Abstract
Background
The unique climatic conditions of the Xinjiang region nurture rich melon and fruit resources, the melon and fruit sugar sources provide sufficient nutrients for the survival of yeast, and the diverse habitats accompanied by extreme climatic conditions promote the production of yeast diversity and strain resources. However, the relationship between yeast species and their relationship with environmental factors in the soil of Xinjiang specialty cash crop Hami melon is not clear. Here, we aimed to characterize the diversity, community structure, and relationship between yeast species and environmental factors in Hami melon orchards soils in different regions of Xinjiang, China.
Results
Based on Illumina MiSeq high-throughput sequencing analysis of the D1 domain of the LSU rRNA genes, the community richness of yeast in the soil of Northern Xinjiang was higher than in the Southern and Eastern Xinjiang, but the community diversity was significantly lower in the Northern Xinjiang than in the Southern and Eastern Xinjiang. A total of 86 OTUs were classified into 59 genera and 86 species. Most OTUs (90.4%) belonged to the Basidiomycota; only a few (9.6%) belonged to Ascomycota. The most dominant species in the Southern, Eastern and Northern Xinjiang were Filobasidium magnum (17.90%), Solicoccozyma aeria (35.83%) and Filobasidium magnum (75.36%), respectively. Principal coordinates analysis (PCoA) showed that the yeast community composition in the soils of the three regions were obviously different, with the Southern and Eastern Xinjiang having more similar yeast community. Redundancy analysis (RDA) showed that soil factors such as conductivity (CO), total phosphorus (TP) and Total potassium (TK) and climate factors such as average annual precipitation (PRCP), relative humidity (RH) and net solar radiation intensity (SWGNT) were significantly correlated with yeast communities (P < 0.05).
Conclusion
There are abundant yeast resources in the rhizosphere soil of Hami melon orchard in Xinjiang, and there are obvious differences in the diversity and community structure of yeast in the three regions of Xinjiang. Differences in climatic factors related to precipitation, humidity and solar radiation intensity and soil factors related to conductivity, total phosphorus and total potassium are key factors driving yeast diversity and community structure.
Similar content being viewed by others
Background
Yeast, a common taxon found in the soil, plays an important role in maintaining the ecological functioning of the soil, promoting plant growth, and protecting plants from pests and diseases [1]. Yeasts isolated from soil (e.g., Filobasidium magnum, Naganishia albida, and Lipomyces spp.) have been found to produce extracellular polymeric substances to resist extreme external environmental disturbances, forming soil aggregates in the process and enhancing the stability of the soil structure [2,3,4]. Plant roots support the survival of yeast species by secreting carbohydrates and organic acids (i.e., amino acids and carboxylic acids). Yeast, in turn, contributes to plant growth and development by dissolving large amounts of nutrients, such as phosphorus and calcium [5,6,7,8]. Additionally, some soil yeasts are also present as antagonists of pathogens, such as Verticillium dahliae and Pythium aphanidermatum, and thus protect the plant from diseases [8, 9]. The size, diversity, and structure of the soil yeast community are known to be influenced by factors, such as soil type, plant species, and geographic location [1]. Moreover, special ecological environments can help yeast species develop tolerance to conditions, such as high / low temperatures-tolerant, drought-tolerant, salinity, etc. [10]. For example, psychrophilic yeasts can be isolated from glaciers [11]. Therefore, the study of yeast diversity, community structure and adaptation strategies in soils under special environments is essential for the development and utilization of yeast resources.
Xinjiang is located in the hinterland of Eurasia, a transition zone between the dry summer zone of Europe and the humid summer belt of East Asia [12]. The special climatic conditions of this region, such as large differences in temperature between day and night and its long hours of daylight, promote the richness of melon and fruit resources [13]. Rich sugar sources in orchard ecosystems promote yeast survival. Meanwhile, the harsh natural environment of dry summers and cold winters has contributed to the evolution of yeast and thus to the accumulation of yeast diversity [12]. Hami melons are popular worldwide and are considered to be a national geographic product and the king of melons in China due to their pleasant aroma, crisp taste, sweetness, and color [14]. The central production areas of Hami melon are the Turpan-Hami Basin, the northwestern and southwestern Tarim Basin, and the north slope of Tianshan Mountain [15, 16]. Currently, the research on Hami melon yeast is mainly focused on the screening of antagonistic yeast to prevent postharvest diseases and control the bacterial fruit blotch disease [17,18,19,20,21]. However, the diversity and composition of yeasts and the ecological factors that influence the yeast community in the soil of Hami melon orchards in different areas of Xinjiang are unknown; such information will provide an in-depth understanding of the adaptation mechanism of Hami melon soil yeast species and the collection and collation of yeast resources in Xinjiang.
In recent years, research on the yeast species from orchard soils has been done using the culture-dependent method. This method is useful for isolating diverse yeast cultures, enriching the resources bank of yeast strains, screening of useful strains for food, industry, medicine, etc.; however, only a few yeast species have been identified in soil samples using culture-dependent methods, and the possibility for studying microbial population dynamics in an individual environment is limited compared with culture-independent methods [22, 23]. Illumina MiSeq high-throughput sequencing is a technology that is now more widely used, which allows comprehensive and accurate detection of the species composition, generates large data volume with greater coverage compared to traditional culture methods [24]. However, its long run times and short read lengths are not optimal for small-scale sequencing [25, 26]. This study aimed to quantitatively analyze the diversity and structure of rhizosphere soil yeast communities in Hami melon orchards in different regions of Xinjiang (Fig. 1) using the Illumina MiSeq high-throughput sequencer and to explore the environmental factors that influenced the differences in the formation of yeast community structures in different regions. Our results offer new insights into the diversity and structure of yeast communities in the soil of Hami melon orchards in different regions of Xinjiang, providing supplemental information on the yeast resources in Xinjiang orchards.
Sampling locations and geographic distribution of all rhizosphere soil samples of Hami melon in Xinjiang, China. Here, SX, EX, and NX represent the sampled areas of Hami melon in Southern Xinjiang, Eastern Xinjiang, and Northern Xinjiang respectively; KS, AK, TL, HM, TC and TS represents the sampled locations in Kashgar and Aksu Prefecture of Southern Xinjiang, Turpan and Hami Prefecture of Eastern Xinjiang, Changji and Shihezi Prefecture of Northern Xinjiang, respectively. Each sample had three replicates (Not shown in the figure). Digital Elevation Model (DEM) provided by the Chinese Academy of Sciences Geospatial Data Cloud Platform
Results
Sequencing analysis and the richness of yeast communities
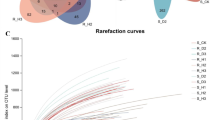
After removing chimeras and sequences with low-quality reads, we obtained 1,952,961 fungal sequence reads of the D1 domain of the large subunit (LSU) rRNA gene from 54 soil samples. After removing non-yeast sequence reads, a total of 31, 948 yeast sequence reads were retained and clustered at 97% sequence similarity yielding 86 operational taxonomic units (OTUs). Rarefaction curves of yeast for all sequences plateaued, indicating that sequencing depth per sample was adequate to capture the diversity in the study sites (Fig. 2). In addition, we divided all samples into three large groups according to their geographical locations: Southern Xinjiang group (SX), Eastern Xinjiang group (EX) and Northern Xinjiang group (NX).
Rarefaction curves of rhizosphere soil samples. Rarefaction curves of OTUs were clustered for a dissimilarity threshold of 3%. Each sample had three replicates (Replicates are not specifically shown in the legend, but have been involved in the analysis). Sample abbreviations are same as presented in Fig. 1
The observed species richness (Sobs), estimated richness (Chao1 and ACE indices) and species diversity (Shannon and Simpson indices) showed that the richness of yeast in the Northern Xinjiang was higher than that in the Southern and Eastern Xinjiang, but the diversity was significantly lower than them (P < 0.05) (Table 1). Based on the analysis of intergroup differences, all the values of Sobs, Chao1 and ACE indices of samples from Northern Xinjiang (NX) were the highest among the three groups, but there was no significant difference. The Shannon index was significantly higher in Southern Xinjiang (SX) and Eastern Xinjiang (EX) than in Northern Xinjiang, and Simpson index was significantly higher in Northern Xinjiang (NX) than in Southern Xinjiang (SX) and Eastern Xinjiang (EX).
Yeast community composition
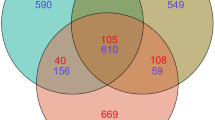
The numbers of yeast sequence reads and OTUs detected in samples from the SX, EX, and NX were 4268 and 57, 5616 and 59, and 22,065 and 55, respectively. We found that 34 OTUs were shared by all three groups; OTUs species were shared between SX and EX; 41 OTUs were shared between SX and NX; 38 OTUs were shared between EX and NX (Fig. 3). We identified 86 OTUs, 59 genera, and 86 species, which belonged to Ascomycota and Basidiomycota. Ascomycota contained 45 OTUs, 27 genera, and 45 species accounting for approximately 9.6% of all yeast sequences, while Basidiomycota had 41 OTUs, 32 genera, and 41 species accounting for approximately 90.4%. These include six genera of yeast-like fungi: Aureobasidium (0.54%), Microglossum (0.15%), Basidioascus (0.04%), Hormonema (0.03%) and Cyphellophora (0.01%), Tilletiopsis (0.01%), and a total of 36 rare species (Species with less than 1% frequency of occurrence) were detected (Tables 2 and 3). The dominant genera that accounted for greater than 1% were Filobasidium (54.97%), Vishniacozyma (7.32%), Solicoccozyma (6.41%), Malassezia (5.13%), Sporobolomyces (4.01%), Cutaneotrichosporon (3.16%), Naganishia (2.07%), Udeniomyces (1.92%), Colacogloea (1.82%), Pichia (1.54%), Saitoella (1.41%), and Mrakia (1.20%). The dominant species that accounted for greater than 1% were Filobasidium magnum (54.97%), Vishniacozyma tephrensis (7.32%), Solicoccozyma aeria (6.41%), Malassezia sp. ‘phylotype 131’ (4.89%), Sporobolomyces carnicolor (3.05%), Naganishia albida (2.07%), Udeniomyces sp. 1 AK-2015 (1.69%), Colacogloea philyla (1.66%), Cutaneotrichosporon curvatum (1.60%), Cutaneotrichosporon cutaneum (1.56%), Saitoella complicata (1.41%), Pichia kudriavzevii (1.29%), and Mrakia gelida (1.18%) (Tables 2, 3, Fig. 4a). The 12 dominant genera and 13 dominant species accounted for 90.96 and 89.1% of all yeast sequences, respectively. Filobasidium magnum, the most dominant species of all yeasts, was detected in the NX accounted for 75.36%, SX and EX having 17.90 and 3.03%, respectively; Vishniacozyma tephrensis was the second dominant species: SX (14.50%), EX (0.05%), and NX (7.78%). The proportion of Solicoccozyma aeria was 35.83, 19.25, and 0.67% in SX, EX, and NX, respectively. The most dominant species in the samples from both South (SX) and North Xinjiang (NX) was Filobasidium magnum, Solicoccozyma aeria was the most dominant species in the Eastern Xinjiang (EX) (Fig. 4b).
Venn diagram at the OTU level of soil samples in Southern Xinjiang (SX), Eastern Xinjiang (EX) and Northern Xinjiang (NX). Each circle with different colors in the diagram represents a group; middle core numbers represent the number of OTUs common to all groups. The shared and unique yeast OTUs were shown at a 0.03 dissimilarity distance after removing singletons
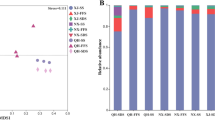
Proportion of dominant yeasts in soil samples. (a) all samples; (b) the samples in Southern Xinjiang (SX), Eastern Xinjiang (EX), and Northern Xinjiang (NX). Others indicated that species accounted for less than 1%. Sample abbreviations are same as presented in Fig. 1
The results of the analysis of species differences between groups based on the phylum level showed that the proportion of Basidiomycota was significantly higher than that of Ascomycota among all soil samples from three groups, Basidiomycota was considered to be the dominant phylum. The proportion of Ascomycota in EX was significantly higher than that in SX and NX (P < 0.05) (Fig. 5b). At the genus level, there were 11 dominant genera with significant differences in relative abundance (P < 0.05) among SX, EX, and NX, except for Udeniomyces. Filobasidium and Vishniacozyma were mainly present in the samples from Southern and Northern Xinjiang; Sporobolomyces, Cutaneotrichosporon and Saitoella were detected mainly in samples from the Southern and Eastern Xinjiang; Solicoccozyma and Mrakia are found mainly in the Eastern and Southern Xinjiang, respectively (Fig. 5a).
Species difference analysis of the samples in Southern Xinjiang (SX), Eastern Xinjiang (EX) and Northern Xinjiang (NX): (a) at the genus level; (b) at the phylum level. The y-axis represents the classification levels of species, and the x-axis represents the percentage of species average relative abundance in each sample group. The red, blue, and green columns represent the average results in the SX, EX, and NX soil samples, respectively. The Kruskal-Wallis rank-sum test was used to show significant differences (*: 0.01 < P < = 0.05, **: 0.001 < P < = 0.01, ***: P < = 0.001)
Relationship between yeast communities in samples from different regions
We performed ordination by PCoA at the OTU level to reveal similarities or differences in community composition among grouped samples (Fig. 6). The first principal coordinates axis (PCoA1) and the second principal coordinates axis (PCoA2) alone explained 23.86 and 10.63% of the variance, respectively. PCoA1 has relatively small eigenvalues, capturing less than 50% of the variation in the input data, and therefore is not considered a very successful PCoA. However, R value (0.6144) greater than 0 indicates that the difference between sample groups is greater than the differences within groups and that the difference is significant (P < 0.05). Overall, most samples from each group were clustered together, with only a slight overlapping among the samples from the three groups on the score plots, indicating significant differences in community composition between groups. For inter-groups, the SX and EX were more similar in community composition, and this result can also be observed visually in the box plot of PCoA (Fig. S1).
Relationship between yeast community structure and environmental factors
The statistical results of soil physicochemical properties showed that the values of conductivity (CO) in the Southern Xinjiang (SX), the levels of organic matter (OM) and total phosphorus (TP) in the Eastern Xinjiang (EX), the pH, total potassium (TK) and available potassium (AK) values in the Northern Xinjiang (NX) are significantly higher than in the other two regions (P < 0.05). Available nitrogen (AN) content of NX was significantly lower than SX and EX (P < 0.05) (Table 4). Redundancy analysis based on yeast genera and soil physicochemical properties in soil samples from different regions showed that the first and second RDA components explained 43.6% of the total variation (Fig. 7a). CO, TP and TK were significantly associated with the yeast community (P < 0.05), and mainly influenced the distribution of samples in the Southern (SX), Eastern (EX) and Northern Xinjiang (NX), respectively. These results suggest a correlation between the yeast community and soil physicochemical properties, particularly total phosphorus (TP) content in the soil. The F-ratio and P values for each soil factor are shown in Table S1.
Redundancy analysis (RDA) of (a) the correlation between the yeast community and soil physicochemical properties in all samples from three regions of Xinjiang, and (b) the correlation between the yeast community and climate factors at the genus level. Red, blue, and green symbols represent samples from SX, EX, and NX, respectively. Red and black arrows represent the soil parameters and genera, respectively. Soil physicochemical properties: pH, Conductivity (CO), Organic matter (OM), Total nitrogen (TN), Total phosphorus (TP), Total potassium (TK), Available nitrogen (AN), Available phosphorus (AP), Available potassium (AK). Climate factors: Average annual precipitation (PRECTP), Average annual temperature (TEMP), Average annual land surface temperature (LST), Average annual relative humidity (RH), The annual average net solar radiation intensity received by the earth’s surface (SWGNT)
The results of the analysis of climatic factors at the sampling sites in different regions show that the average annual precipitation (PRCP) and relative humidity (RH) were significantly higher in Northern Xinjiang (NX) than in Southern (SX) and Eastern Xinjiang (EX), and the lowest in Eastern Xinjiang (EX) (P < 0.05). The average annual temperature (TEMP), land surface temperature (LST) and net solar radiation intensity (SWGNT) in Southern (SX) and Eastern Xinjiang (NX) are higher than in Northern Xinjiang, but there is no significant difference (Table 5). Redundancy analysis (RDA) of the correlation between the yeast community and climate factors showed that the first and second RDA components explained 39.4% of the total variation. PRCP, RH and SWGNT were the climatic factors that have significant effects on the distribution of yeast communities (P < 0.05) (Fig. 7b). PRCP and RH were negatively correlated with SWGNT, and were positively correlated with Filobasidium and Vishniacozyma but negatively correlated with Solicoccozyma. SWGNT was positively correlated with Sporobolomyces, Cutaneotrichosporon and Saitoella. The F-ratio and P values for each climate factor are shown in Table S2.
Discussion
Yeast diversity in rhizosphere soils of Hami melon orchards
A total of two phyla, 59 genera and 86 species of yeasts were detected based on high-throughput sequencing technology in this study (Tables 2 and 3). Using a combination of MALDI-TOF MS and rDNA sequencing, previous scholars identified a total of 60 yeast species from 200 soil samples of five fruit trees (apple, pear, plum, peach and apricot) from two locations in southwest Slovakia [27]. Moreover, only 16 species of yeast were detected in 493 samples of Cameroon-based agricultural soil from nine locations using the culture-dependent method [28]. This indicates that there are rich yeast resources with a relatively high yeast diversity in the rhizosphere soil of Xinjiang Hami melon. On the one hand, the high level of yeast diversity may be related to the high sugar content of Xinjiang Hami melon (15–18%) compared to other fruits and vegetables such as watermelon (7–11%), tomatoes (7–10%) and apples (10–14.2%) [29,30,31,32]. On the other hand, tillage practices also influence the diversity and abundance of soil microorganisms, for example, crop rotation is more conducive to the accumulation of mycorrhizal species than continuous cropping [33,34,35]. During our sampling, we learned that crop rotation is commonly used in Xinjiang Hami melon fields to avoid pests and soil micronutrient deficiencies [36]. Furthermore, epiphytic yeasts from the surfaces of various plant species entering the soil with humus during crop rotation may also further increase soil yeast diversity in Hami melon orchards [37]. This is because, the Basidiomycete genera Vishniacozyma, Sporobolomyces, Kockovaella, Rhodotorula and Cystobasidium in this study were usually isolated from plant surfaces in most studies [33, 37].
Ascomycota was the more diverse phylum, but its abundance was much lower than that of Basidiomycetes (Tables 2, 3 and Fig. 5b), which challenged the traditional view that Ascomycetous yeasts were generally more frequent and abundant in agricultural soils, orchards, and grasslands [38, 39]. Other studies revealed that Basidiomycetes were dominant in forest soils [24, 38, 40]. This may be the result of differences in research methods. Although in this study Basidiomycetes were found to have a greater advantage by high-throughput sequencing analysis, the opposite result may be obtained by culture-dependent method: since Ascomycetous have the advantage of faster growth than Basidiomycetes yeasts during culture [33]. In fact, the conclusion obtained in this study is not an isolated case, as there are previous studies on yeast in citrus orchards soil in which Basidiomycetes yeast is also dominant [41]. In addition, another study has shown that the rhizosphere of maize seedlings (20 d) was harbored only by yeasts of the phylum “Ascomycota”, whereas the rhizosphere of senescent plants (90 d) was inhabited by basidiomycetous yeasts [42]. The samples collected in this study were from rhizosphere soil at the ripening stage of Hami melon, which may also account for the higher abundance of basidiomycetous yeasts.
The rare yeast found in this study accounted for approximately 41.86% of the yeast species in all soil samples of Hami melon (Tables 2, 3), a value within the range of the proportion of rare yeast isolated by other studies from fruit trees, forests, grasslands, and shrub soils [27, 43, 44]. Cutaneotrichosporon cutaneum and Cutaneotrichosporon curvatum were also found in most samples, suggesting that the genus Cutaneotrichosporon may be resident yeast in the rhizosphere soil of Hami melon orchards. A strain of Cutaneotrichosporon cutaneum was found to be highly tolerant to tetracycline antibiotics, chloramphenicol, copper and zinc ions, and to degrade oxytetracycline with high efficiency, which could play a positive role in the prevention of environmental antibiotic contamination [45]. Cutaneotrichosporon curvatus belongs to the oleaginous yeast, which can be used as a biofuel [3, 9, 46]. So, the rhizosphere soil of Hami melon orchards is a potential bioprospecting soil for oleaginous yeasts for biodiesel production. Filobasidium magnum, Naganishia albida and Mrakia gelida belong to three of the dominant species in this study. The first two are capable of producing extracellular polymerases that contribute to the stabilisation of the soil structure and the last can be used in the food industry for brewing low-alcohol beer [47, 48]. In addition, we also detected the pathogenic fungus Malassezia restricta, which causes aggravate atopic dermatitis (AD), and Tilletiopsis washingtonensis, which produces hydrolases and antifungal compounds that can be used as antagonists of powdery mildew fungi in agricultural production, but their abundance was low [49, 50].
Drivers affecting differences in yeast diversity and community structure in different regions
Alpha diversity analysis revealed differences in species richness and diversity among the three regions (Table 1), and the structure of the rhizosphere soil yeast communities of Hami melon also showed geographical differences among the three regions, with the Eastern and Southern Xinjiang being more similar (Fig. 6). We hypothesize that differences in soil physicochemical properties and environmental climate may be the main drivers of the differences in yeast community composition among the three regions of Xinjiang. The Changji and Shihezi areas are in the temperate grey-brown desert soil grey desert soil zone, while Tarim Basin and the Turpan-Hami Basin are in the Warm temperate brown desert soil zone [51]. Soil physicochemical properties analysis also revealed significant differences in soil type between the three areas (Table 4), and RDA analysis showed that electrical conductivity (CO), total phosphorus (TP) and total potassium (TK) were significantly correlated with the yeast community (Fig. 7a). These three factors were positively correlated with the dominant yeast genus in the Southern, Eastern, and Northern Xinjiang, respectively. The strongest correlation between total phosphorus (TP) and the yeast community may be due to the fact that phosphorus is a key element in the nutrient conversion between plants and yeast [52,53,54].
The meteorological data show that radiation intensity and precipitation considerably differed among three regions in Xinjiang (Table 5). And the results of redundancy analysis showed that the average annual precipitation (PRCP), relative humidity (RH) and net solar radiation intensity (SWGNT) were significantly correlated with yeast communities (Fig. 7b). There are differences in radiation levels in the Southern (293–322 KJ/cm2 per year), Eastern (304–307 KJ/cm2 per year) and Northern Xinjiang (262–277 KJ/cm2 per year), with the former two being hotter and more evaporative than the latter [55]. However, the precipitation situation was the opposite. Due to the influence of the warm and humid air currents from Siberia, the climate in Northern Xinjiang is relatively humid, with a little more rainfall; while Southern and Eastern Xinjiang is surrounded by mountains and is characterised by an arid climate with little rainfall; the more complex topography of Eastern Xinjiang creates a variety of habitat types [56, 57]. Additionally, previous study has shown that the abundance of yeast in soil is positively correlated with soil water content [39]. The high proportion of yeast sequence reads in this region and the fact that Filobasidium magnum, which is often isolated from wetter habitats and is the dominant species, was also isolated in the Northern Xinjiang and exists as a dominant species confirm the relatively wetter climate of the Northern Xinjiang [58, 59]. The higher precipitation and relative humidity of soils in the Northern border may have led to a slower decomposition of organic matter in the soil, and Filobasidium magnum is able to degrade or transform various organic compounds [4]. On the contrary, the most dominant species in the genus Solicoccozyma, Solicoccozyma aeria, has a preference for arid environments, mainly in the Eastern Xinjiang [52]. Combining the above information, Filobasidium magnum and Solicoccozyma aeria have the potential to serve as indicator species of ambient humidity. In addition, the Southern and Eastern Xinjiang have a high diversity of yeasts, probably due to the high level of environmental heterogeneity in Southern and Eastern Xinjiang facilitates the generation of genetic mutations and the accumulation of genetic variation in yeast [60]. Furthermore, the high quality and strong landrace of Hami melon in Southern and Eastern Xinjiang also reflect the good interplay between rhizosphere yeast community and plants [15].
Conclusions
Our results showed that yeast resources were abundant in the soil of Hami melon orchards, and there were noticeable differences in yeast diversity and community structure among Southern, Eastern, and Northern Xinjiang. The results of this study provided interesting insights into the relationship between the yeast composition of rhizosphere soil in Hami melon orchards and their geographic regions. The results also demonstrated that both conductivity (CO), total phosphorus (TP) and Total potassium (TK) in soil factors and the average annual precipitation (PRCP), relative humidity (RH) and net solar radiation intensity (SWGNT) in climate factors have an influence on yeast community structure. The results of this study will provide a theoretical basis for better exploitation of soil yeast resources and understanding of their adaptive mechanisms.
Methods
Study sites and sampling
We collected rhizosphere soil samples from Hami melon orchards from six different areas within three big regions of Xinjiang between July and August 2019. Study sites included the Kashgar (35°20′ - 40°18′ N and 73°20′ - 79°57 E) and Aksu (39°30′ - 42°41′ N and 78°03′ - 84°07 E) Prefecture (SX, Southern Xinjiang), the Turpan (41°12′ - 43°40′ N and 87°16′ - 91°55 E) and Hami (40°52′ - 45°05′ N and 91°06′ - 96°23 E) Prefecture (EX, Eastern Xinjiang), the Changji (43°20′ - 45°00′ N and 85°17′ - 91°32 E) and Shihezi (43°20′ - 45°20′ N and 84°45′ - 86°40 E) Prefecture (NX, Northern Xinjiang). Then three locations have Hami melon orchards with a planting area of not less than 3 ha were selected from each prefecture for sampling, and soil samples were collected in triplicates from each orchard (Fig. 1). In total, 54 rhizosphere soil samples were studied. The five-point sampling method was used for sample collection. Briefly, five Hami melons at maturity were randomly selected from each orchard to collect soil samples around their roots, at approximately 10 cm depth, using a shovel and sieved to remove plant residues and stones. The rhizosphere soil samples of five Hami melon plants were then mixed evenly and divided into three equal portions. Each sample was stored individually in sterile self-sealing bags and transported to the laboratory in an ice box (< 10 °C). After each soil sample was crushed and filtered using a 2 mm sieve, they were divided into two parts: one part was air dried and used for soil physicochemical analysis; the other part was stored in a − 80 °C refrigerator for DNA extraction.
The soil types in Kashgar, Aksu, Turpan and Hami Prefecture are clay loam, brown-gray clay loam, sandy loam and sandy clay loam, respectively. The soil types in Changji and Shihezi Prefecture are both loamy clay. Xinjiang has a variety of climate types, with a clear distinction between warm, cold and temperate from south to north, and dry and wet from east to west. Therefore, we divided all samples into Southern (SX), Eastern (EX) and Northern Xinjiang (NX) groups according to their geographical distribution for subsequent analysis. The climate information for each sampling site is shown in Table 5. The data of precipitation (PRCP) and temperature (TEMP) from NOAA - Climate Prediction Center (https://www.cpc.ncep.noaa.gov/), land surface temperature (LST) and relative humidity (RH) from NASA GES DISC MERRA2 - inst1_2d_asm_Nx (https://disc.gsfc.nasa.gov/), net solar radiation intensity (SWGNT) from NASA GES DISC MERRA2 - tavg1_2d_rad_Nx (https://disc.gsfc.nasa.gov/).
DNA extraction and Illumina MiSeq
E.Z.N.A.® soil DNA Kit (Omega Biotek, USA) was used to extract total DNA from soil samples (0.5 g) following the manufacturer’s protocol. The final DNA concentration was detected using a NanoDrop 2000 UV-Vis spectrophotometer (Thermo Scientific, USA). The integrity of the DNA was assessed using 1% agarose gel electrophoresis. The yeast 26S rDNA was amplified with a pair of specific primers with barcode NL1F (forward primer) (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL2R (reverse primer) (5′-CTTGTTCGCTATCGGTCTC-3′) [61]. The PCR reaction system (20 μL) contained 5× FastPfu Buffer (4 μL), 2.5 mM dNTPs (2 μL), primer (5 μM; 0.8 μL each), FastPfu Polymerase (0.4 μL), BSA (0.2 μL), and template DNA (10 ng). The PCR reaction was performed using a thermocycler PCR system as follows: 5 min at 98 °C (denaturation), 30 cycles at 98 °C for 30 s, 52 °C for 30 s, and 72 °C for 45 s, and finally, at 72 °C for 5 min (elongation). The PCR products were analyzed using 2% agarose gel electrophoresis, purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, USA). The DNA fragments were quantified using QuantiFluor™-ST (Promega, USA) [62]. Equimolar amounts of purified DNA fragments were pooled after individual samples were tagged with indexes through an index PCR, and the Illumina MiSeq PE300 platform (Illumina, USA) was used to perform paired-end sequencing (2 × 300) following the protocol by Meiji Biomedical Technology Co. Ltd. (Shanghai, China).
Sequence processing
Raw sequence files were demultiplexed and quality filtered by Trimmomatic and merged by FLASH based on the following criteria: (i) reads with an average quality score < 20 over a 50-bp sliding window were truncated; (ii) sequences with an overlap longer than 10 bp were merged based on their overlapping sequences; (iii) the maximum mismatch ratio allowed in the overlap region of a spliced sequence was 0.2, and non-conforming sequences were eliminated; (iv) the samples were differentiated according to the barcode and primers at the beginning and end of the sequence; the sequence orientation was adjusted, the number of mismatches allowed by the barcode was 0, and the maximum number of primer mismatches was 2 [63,64,65]. OTUs were clustered with a 97% similarity cutoff using UPARSE (version 7.1 http://drive5.com/uparse/), and chimeric sequences were identified and removed using the UCHIME software [66, 67]. The classification of each D1 domain of the LSU rRNA sequence was analyzed by the Ribosomal Database Project (RDP) Classifier algorithm (version 2.2 http://sourceforge.net/pro-jects/rdp-classifier/) [66]. The NCBI database (National Centre for Biotechnology Information, https://www.ncbi.nlm.nih.gov/public/) database using a confidence threshold of 0.7 [68]. The observed richness (Sobs), the ACE index the Chao1 estimator, the Shannon diversity (H) index and the Simpson index were calculated using the mothur (version v.1.30.2 https://mothur.org/wiki/chao/, https://mothur.org/wiki/ace/, https://mothur.org/wiki/shannon/, http://mothur.org/wiki/Simpson) index analysis with Operational Taxonomic Units (OTUs) at 0.97 level [69]. Next, we plotted the rarefaction curves to observe the community abundance of each sample and the sequencing data [62, 66].
Determination of soil chemical properties
Here, we evaluated nine soil physicochemical factors (Table 4). The soil water suspension was shaken for 30 min, followed by measurement of pH using a glass electrode meter. A naturally dried soil sample was mixed with water at a ratio of 1:5 (M/V), and conductivity (CO) was determined using the electrode method. The organic matter (OM) was determined by titration with ferrous sulfate, using o-phenanthroline as the indicator, by adding a potassium dichromate-sulfuric acid solution to a test tube containing the soil samples. The available nitrogen (AN) and total nitrogen (TN) were determined by the Kjeldahl method. The available phosphorus (AP) in the soil was extracted with sodium bicarbonate and then determined using the molybdenum blue method. The available potassium (AK) in the soil was extracted with ammonium acetate and determined by flame photometry. Total phosphorus (TP) and total potassium (TK) were measured by acid solubilization [70, 71].
Data analysis
SPSS Statistics v25.0 software (IBM, USA) was used to analyze the data of soil physicochemical properties and climatic factors. All values are presented as mean ± standard error (mean ± SE). Since the data were not normally distributed, Kruskal-Wallis test for independent samples was used to compare the physicochemical properties of the soil and climatic factors among different groups. Differences were taken statistically significant at P < 0.05. The dilution curve was drawn using the “vegan” and “ggplot2” packages in R (v4.0.2); Venn diagram using the “VennDiagram” package; community bar graph was plotted using “ggplot2” and “ggalluvial” packages in R (v4.0.2). Since the data of alpha diversity indices did not follow a normal distribution, the Kruskal-Wallis test was used to detect whether there were significant differences in alpha diversity indices among the groups. Analysis of the species that showed differences between groups based on genus level and phylum level was performed by Kruskal-Wallis rank-sum test, followed by plotting through the “ggplot2” package in R (v4.0.2). In this process the P-values are corrected for multiple testing by the false discovery rate (FDR) and further testing by Post-hoc testing after the Kruskal-Wallis H-test, with a further two-way comparison of the multiple groups, which is done by the stats package for R and the scipy package for Python. Principal co-ordinate analysis (PCoA) was done based on Bray-Curtis at OTU level to analyze similarities or differences in the community composition of samples using “vegan” and “ape” packages in R (v4.0.2). Tests for differences between groups in PCoA were analyzed using ANOSIM (analysis of similarities) by vegan package in R. Redundancy analysis (RDA) was used to evaluate the relationships between soil factors and yeast communities and between climatic factors and yeast communities respectively, based on sample soil physicochemical properties, local meteorological data and sample genus level data and calculated using the software Canoco for Windows 5 (Microcomputer Power, USA) [62, 65, 66]. Monte Carlo permutation test in Canoco was used to identify environmental factors that were significantly associated with yeast community structure.
Availability of data and materials
Sequence data of this project have been deposited in the Sequence Read Archive (SRA) of the National Center for Biotechnology Information (NCBI) under the accession number PRJNA725370 (https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA725370).
Abbreviations
- SX:
-
Southern Xinjiang
- EX:
-
Eastern Xinjiang
- NX:
-
Northern Xinjiang
- OTU:
-
Operational taxonomic units
- CO:
-
Conductivity
- OM:
-
Organic matter
- TN:
-
Total nitrogen
- TP:
-
Total phosphorus
- TK:
-
Total potassium
- AN:
-
Available nitrogen
- AP:
-
Available phosphorus
- AK:
-
Available potassium
- PRCP:
-
Average annual precipitation
- TEMP:
-
Average annual temperature
- LST:
-
Average annual land surface temperature
- RH:
-
Average annual relative humidity
- SWGNT:
-
Average annual net solar radiation intensity
- PCoA:
-
Principal coordinates analysis
- RDA:
-
Redundancy analysis
References
Vadkertiová R, Dudášová H, Balaščáková M. Yeasts in agricultural and managed soils. 2017;117–44. https://doi.org/10.1007/978-3-319-62683-3_4.
Deng J, Orner E, Chau J, Anderson E, Kadilak A, Rubinstein R, Bouchillon G, Goodwin R, Gage D, Shor L. Synergistic effects of soil microstructure and bacterial EPS on drying rate in emulated soil micromodels. Soil Biol Biochem. 2015;83:116. https://doi.org/10.1016/j.soilbio.2014.12.006.
Vishniac HS. Simulated in situ competitive ability and survival of a representative soil Yeast, Cryptococcus albidus. Microb Ecol. 1995;30(3):309-20. https://doi.org/10.1007/BF00171937.
Botha A. Yeasts in Soil. In: Péter G, Rosa C, editors. Biodiversity and Ecophysiology of Yeasts. Berlin, Heidelberg: Springer Berlin Heidelberg; 2006. p. 221–240. https://doi.org/10.1007/3-540-30985-3_11.
Amprayn K-o, Rose M, Kecskés M, Pereg L, Nguyen H, Kennedy I. Plant growth promoting characteristics of soil yeast (Candida tropicalis HY) and its effectiveness for promoting rice growth. Appl Soil Ecol. 2012;61:295–9. https://doi.org/10.1016/j.apsoil.2011.11.009.
Berendsen R, Pieterse C, Bakker P. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17:478–6. https://doi.org/10.1016/j.tplants.2012.04.001.
Fu S-F, Sun P-F, Lu H-Y, Wei J-Y, Xiao H-S, Fang W-T, Cheng B-Y, Chou J-Y. Plant growth–promoting traits of yeasts isolated from the Phyllosphere and Rhizosphere of Drosera spathulata lab. Fungal Biol. 2016;120. https://doi.org/10.1016/j.funbio.2015.12.006.
Mestre M, Fontenla S, Bruzone M, Fernández N, Dames J. Detection of plant growth enhancing features in psychrotolerant yeasts from Patagonia (Argentina): plant growth promoter yeasts from Patagonia. J Basic Microbiol. 2016;56. https://doi.org/10.1002/jobm.201500728.
Azcón R, Perálvarez M, Roldán A, Barea J-M. Arbuscular Mycorrhizal Fungi, Bacillus cereus, and Candida parapsilosis from a multicontaminated soil alleviate metal toxicity in plants. Microb Ecol. 2009;59:668–77. https://doi.org/10.1007/s00248-009-9618-5.
Wei D. Significance and recommendation of Xinjiang special environmental microorganism resources research and exploitation. Xingjiang Agric Sci. 2004;41(s1):1–4. https://doi.org/10.3969/j.issn.1001-4330.2004.z1.001.
Turchetti B, Hall SRT, Connell LB, et al. Psychrophilic yeasts from Antarctica and European glaciers: description of Glaciozyma gen. nov., Glaciozyma martinii sp. nov. and Glaciozyma watsonii sp. nov. Extremophiles. 2011;15(5):573. https://doi.org/10.1007/s00792-011-0388-x.
Wang QG. Studies on species diversity and application of wild yeast in Xinjiang. Jinan, China: Shandong Institute of Light Industry; 2007.
Chen YL. Melon and Fruit Plant Resources in Xinjiang. Jiangxi Forestry Sci Technol. 1996;(01):45. https://doi.org/10.16259/j.cnki.36-1342/s.1996.01.017.
Zhao XX, Ning M, Zhang Q, Cai WC, Shan CH, Tang FX. Isolation and identification of pathogenic microorganisms causing post-harvest spoilage in Hami melo. Food Sci. 2021;42(06):325-35. https://doi.org/10.7506/spkx1002-6630-20200102-023.
Wang J, Jiang M, Yuchao LI, et al. Influence of climatic conditions on planting of Hami Melon. 2018;10(02):86–90. https://doi.org/10.19601/j.cnki.issn1943-9903.2018.2.019.
Lim D. Cucumis melo L. (Reticulatus Group) ‘Hami melon’. 2012. p. 231–4. https://doi.org/10.1007/978-94-007-1764-0_36.
Pei SQ, Tong JM, Kou XT, Wang XT. Biological control of postharvest diseases of postharvest Hami-melon using antagonistic yeasts. Anhui Agric Sci Bull. 2009;15(01):134–5,75. https://doi.org/10.16377/j.cnki.issn1007-7731.2009.01.076.
Zhi-Hao W, Chun-Hui S, Jian W, Cai B, Li-Fang Z, Jing-Yun W, Pei-Pei F. Antagonistic Yeast Adding Method on Preservation Effect of Cantaloupe. Agri-processing. 2013;(5):34-36. https://doi.org/10.3969/j.issn.1671-9646(X).2013.03.009.
Li BK, Mao XY, Chen J, Zhang J. Identification of yeast from the melon juice of spontaneous fermentation. Liquor-making Sci Technol. 2009;36(01):41–43. https://doi.org/10.3969/j.issn.1002-8110.2009.01.015.
Shen T, Chen HZ, Yang J. Selection of aroma-producing yeast for Hami melon wine and its properties. Liquor-making Sci Technol. 2004;(03):36–38. https://doi.org/10.3969/j.issn.1001-9286.2004.03.008.
Wang X, Li G, Jiang D, Huang H-C. Screening of plant epiphytic yeasts for biocontrol of bacterial fruit blotch (Acidovorax avenae subsp. citrulli) of hami melon. Biol Control. 2009;50:164–71. https://doi.org/10.1016/j.biocontrol.2009.03.009.
Hrynkiewicz K. Belowground microbiota and the health of tree crops. Front Microbiol. 2018;9. https://doi.org/10.3389/fmicb.2018.01006.
Yurkov A. Yeasts in Forest soils. 2017;87–116. https://doi.org/10.1007/978-3-319-62683-3_3.
Chen Z, Xia T, Wang D, Wu G, Liu J, Zhang Y, et al. Diversity analysis of Endophytic Fungi in Mentha using Illumina MiSeq high-throughput sequencing technology. Med Plant. 2016;7(Z1):46–50 https://doi.org/CNKI:SUN:MDPT.0.2016-Z1-015.
Huang Y. High-throughput sequencing-based microbial genomics. Chinese People's Liberation Army Academy of Military Medical Sciences; 2014. https://doi.org/10.7666/d.D358012.
Guo HY, Cheng GH, Li YJ, Zhang H, Qin KL. High-throughput sequencing technologies and their applications in biology. Contemp Anim Husbandry. 2016;000(004):61–65. https://doi.org/CNKI:SUN:DDXM.0.2016-12-035.
Vadkertiová R, Dudášová H, Stratilová E, Balaščáková M. Diversity of yeasts in the soil adjacent to fruit trees of the Rosaceae family. Yeast. 2019;36(10):617–31. https://doi.org/10.1002/yea.3430.
Aljohani R, Samarasinghe H, Ashu T, Xu J. Diversity and relationships among strains of culturable yeasts in agricultural soils in Cameroon. Sci Rep. 2018;8(1):15687. https://doi.org/10.1038/s41598-018-34122-2.
Li FC, AP·YSK, MHMT·YSJ, AHMT·HLL, Zao RM, Chen SL, et al. Key points on cultivation techniques for fine Hami melon in continuous greenhouses Xinjiang Agric Sci Technol. 2009;(1):48. https://doi.org/10.3969/j.issn.1007-3574.2009.01.045.
Han WF, Qiu P. Watermelon jelly preparation. Farm Products Processing (top). 2014. (9): 50–50.
Zhang MC, Zhang KX, Jiang HF, Zhou ZW, Che XJ, Zhang JL. Effect of temperature on the quality of red Fuji apples in the Longdong region and quality zoning. Chinese Agronomy Bulletin. 2020;v.36;No.555(12):52–58. https://doi.org/CNKI:SUN:ZNTB.0.2020-12-007.
OuYang W. Introduction of new cherry tomato varieties for planting trials in Urumqi. Xinjiang Agric Sci Technol. 2015;03(3):33 https://doi.org/CNKI:SUN:XJNK.0.2015-03-020.
Yurkov AM. Yeasts of the soil - obscure but precious. Yeast (Chichester, England). 2018;35(5):369–78. https://doi.org/10.1002/yea.3310.
Singh B, Dawson L, Macdonald C, Buckland S. Impact of biotic and abiotic interaction on soil microbial communities and functions: a field study. Appl Soil Ecol. 2009;41:239–48. https://doi.org/10.1016/j.apsoil.2008.10.003.
Spehn EM, Joshi J, Schmid B, Alphei JR, Körner C. Plant diversity effects on soil heterotrophic activity in experimental grassland ecosystems. Plant & Soil 2000;224(2):217–230. https://doi.org/10.1023/A:1004891807664.
He X. Study on standardization Production Base model of Hami melon in Xinjiang. Xinjiang Agricultural University; 2012.
Botha A. The importance and ecology of yeasts in soil. Soil Biol Biochem. 2011;43:1–8. https://doi.org/10.1016/j.soilbio.2010.10.001.
Yurkov A, Kemler M, Begerow D. Assessment of yeast diversity in soils under different management regimes. Fungal Ecol. 2012;5:24–35. https://doi.org/10.1016/j.funeco.2011.07.004.
Sláviková E, Vadkertiová R. The diversity of yeasts in the agricultural soil. J Basic Microbiol. 2003;43(5):430–6. https://doi.org/10.1002/jobm.200310277.
Yurkov AM, Röhl O, Pontes A, Carvalho C, Maldonado C, Sampaio JP. Local climatic conditions constrain soil yeast diversity patterns in Mediterranean forests, woodlands and scrub biome. FEMS Yeast Res. 2016;16(1):fov103. https://doi.org/10.1093/femsyr/fov103.
Spencer JF, Gorin PA. Yeasts isolated from soils of citrus orchards and citrus waste disposal areas in California and Florida: flavonoid utilization. Can J Microbiol. 1971;17(7):871–7. https://doi.org/10.1139/m71-140.
Gomes NC, Fagbola O, Costa R, Rumjanek NG, Buchner A, Mendona-Hagler L, Smalla K. Dynamics of fungal communities in bulk and maize rhizosphere soil in the tropics. Appl Environ Microbiol. 2003;69(7):3758–66. https://doi.org/10.1128/aem.69.7.3758-3766.2003.
França L, Sannino C, Turchetti B, Buzzini P, Margesin R. Seasonal and altitudinal changes of culturable bacterial and yeast diversity in Alpine forest soils. Extremophiles. 2016;20(6):855–73. https://doi.org/10.1007/s00792-016-0874-2.
Vishniac HS. A multivariate analysis of soil yeasts isolated from a latitudinal gradient. Microb Ecol. 2006;52(1):90–103. https://doi.org/10.1007/s00248-006-9066-4.
Shanshan L, Yuli C, Wen H, Qixia W, Cunlong L, Aimin L. Screening and identification of an oxytetracycline degradation strain resistant to Cu2+ and Zn2+ and its characteristics. J S China Agric Univ. 2020;41(3):56–62. https://doi.org/10.7671/j.issn.1001-411X.201909025.
Pan L-X, Yang D-F, Shao L, Li W, Chen G-G, Liang Z-Q. Isolation of the oleaginous yeasts from the soil and studies of their lipid-producing capacities. Food Technol Biotechnol. 2009;47. https://doi.org/10.1016/j.fm.2008.11.001.
García-Martos P, Marín P, Hernández-Molina JM, García-Agudo L, Aoufi S, Mira J. Extracellular enzymatic activity in 11 Cryptococcus species. Mycopathologia. 2001;150(1):1–4. https://doi.org/10.1023/a:1010868223582.
De Francesco G, Sannino C, Sileoni V, Marconi O, Filippucci S, Tasselli G, Turchetti B. Mrakia Gelida in brewing process: an innovative production of low alcohol beer using a psychrophilic yeast strain. Food Microbiol 2018 Dec; 76:354–362. https://doi.org/https://doi.org/10.1016/j.fm.2018.06.018.
Sugita T, Tajima M, Amaya M, Tsuboi R, Nishikawa A. Genotype analysis of Malassezia restricta as the major cutaneous flora in patients with atopic dermatitis and healthy subjects. Microbiol Immunol. 2013;48(10):755–9. https://doi.org/10.1111/j.1348-0421.2004.tb03601.x.
Urquhart E J, Punja Z K. Hydrolytic enzymes and antifungal compounds produced by Tilletiopsis species, phyllosphere yeasts that are antagonists of powdery mildew fungi. Can J Microbiol. 2002;48(3):219–29. https://doi.org/10.1139/w02-008.
Gong XM. The analysis of the features of emporal-spatial variation of desertification and its driving factors in Xinjiang. Chian: Xinjiang University; 2007.
Khan MS, Zaidi A, Ahemad M, Oves M, Wani PA. Plant growth promotion by phosphatesolubilizing fungi – current perspective. Arch Agron Soil Sci. 2010;56:73–98. https://doi.org/10.1080/03650340902806469.
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus. 2013;2:587. https://doi.org/10.1186/2193-1801-2-587.
Narsian V, Samaha SMAA, Patel HH. Rock phosphate dissolution by specific yeast. Indian J Microbiol. 2010;50:57–62. https://doi.org/10.1007/s12088-009-0019-8.
Lin C, Qin Y, Chen QJ, Lin CY. Climate characteristics and protected area vegetable production in Xinjiang. Chin Vegetables 1994;1(4): 16–20. http://www.cnveg.org/CN/Y1994/V1/I4/16.
Xu GQ, Wei WS. Climate change of Xinjiang and its impact on eco-environment. Arid Land Geography 2004; 27:14–18. https://doi.org/10.13826/j.cnki.cn65-1103/x.2004.01.003.
Wang WT, Tian B, Li J. Climate change in eastern region of Xinjiang in the past 50 years and its effects on the local ecological environment. Res Soil Water Conserv. 2014;21(05):249–54. https://doi.org/10.13869/j.cnki.rswc.2014.05.045
Guo XF, De J, Long QW, Bai BJ, Wang HJ, Zeng YP. Spatial dynamics of yeast community and its relationship with environmental factors in Lhalu wetland, Tibet. Acta Microbiol Sin. 2018;58(07):1167–81. https://doi.org/10.13343/j.cnki.wsxb.20170311.
Han L. Diversity and spatial distribution of fungi in Dianchi Lake of Yunnan plateau. Yunnan University. 2018.
Pan HX, Chen ZM, Wang LX. Studies on the micro-organisms resources in special ecological environment in Xinjiang Turpan Basin. J Arid Land Resour Environ 2002;16(4):91–94. https://doi.org/10.3969/j.issn.1003-7578.2002.04.019.
Liu KP, Wei YM, Xiong J, Sun YF, Lei YH. Analysis of diversity of endobiotic yeasts isolated from Locusta migratoria manilensis and Ischiodon scutellaris Fabricius. Guangdong Agric Sci. 2018;45(09):90–5. https://doi.org/10.16768/j.issn.1004-874X.2018.09.015.
Zhang H, Wang Y, Chen S, Zhao Z, Feng J, Zhang Z, et al. Water bacterial and fungal community compositions associated with Urban Lakes, Xi’an, China. Int J Environ Res Public Health. 2018;15(3). https://doi.org/10.3390/ijerph15030469.
Bolger, Anthony M, Lohse, Marc, Usadel, Bjoern. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014. https://doi.org/10.1093/bioinformatics/btu170.
Mago T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–2963. https://doi.org/10.1093/bioinformatics/btr507.
Zhang H, Feng J, Chen S, Li B, Raju S, Zhao Z, Jia J, Wang Y, Kang P. Disentangling the drivers of diversity and distribution of fungal community composition in wastewater treatment plants across spatial scales. Front Microbiol. 2018;9:1291. https://doi.org/10.3389/fmicb.2018.01291.
Zhang H, Jia J, Chen S, Huang T, Wang Y, Zhao Z, Feng J, Hao H, Li S, Ma X. Dynamics of bacterial and fungal communities during the outbreak and decline of an algal bloom in a drinking water reservoir. Int J Int J Environ Res Public Health. 2018;15(2). https://doi.org/10.3390/ijerph15020361.
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011; 27(16):2194–2200. https://doi.org/10.1093/bioinformatics/btr381.
Li J, Hu W, Huang X, Xu Y. Investigation of yeast population diversity and dynamics in spontaneous fermentation of Vidal blanc icewine by traditional culture-dependent and high-throughput sequencing methods. Food Res Int 2018; 112:66–77. https://doi.org/10.1016/j.foodres.2018.06.011.
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister E, et al. Introducing mothur: open-source, platform-independent, Community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537. https://doi.org/10.1128/AEM.01541-09.
Sanguin H, Sarniguet A, Gazengel K, Moënne-Loccoz Y, Grundmann GL. Rhizosphere bacterial communities associated with disease suppressiveness stages of take-all decline in wheat monoculture. New Phytol. 2009;184(3):694–707. https://doi.org/10.1111/j.1469-8137.2009.03010.x.
Bao SD. Soil agro-chemistrical analysis, vol. 268-270. Beijing: China Agriculture Press; 2007. p. 389–91.
Acknowledgments
The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Funding
The work was funded by the national natural science foundation of China (project number: 31860003). The funding agency has no role in the design, data collection, analysis or interpretation of the research or in the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
SS.Z designed and performed the experiments, analyzed the data, and drafted the manuscript. YH.L helped design experiments, analyzed the data and drafted the manuscript. C.W performed sample collection, DNA extraction, PCR amplification and analyzed part of the data. YM.W performed sample collection, and soil chemical property analysis. CC.W performed sample collection, DNA extraction and PCR amplification. YF.S designed and performed the experiments and analyzed data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval is not applicable in the case of the study. However, the collection of the soil samples from Hami melon orchards was verbally permitted by each farm owner.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Box plot of Principal Coordinates analysis (PCoA) based on Bray-Curtis distance method at the OTU level. Red, blue and green represent samples from SX, EX, and NX, respectively. and the box plots in the figure represent the discrete distribution of different groups of samples on the PCoA1 axis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhu, S., Lei, Y., Wang, C. et al. Patterns of yeast diversity distribution and its drivers in rhizosphere soil of Hami melon orchards in different regions of Xinjiang. BMC Microbiol 21, 170 (2021). https://doi.org/10.1186/s12866-021-02222-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-021-02222-1