceptibility testing of Staphylococcus isolates
In vitro antimicrobial susceptibility testing determined by Kirby-Bauer disk diffusion according to the CLSI guidelines [13]. The 10 antimicrobial disks tested included: cefoxitin (FOX, 30 μg), clindamycin (CD, 2 μg), ciprofloxacin (CIP, 5 μg), cotrimaxazole (TS, 25 μg), rifampicin (RP, 5 μg), teicoplanin (TEC, 30 μg), tetracycline (T, 10 μg), erythromycin (E, 15 μg), quinupristin/dalfopristin (SYN, 15 μg), and linezolid (LZD, 30 μg) [MAST Diagnostics, Merseyside UK].
.Also, vancomycin susceptibility testing was performed by vancomycin agar screen method with brain heart infusion agar containing 6 μg/ml. Finally, the minimum inhibitory concentration (MIC) of vancomycin was determined with the broth microdilution method according to CLSI 2016 instructions [13]. The vancomycin powder was purchased from Sigma (USA) Company. S. aureus ATCC 29213 and ATCC 33591 were used as methicillin-sensitive and resistant control strains, respectively.
Detection of superantigenic toxin genes (etA, etA, tsst-1, and pvl)
Extraction of genomic DNA
Genomic DNA was extracted from pure colonies of MRSA strains using the boiling method as described previously [15]. A Biophotometer (Eppendorf, Germany) at 260 nm, was used to measure the concentration of DNA. The extracted DNA were stored at -20 ° C for further molecular investigation.
PCR protocol
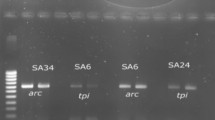
Several PCR amplifications were carried out for the detection of superantigenic toxin genes using specific primers based on etA, etB, tsst-1, and pvl genes as previously described [4, 16]. The sequences of primers are listed in Table 1. The synthesis of primers was carried out by the TAG Copenhagen Company, Denmark. The M-PCR was optimized on an Eppendorf thermocycler (Roche Co., Germany), in a final volume of 25 μl containing 12.5 μl of PCR Master Mix, 0.4 mM (1 μl) of each forward and reverse primers, 5 μl of DNA template, and 9.5 μl of nuclease-free water. The amplicons products were analyzed using an ultraviolet gel documentation device (Protein Simple, San Jose, CA, USA), after loading in the gel electrophoresis tank on a 1.5% agarose gel (w/v) with 2 μl safe stain (Sinaclon Co., Tehran, Iran).
Staphylococcal protein a (spa) typing
The spa typing was performed for MRSA and MSSA strains with some modification by PCR as previously described [17]. The polymorphic X regions of the spa gene from the extracted chromosomal DNAs were amplified using the primers spa 1113F (5′-TAAAGACGATCCTTCGGTGAGC-3′) and spa-1514R (5′-CAGCAGTAGTGCCGTTTGCTT-3′). The variable X region of the spa gene was amplified in a 25 μl reaction volume and optimized on an Eppendorf thermo-cycler (Roche Co., Berlin, Germany). The PCR mixture contained 12.5 μl 1.5×TaqMaster Mix, 2 μl of each spa primers (10 μM), 1 μl chromosomal DNA template and 7.5 μl double distilled water. The PCR conditions were as follow: initial denaturation at 94 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 30s, annealing at 60 °C for 60s, and extension at 72 °C for 30s, and a final extension at 72 °C for 10 min. The amplicons products were subjected to DNA sequencing for both strands by Macrogen Seoul, South Korea). The sequences obtained were edited using Chromas software (version 1.45, Australia). The Ridom Spa Server database (http://www.spaserver.ridom.de) (Ridom, Wurzburg, Germany) was used to assign the edited sequences to particular spa types [17].
Statistical analysis
Data were analyzed using SPSS software version 25 (IBM Corporation, Armonk, NY, USA). The Chi-square test and Fisher’s exact test was used for the association between the studied genes and the presence of MRSA strains. A p-value < 0.05 was considered statistically significant.
Result
Studied isolates
In current study, out of the 307 clinical bacterial isolates, 79 (25.7%) isolates were confirmed as Staphylococcus species and the rest of the 228 isolates (74.3%) were Gram-negative bacteria based on culture and standard biochemical criteria. All Staphylococcus isolates were evaluated for the presence of nuc and 16S rRNA genes to confirm and select the S. aureus isolates. According to PCR analysis, from 79 Staphylococcus isolates, 38 (48.1 %) were molecularly confirmed as S. aureus by the presence of 16S rRNA and nuc genes, while the remaining 41 isolates (51.9 %) were different coagulase-negative staphylococci (CoNS) species. The isolates were collected from 35 male (44.3 %) and 44 female patients (55.7 %), respectively. There was no statistically significant difference in gender distribution (P = 0.75). The total distribution of 79 Staphylococcus isolates in various types of clinical specimens was as follows: blood 64 (81 %), urine 7 (8.9 %), wound 5 (6.3 %) and catheters 3 (3.8 %). The distribution of isolated Staphylococcus species according to the hospital wards was as follows: hematology intensive care unit (HICU) 31 (39.3 %), transplantation and bone marrow transplantation (BMT) 12 (15.2%), pediatric 17 (21.5 %), women 11 (13.9 %), men 5 (6.3 %), and thalassemia unit 3 (3.8 %).
MRSA and MRCoNS screening
The results of cefoxitin test and mecA PCR amplification confirmed 76.3% (29/38) of S. aureus strains as MRSA, while the 9 (23.7 %) remaining isolates were considered as methicillin-sensitive S. aureus (MSSA). Also, among the CoNS species, 65.9% (27/41) and 34.1% (14/41) of isolates were confirmed as MRCoNS and methicillin-sensitive CoNS (MSCoNS), respectively. The both methods (cefoxitin test and mecA PCR) showed similar results. The prevalence rate of MRSA in different clinical specimens was as follows: blood samples (n=22/29, 75.9%), catheters and urine each one (n =3/29, 10.3%), and wound (n=1/29, 3.5%). The most frequency of MRSA isolates (75.9%) were seen in blood samples. The highest prevalence of MRSA isolates was seen in HICU with 41.5% (n=12/29) rates. Although the prevalence of MRSA strains was higher in blood culture samples (91.7 %, n = 11/12), but no significant difference was observed in the frequency of strains in different cancer patients admitted to HICU (P > 0.05). The frequency of MRSA in other wards was as follows: pediatric and BMT: (17.2%, n=5/29 for each wards), women and men wards (10.3%, n=3/29 for each wards), and thalassemia unit (3.5%, n=1/29).
Antimicrobial susceptibility testing of S. aureus and CoNS
The susceptibility patterns of total 79 Staphylococcus isolates demonstrated highly resistant to erythromycin (77.1%) and cefoxitin (70.8%). The linezolid with susceptibility rate of 80%, was the most effective antibiotic. The resistance rates of all Staphylococcus isolates are shown in Fig. 1.
The susceptibility patterns of MRSA strains against 10 various antibiotics are presented in Table 2. A high rate of resistance was detected against ciprofloxacin (n=25/29, 86.2 %), erythromycin (n=24/29, 82.8 %), and clindamycin (n=20/29, 69 %). The resistance to clindamycin was constitutive phenotype and no inducible phenomenon was detected. MRSA isolates had the lowest prevalence of resistance against teicoplanin and linezolid (n=3/29, 10.4% for each one). From a total of 29 MRSA isolates, 3 strains (10.3 %) were resistant to vancomycin using vancomycin agar screen test. Subsequently, the broth microdilution assay confirmed that these isolates had MIC values of 128 μg/ml (Table 3). All VRSA strains were isolated from blood culture of hospitalized patients in the ICU. They were susceptible to linezolid. The resistance rates of different antibiotics against CoNS isolates were as follows: erythromycin 90.2%, clindamycin 70.7 %, cefoxitin 65.9 %, tetracycline 48.2 %, rifampicin 46.3 %, ciprofloxacin 46.3 %, cotrimoxazole 39.1 %, teicoplanin 12.2 %, and linezolid 9.8%.
Detection of etA, etB, tsst-1, and pvl genes
A total of 38 S. aureus isolates were subjected to superantigenic toxin genes etA, etB, tsst-1, and pvl analysis. Twenty isolates (n=20/38, 52.6%) harbored at least one superantigenic toxin genes. Distribution of these genes was as follows: 36.8% (n=14/38), 23.7% (n=9/38), 23.7% (n=9/38), and 10.5% (n=4/38) for tsst-1, etA, etB, and pvl, respectively. Finally, 17 (n=17/29, 58.6%) of MRSA and 3 (n=3/9, 33.3%) of MSSA isolates produced one of these toxins. Also, 13.8 % of MRSA isolates, (n=4/29) had 3 tsst-1, etA and etB genes, simultaneously. Also, prevalence rate of concurrent tsst-1 and etA genes in MRSA isolates were 3.4 % (n=1/29). The tsst-1 and etB genes were simultaneously detected in 10.3 % (n=3/29). Also, no association was found among the presence of superantigenic toxins and antibiotic resistance (data not shown).
Spa typing
According to spa typing in this study, 12 different spa types were observed among the 38 S. aureus strains. The spa type t14870 was the most common type (39.5%) among the isolates. According to statistical tests, there was no significant difference in the distribution of spa types with different hospital wards and clinical specimens. Most of the samples in this study were blood and the most common type t14870 was isolated from these samples (n=13/38, 34.2%). There was no significant relationship between the distribution of different types and sample type (P = 0. 72). Also, among the isolates that were positive for pvl, tsst-1, etA, etB, and mec genes, the type t14870 was the most prevalent compared to all other types. Two VRSA isolates had spa type t14870 and one of them had spa type t030. The distribution of different spa types among Staphylococcus isolates is shown in Table 4.
Discussion
The circulation of MRSA in health systems and community leads to increase of the costs of antibiotic therapy and limits the cure options. During the recent decades, there have been extensive studies on the resistance pattern of MRSA strains [18]. It has been observed that some MRSA clones spread very rapidly in different regions [19]. Hence, the determination of the regional pattern of resistance is helpful in selecting the appropriate drug. The current study was the first that performed to investigate the resistance patterns and molecular epidemiology of MRSA common clones among cancer patients in the Ahvaz city, southwest region of Iran. So far, no epidemiologic data about MRSA strains and their different clone types have been reported in cancer patients in aforesaid area.
Recent data from several cancer centers/organizations indicated the CoNS strains as the predominant Gram-positive pathogens isolated from various infections in cancer patients [20, 21]. The current research in agreement with previous studies showed the CoNS species as the most common Gram-positive bacteria with frequency of 51.9 % (n = 41/79). However, the total frequency rate of CoNS with 13.4 % (n = 41/307) was much lower than a study by Garcia-Vidal et al. [21] from Spain who reported an incidence of 35.7% for these species. In another study by Islas-Muñoz et al. [20] from Mexico, the frequency rate of CONS isolates (11 %) was almost close to our result. CoNS isolates showed the most resistance rates (more than 60 %) against erythromycin and clindamycin. Similar to the current research, Ghadiri et al. [22] from Tehran, Iran, and Fahim et al. [23] from Egypt reported a high resistance rate against erythromycin and clindamycin.
The current study showed the total prevalence of 9.4 % (n = 29/307) for MRSA in the cancer patients that was lower than several previous studies from Japan 77.8% [24], Egypt 70% [25], Libya 35.5% [26], France 44.4% [27], and Kerman city in Iran 28% [28]. Besides, the total prevalence of 8.8 % for MRCoNS isolates in this study was lower than the total incidence rate of a study by Fentie et al. [29] who revealed a prevalence of 11.6 %. These dissimilarities of the results of various studies may be due to the differences in studied sample size, epidemiology of the study area, the bacterial detection methods, and the type of studied samples. In this research, the prevalence of MRSA strains in HICU ward (41.5%) was higher than other wards that was in line with the study by Srinivasan et al. [30] who reported the highest frequency of MRSA in the ICU ward (15%) of the hospital.
In the current research, the results of disk diffusion method showed the highest resistance rate of MRSA isolates against ciprofloxacin (86.2%), erythromycin (82.8%), and clindamycin (69%). The resistance rate of > 50 % against ciprofloxacin, erythromycin, and clindamycin was in parallel with the results of Bai et al. [31] from China. Also, in their study the resistance rate of cotrimoxazole (37%) was almost close to our result. In our studied region, the lowest resistance rates were seen against teicoplanin and linezolid that was in line with several previous reports from Iran and other countries [31,32,33,34]. In this study, 3 (10.3 %) MRSA isolates were resistant to vancomycin. The overall vancomycin-resistant Staphylococcus aureus (VRSA) prevalence was 7.9 % (3/38) that was meaningly different from its global occurrence (2.4 %) [35]. More frequent use of vancomycin for treatment of MRSA infections, improved diagnostic methods, inadequate surveillance for drug-resistant strains, a possible change in the vancomycin-resistance breakpoints since 2006, and the increased use of antibiotics as a food supplement are all possible reasons for the emergence or detection of more VRSA strains in recent years [35].
Another notable finding in the current study was the high resistance rate of MRSA to ciprofloxacin, which may be due to the increased use of fluoroquinolone antibiotics as prophylaxis to prevent infection in neutropenic cancer patients [36]. Overall, the results emphasize that a new surveillance program should be designed to determine the correct pattern of antibiotic use for cancer patients in the study area. Also, the indiscriminate use of ineffective antibiotic classes such as quinolones that may lead to Clostridioides difficile selection should be avoided [37].
To date, very little information is available on the prevalence of S. aureus superantigen genes in cancer patients, especially in Iran country. The findings of the current experiment showed that 58.6 % of MRSA isolates had at least one toxin genes that was much lower than a study by Jaradat et al. [38] from Jordan who showed 100 % of isolates harbored at least one toxin gene. In this study the prevalence of pvl, tsst-1, etA, and etB superantigenic toxins in S. aureus strains were 10.5 %, 36.8 %, 23.7 %, and 23.7 % respectively. Campo et al. [39] from USA reported frequency rate of 40 % for pvl gene in MRSA strains in cancer patients. The frequency rates of pvl gene in other studies that were performed in non-cancer patients were as follows: Iran 26.3 % [4], Jordan 100 % [38], China 45.2 % [40], and Germany 6.2 % [41]. Also, the prevalence of the tsst-1 gene in the previous studies in non-cancer patients was as follows: Iran 32.6 % [4], Jordan 29.5 % [38], and Greece 3.5 % [42]. The frequency rate of etA gene in this research was lower than a report by Salas et al. [43] from Spain (71.3 %) and Jaradat et al. [38] from Jordan (42%). However, the detection rate of etB gene was so higher than Jaradat et al. [38] from Jordan and Horváth et al. [44] from Hungary who reported 0.0 % and 1.3 % of prevalence for aforesaid gene, respectively. While the frequency rates of two etA and etB genes were similar in our research, some studies reported the more frequency of etA compared to etB [43, 45]. According to this study, the incidence of superantigen genes showed that these virulence factors play an efficient role in MRSA infections in the cancer patients.
In this study, 12 different spa types were found that t14870, t386, t030, and t1671 with frequency rates of 39.5 %, 7.8%, 5.2%, and 5.2% were the most prevalent types, respectively. There was no significant association between patterns of spa gene with different parts of hospital and clinical samples (P value > 0.05). In Iran, many studies have been done on the typing of S. aureus strains. In a previous study from Tehran, Iran the t030 and t037 spa types accounted for 43% of all 11 different detected types [46]. In contrast to previous study from southwest of Iran by Hashemizadeh et al. [47] who showed the t030 as the most prevalent spa type among S. aureus isolates, this study revealed the t14870 as the most circulating spa type in the cancer patients. These discrepancies may be due to the study population, the type of sample being tested, and the pattern of used antibiotics that has enabled species with greater adaptability to form the dominant population. All different spa types that were detected in this research have been reported previously from Iran and other countries [17, 46,47,48]. Although, the spa type t14870 was previously reported in Iran and other regions, the current study was the first report for this spa type in cancer patients in southwest Iran. In line with the current study, Faramand et al. [49] reported the t14870 as the most prevalent spa from S. aureus isolated from raw beef and chicken meat samples.
Conclusions
This study showed a high rate of MRSA circulation in cancer patients from southwest of Iran. Also, the tsst-1 and etA were the most prevalent virulence genes. This study indicated a high prevalence of t14870 MRSA isolates in cancer patients from southwest of Iran. Moreover, this investigation showed a relatively high resistance rate against ciprofloxacin, erythromycin, and clindamycin among MRSA isolates and these antibiotics should be used with extreme caution based on the results of antibiogram tests. It is recommended that regular monitoring programs be among the priorities of health policy makers in order to reduce the faster spread of resistant strains in the population of cancer patients and reduce the resulting mortality.
Availability of data and materials
Not applicable.
Abbreviations
- BMT:
-
Bone Marrow Tranplantation
- CSF:
-
Cerebrospinal Fluid
- MRCoNS:
-
Methicillin-Resistant Coagulase-Negative Staphylococci
- MRSA: MSSA:
-
Methicillin-Sensitive Staphylococcus aureus; Methicillin-Resistance Staphylococcus aureus
- PVL:
-
Panton-Valentine leukocidin
- PCR:
-
Polymerase Chain Reaction
- spa :
-
Staphylococcal protein A
References
Garbacz K, Wierzbowska M, Kwapisz E, Kosecka-Strojek M, Bronk M, Saki M, et al. Distribution and antibiotic-resistance of different Staphylococcus species identified by matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) isolated from the oral cavity. J Oral Microbiol. 2021;13(1):1983322.
Li Z, Zhuang H, Wang G, Wang H, Dong Y. Prevalence, predictors, and mortality of bloodstream infections due to methicillin-resistant Staphylococcus aureus in patients with malignancy: systemic review and meta-analysis. BMC Infect Dis. 2021;21:74.9.
Turner NA, Sharma-Kuinkel BK, Maskarinec SA, Eichenberger EM, Shah PP, Carugati M, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019;17(4):203–18.
Rashidi Nezhad R, Meybodi SM, Rezaee R, Goudarzi M, Fazeli M. Molecular characterization and resistance profile of methicillin resistant Staphylococcus aureus strains isolated from hospitalized patients in intensive care unit, Tehran-Iran. Jundishapur J Microbiol. 2017;10(3):e41666.
Rahimi F, Katouli M, Karimi S. Biofilm production among methicillin resistant Staphylococcus aureus strains isolated from catheterized patients with urinary tract infection. Microb Pathog. 2016;98:69–76.
Abdurrahman G, Schmiedeke F, Bachert C, Bröker BM, Holtfreter S. Allergy-a new role for T cell superantigens of Staphylococcus aureus? Toxins. 2020;12(3):176.
Tam K, Torres VJ. Staphylococcus aureus secreted toxins and extracellular enzymes. Microbiol Spectr. 2019;7(2):https://doi.org/10.1128/microbiolspec. GPP3-0039-2018.
Jiang AM, Shi X, Liu N, Gao H, Ren MD, Zheng XQ, et al. Nosocomial infections due to multidrug-resistant bacteria in cancer patients: a six-year retrospective study of an oncology Center in Western China. BMC Infect Dis. 2020;20(1):1–2.
Perdikouri EI, Arvaniti K, Lathyris D, Apostolidou Kiouti F, Siskou E, Haidich AB, et al. Infections due to multidrug-resistant bacteria in oncological patients: insights from a five-year epidemiological and clinical analysis. Microorganisms. 2019;7(9):277.
Cornejo-Juárez P, Vilar-Compte D, García-Horton A, López-Velázquez M, Ñamendys-Silva S, Volkow-Fernández P. Hospital-acquired infections at an oncological intensive care cancer unit: differences between solid and hematological cancer patients. BMC Infect Dis. 2016;16:274.
Tille P. Bailey & Scott’s diagnostic microbiology-E-book. St. Louis: Elsevier Health Sciences; 2015. p. 307–27.
McClure JA, DeLongchamp JZ, Conly JM, Zhang K. Novel multiplex PCR assay for detection of chlorhexidine-quaternary ammonium, mupirocin, and methicillin resistance genes, with simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J Clin Microbiol. 2017;55(6):1857–64.
CLSI, editor. Editor. Performance standards for antimicrobial suscepetibility testing, 26th ed. CLSI supplement M100. Clinical and Laboratory Standards Institute: Wayne; 2016.
McClure JA, Conly JM, Lau V, Elsayed S, Louie T, Hutchins W, et al. Novel multiplex PCR assay for detection of the staphylococcal virulence marker Panton-valentine leukocidin genes and simultaneous discrimination of methicillin-susceptible from-resistant staphylococci. J Clin Microbiol. 2006 Mar;44(3):1141.
Khosravi AD, Jenabi A, Montazeri EA. Distribution of genes encoding resistance to aminoglycoside modifying enzymes in methicillin-resistant Staphylococcus aureus (MRSA) strains. Kaohsiung J Med Sci. 2017;33(12):587–93.
Bhatta DR, Cavaco LM, Nath G, Kumar K, Gaur A, Gokhale S, et al. Association of Panton valentine Leukocidin (PVL) genes with methicillin resistant Staphylococcus aureus (MRSA) in Western Nepal: a matter of concern for community infections (a hospital based prospective study). BMC Infect Dis. 2016;16(1):1–6.
Liu B, Sun H, Pan Y, Zhai Y, Cai T, Yuan X, et al. Prevalence, resistance pattern, and molecular characterization of Staphylococcus aureus isolates from healthy animals and sick populations in Henan Province, China. Gut pathogens. 2018;10(1):31.
Goudarzi M, Fazeli M, Goudarzi H, Azad M, Seyedjavadi SS. Spa typing of Staphylococcus aureus strains isolated from clinical specimens of patients with nosocomial infections in Tehran, Iran. Jundishapur J Microbiol. 2016;9(7):e35685.
Lakhundi S, Zhang K. Methicillin-resistant Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev. 2018;31(4):e00020–18.
Islas-Muñoz B, Volkow-Fernández P, Ibanes-Gutiérrez C, Villamar-Ramírez A, Vilar-Compte D, Cornejo-Juárez P. Bloodstream infections in cancer patients. Risk factors associated with mortality. Int J Infect Dis. 2018;71:59–64.
Garcia-Vidal C, Cardozo-Espinola C, Puerta-Alcalde P, Marco F, Tellez A, Agüero D, et al. Risk factors for mortality in patients with acute leukemia and bloodstream infections in the era of multiresistance. PLoS One. 2018;13(6):e0199531.
Ghadiri H, Vaez H, Khosravi S, Soleymani E. The antibiotic resistance profiles of bacterial strains isolated from patients with hospital-acquired bloodstream and urinary tract infections. Crit Care Res Pract. 2012;2012:890797.
Fahim NA. Prevalence and antimicrobial susceptibility profile of multidrug-resistant bacteria among intensive care units patients at Ain Shams University hospitals in Egypt—a retrospective study. J Egypt Public Health Assoc. 2021;96(1):7.
Miyake M, Ohbayashi Y, Iwasaki A, Ogawa T, Nagahata S. Risk factors for methicillin-resistant Staphylococcus aureus (MRSA) and use of a nasal mupirocin ointment in oral cancer in patients. J Oral Maxillofac Surg. 2007;65(11):2159–63.
Khamees AA, Abdelbary NM, Elmasry EA, Gohar SF. Incidence of MRSA infection in pneumonia in cancer patients with phenotyping and genotyping study. Egypt J Chest Dis Tuberc. 2015;64(3):689–91.
Zorgani AA, Belgasim Z, Ziglam H, Ghenghesh KS. Antimicrobial susceptibility profiles of gram-negative bacilli and gram-positive cocci isolated from cancer patients in Libya. Arch Clin Infect Dis. 2012;3(3):1–8.
Lanoix JP, Pluquet E, Lescure FX, Bentayeb H, Lecuyer E, Boutemy M, et al. Bacterial infection profiles in lung cancer patients with febrile neutropenia. BMC Infect Dis. 2011;11(1):183.
Nejad ZE, Ghafouri E, Farahmandi-Nia Z, Kalantari B, Saffari F. Isolation, identification, and profile of antibiotic resistance of bacteria in patients with cancer. Iran J Med Sci. 2015;35(2):109–15.
Fentie A, Wondimeneh Y, Balcha A, Amsalu A, Adankie BT. Bacterial profile, antibiotic resistance pattern and associated factors among cancer patients at University of Gondar Hospital, Northwest Ethiopia. Infect Drug Resist. 2018;11:2169.
Srinivasan A, Seifried S, Zhu L, Srivastava DK, Flynn PM, Bankowski MJ, et al. Staphylococcus aureus bacteremia in pediatric patients with cancer. Arch Pediatr Infect Dis. 2010;29(2):172.
Bai C, Li D, Zhang Q, Zheng S, Li Z, Wang H, et al. Prognostic analysis of cancer patients with Staphylococcus aureus infection: five-year experience at a comprehensive cancer center. Int J Clin Exp Med. 2018;11(8):8640–5.
Vahedian-Ardakani HA, Moghimi M, Shayestehpour M, Doosti M, Amid N. Bacterial spectrum and antimicrobial resistance pattern in cancer patients with febrile neutropenia. Asian Pac J Cancer Prev. 2019;20(5):1471.
Prabhash K, Bajpai J, Gokarn A, Arora B, Kurkure PA, Medhekar A, et al. Comparison of isolates and antibiotic sensitivity pattern in pediatric and adult cancer patients; is it different? Indian J Cancer. 2014;51(4):496.
Babu KG, Lokanatha D, Lakshmaiah KC, Suresh Babu MC, Jacob LA, et al. Bloodstream infections in febrile neutropenic patients at a tertiary cancer institute in South India: a timeline of clinical and microbial trends through the years. Indian J Med Paediatr Oncol. 2016;37:174–82.
Shariati A, Dadashi M, Moghadam MT, van Belkum A, Yaslianifard S, Darban-Sarokhalil D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: a systematic review and meta-analysis. Sci Rep. 2020;10(1):1–6.
Watanabe K, Kosaka T, Hongo H, Oya M. Utility of prophylactic antibiotics for preventing febrile neutropenia during cabazitaxel therapy for castration-resistant prostate cancer. Sci Rep 2021;11(1):1–7.
Sartelli M, Di Bella S, McFarland LV, Khanna S, Furuya-Kanamori L, Abuzeid N, et al. 2019 update of the WSES guidelines for management of Clostridioides (Clostridium) difficile infection in surgical patients. World J Emerg Surg. 2019;14(1):1–29.
Jaradat ZW, Hamdan TA, Hayajneh W, Al Mousa W, Al SA. Antibiograms, toxin profiling and molecular typing of Staphylococcus aureus isolates from two tertiary hospitals in Jordan. J Infect Dev Ctries. 2017;11(11):876–86.
Campo M, Hachem R, Jiang Y, Dvorak T, Carrillo-Marquez M, Hulten K, et al. Panton valentine Leukocidin exotoxin has no effect on the outcome of cancer patients with methicillin-resistant Staphylococcus aureus (MRSA) infections. Medicine (Baltimore). 2011;90(5):312–8.
Xie X, Bao Y, Ouyang N, Dai X, Pan K, Chen B, et al. Molecular epidemiology and characteristic of virulence gene of community-acquired and hospital-acquired methicillin-resistant Staphylococcus aureus isolates in Sun Yat-sen memorial hospital, Guangzhou, Southern China. BMC Infect Dis. 2016;16(1):339.
Klein S, Hannesen J, Zanger P, Heeg K, Boutin S, Nurjadi D. Entry of Panton–valentine leukocidin-positive methicillin-resistant Staphylococcus aureus into the hospital: prevalence and population structure in Heidelberg, Germany 2015–2018. Sci Rep. 2020;10(1):1–7.
Papadimitriou-Olivgeris M, Drougka E, Fligou F, Dodou V, Kolonitsiou F, Filos KS, et al. Spread of tst-positive Staphylococcus aureus strains belonging to ST30 clone among patients and healthcare workers in two intensive care units. Toxins (Basel). 2017;9(9):270.
Salas M, Wernecki M, Fernández L, Iglesias B, Gutiérrez D, Álvarez A, et al. Characterization of clinical MRSA isolates from northern Spain and assessment of their susceptibility to phage-derived antimicrobials. Antibiotics. 2020;9(8):447.
Horváth A, Dobay O, Sahin-Tóth J, Juhász E, Pongrácz J, Iván M, et al. Characterisation of antibiotic resistance, virulence, clonality and mortality in MRSA and MSSA bloodstream infections at a tertiary-level hospital in Hungary: a 6-year retrospective study. Ann Clin Microbiol Antimicrob. 2020;19(1):17.
Li X, Fang F, Zhao J, Lou N, Li C, Huang T, et al. Molecular characteristics and virulence gene profiles of Staphylococcus aureus causing bloodstream infection. Braz J Infect Dis. 2018;22(6):487–94.
Mirzaii M, Emaneini M, Jabalameli F, Halimi S, Taherikalani M. Molecular investigation of Staphylococcus aureus isolated from the patients, personnel, air and environment of an ICU in a hospital in Tehran. J Infect Public Health. 2015;8(2):202–6.
Hashemizadeh Z, Bazargani A, Kalantar-Neyestanaki D, Mohebi S, Hadi N. Determining spa-type of methicillin-resistant Staphylococcus aureus (MRSA) via high-resolution melting (HRM) analysis, shiraz, Iran. BMC Res Notes. 2020;13(1):1–4.
Aggarwal S, Jena S, Panda S, Sharma S, Dhawan B, Nath G, et al. Antibiotic susceptibility, virulence pattern and typing of Staphylococcus aureus strains isolated from variety of infections in India. Front Microbiol. 2019;10:2763.
Farahmand S, Haeili M, Darban-Sarokhalil D. Molecular typing and drug resistance patterns of Staphylococcus aureus isolated from raw beef and chicken meat samples. Iran J Med Microbiol. 2020;14(5):478–89.
Acknowledgements
This study was a part of the MSc. thesis of Saeedeh Khazaei, which was approved in the Infectious and Tropical Diseases Research Center, and was financially supported by a grant from Research Affairs, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (grant no: OG-9703). We would like to thank all staff of the Microbiology Ward of the laboratory of Shahid Baghaei hospitals for their noteworthy cooperation in collecting the samples.
Funding
Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (grant no: OG-9703).
Author information
Authors and Affiliations
Contributions
E.A.M. and A.D.K. wrote the main manuscript text. E.A.M., A.D.K., and S.K. contributed to the study conception and design. S.K. and A.S. contributed to laboratory experiments and data analysis. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was settled and performed according to the Declaration of Helsinki and obtained approval from the Research Ethics Committee (REC) of the Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (No: IR.AJUMS.REC.1397.036). All patients provided written informed consent. The REC has an assignment to protect the dignity, rights, safety, and well-being of subjects who participate in biomedical research and to offer public accountability through the publication of their decisions.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abbasi Montazeri, E., Khosravi, A.D., Khazaei, S. et al. Prevalence of methicillin resistance and superantigenic toxins in Staphylococcus aureus strains isolated from patients with cancer. BMC Microbiol 21, 262 (2021). https://doi.org/10.1186/s12866-021-02319-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-021-02319-7