Abstract
Background
Acinetobacter baumannii is an important opportunistic pathogen responsible for various nosocomial infections. The BfmRS two-component system plays a role in pathogenesis and antimicrobial resistance of A. baumannii via regulation of bacterial envelope structures. This study investigated the role of the sensor kinase, BfmS, in localization of outer membrane protein A (OmpA) in the outer membrane and production of outer membrane vesicles (OMVs) using wild-type A. baumannii ATCC 17978, ΔbfmS mutant, and bfmS-complemented strains.
Results
The ΔbfmS mutant showed hypermucoid phenotype in the culture plates, growth retardation under static culture conditions, and reduced susceptibility to aztreonam and colistin compared to the wild-type strain. The ΔbfmS mutant produced less OmpA in the outer membrane but released more OmpA via OMVs than the wild-type strain, even though expression of ompA and its protein production were not different between the two strains. The ΔbfmS mutant produced 2.35 times more OMV particles and 4.46 times more OMV proteins than the wild-type stain. The ΔbfmS mutant OMVs were more cytotoxic towards A549 cells than wild-type strain OMVs.
Conclusions
The present study demonstrates that BfmS controls production of OMVs in A. baumannii. Moreover, BfmS negatively regulates antimicrobial resistance of A. baumannii and OMV-mediated host cell cytotoxicity. Our results indicate that BfmS negatively controls the pathogenic traits of A. baumannii via cell envelope structures and OMV production.
Similar content being viewed by others
Background
Acinetobacter baumannii is a clinically important opportunistic pathogen responsible for various nosocomial infections, including ventilator-associated pneumonia, bacteremia, skin and soft tissue infections, urinary tract infections, and meningitis, especially in critically ill patients [1,2,3]. Treatment of this microorganism is challenging due to antimicrobial resistance, particularly to carbapenems and colistin [4, 5]. A. baumannii is one of the ‘ESKAPE’ pathogens, which are potentially antimicrobial resistant bacteria [6]. Despite their growing clinical importance, the pathogenic mechanisms of A. baumannii remain to be characterized. Of the identified virulence factors, outer membrane protein A (OmpA) is the most abundant outer membrane protein and plays a role in the pathogenesis of A. baumannii infections through biofilm formation, outer membrane vesicle (OMV) production, adherence and invasion in host cells, inactivation of the complement cascade, and host cell death [7,8,9,10,11,12,13,14]. In addition, OmpA is a major protein component in A. baumannii OMVs, in which OmpA contributes to host cell cytotoxicity and innate immune responses [13, 15]. OmpA production is tightly regulated by posttranscriptional ribo-regulation in Escherichia coli [16]. The production of OmpA is dependent on bacterial growth rate and is controlled by many environmental stresses [16,17,18]. However, little is known about the mechanisms that control localization of OmpA in either the outer membrane or OMVs.
Bacterial two-component systems (TCSs) are key factors that regulate virulence and antimicrobial resistance, and bacterial adaptation and survival in response to environmental stimuli [19, 20]. TCSs consist of a sensor kinase that senses extracellular or intracellular stimuli embedded in the cytoplasmic membrane, and a response regulator that relays signals in the cytoplasm [21]. The response regulator is a transcription factor that undergoes a conformational change upon phosphorylation and facilitates DNA binding. In A. baumannii, BfmRS regulates cell envelope structures important for virulence and antimicrobial resistance [22, 23]. The response regulator BfmR controls expression of the K locus that harbors genes for exopolysaccharide production and expression of the csuA/BABCDE operon for pili production [22, 24]. The ΔbfmR mutant showed complete loss of biofilm formation, reduced survival in human ascitic fluid and serum, and increased susceptibility to certain antimicrobial agents [24,25,26,27], whereas the ΔbfmS mutant exhibited enhanced virulence via hyperproduction of exopolysaccharides [22, 23], suggesting that BfmS negatively regulates its cognate response regulator BfmR. However, other studies demonstrated that Tn-inserted bfmS mutants showed a reduction in surface motility and bacterial growth in Galleria mellonella larvae [28, 29]. Interestingly, one previous study demonstrated that the BfmS-deficient mutant increasingly released OmpA, TEM-1 β-lactamase, and CarO into the supernatant compared to the wild-type A. baumannii strain [30]. This observation suggests that BfmS possibly controls production of OMVs, because a large amount of OmpA in culture supernatant is found in OMVs [13]. The present study was conducted to investigate whether sensor kinase BfmS controls localization of OmpA in either the outer membrane or OMVs, which subsequently affects OMV production, using wild-type A. baumannii ATCC 17978, ΔbfmS mutant, and bfmS-complemented strains.
Results
Low production of OmpA in the outer membrane of A. baumannii mutant with Tn-inserted bfmS gene
To identify genes controlling OmpA production or localization in the outer membrane, random transposon mutagenesis was performed in A. baumannii ATCC 17978. The mutant library was screened for biofilm formation at an optical density of 570 nm (OD570), because ΔompA mutant showed a significant reduction in biofilm formation [31]. Tn-inserted mutant strains that was inhibited ≥50% of biofilm formation compared with biofilm formation of the wild-type strain were then screened for OmpA production in the outer membrane using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Two mutant strains (#691 and #692), in which Tn was inserted between nucleotide 954 and 955 in the A1S_0749 (bfmS) gene, exhibited low production of OmpA in the outer membrane as compared to wild-type A. baumannii ATCC 17978 (Fig. 1).
Production of OmpA in the outer membrane fraction of transposon-inserted A. baumannii mutant strains. Bacteria were cultured in LB broth for 24 h and proteins (10 μg) in the outer membrane fractions were separated on a 12% SDS-PAGE gel. MW, molecular weight marker; WT, A. baumannii ATCC 17978; #691 and #692 mutant strains, Transposon was inserted in the open reading frame of the A1S_0749 (bfmS) gene. Western blot analysis was performed to identify 38 kDa-OmpA. Protein samples resolved on 12% SDS-PAGE gel were transferred to nitrocellulose membranes and immunoblotted with a polyclonal anti-rabbit OmpA immune sera
Construction of the ΔbfmS mutant and its protein profile in the outer membrane
To determine whether the ΔbfmS mutant increasingly released outer membrane proteins, including CarO and OmpA, in the supernatants as previously described [30], the ΔbfmS mutant (OH0790) of A. baumannii ATCC 17978 was constructed using a markerless gene deletion method [31]. The bfmS-complemented OH0883 strain was constructed (Table 1). SDS-PAGE analysis was performed in the wild-type, ΔbfmS mutant, and bfmS-complemented strains. Protein profiles in bacterial lysates were not different among the three A. baumannii strains (Fig. 2a). However, production of OmpA and ca. 33 kDa-sized proteins in the outer membrane was different between the wild-type and ΔbfmS mutant strains. The ΔbfmS mutant released more proteins, including OmpA and ca. 25 kDa-sized proteins, in the supernatants than the wild-type strain. The expression of ompA was not different among the wild-type, ΔbfmS mutant, and bfmS-complemented strains (Fig. 2b).
Production of OmpA protein and expression of ompA gene in A. baumannii strains. a SDS-PAGE analysis of bacterial proteins. The bacterial lysates and outer membrane fractions corresponding to 10 μg of protein were separated on a 12% SDS-PAGE gel. Proteins precipitated from the culture supernatants (200 ml) were resuspended in 200 μl of PBS and then 15 μl of the samples were separated on 12% SDS-PAGE gel. Lane MW, molecular weight marker; 1, A. baumannii ATCC 17978; 2, ΔbfmS mutant OH0790; 3, ΔbfmS-complemented OH0883. b Transcription levels of ompA in the three A. baumannii strains were determined using qPCR. The data are mean ± SD expression levels of the target gene in each strain relative to expression of this gene in A. baumannii ATCC 17978. Data were obtained from three independent experiments
Phenotypic characteristics of the ΔbfmS mutant strain
To determine whether bfmS affected the growth of A. baumannii strains, bacterial growth was measured at OD600. The growth rate was not different between the wild-type and ΔbfmS mutant strains cultured under shaking conditions, but growth retardation was observed in the ΔbfmS mutant cultured under static conditions (Fig. 3a). To investigate whether deletion of bfmS led to hyperproduction of exopolysaccharides as previously described [23], A. baumannii strains were cultured in blood agar plates for 24 h. The ΔbfmS mutant OH0790 was more viscous than the wild-type strain (Fig. 3b). Deletion of the bfmS gene did not alter the expression of bfmR in A. baumannii (Fig. 3c). Bacterial growth in static and shaking culture conditions, the production of exopolysaccharides, and the expression of bfmS were restored in the bfmS-complemented OH0883 strain.
Characteristics of the ΔbfmS mutant strain. a A. baumannii strains were grown in LB broth under shaking or static conditions and then OD600 was determined at the indicated times. The data are representative of three experiments with similar results. b A. baumannii strains were cultured overnight on blood agar plates. c Transcription levels of bfmS and bfmR in A. baumannii strains were determined using qPCR. The data are mean ± SD expression levels of the target genes in each strain relative to expression of these genes in A. baumannii ATCC 17978. Data were obtained from three independent experiments
Effect of bfmS on pathogenic traits of A. baumannii
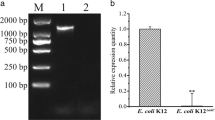
To investigate the role of bfmS in pathogenic traits of A. baumannii, the ability of wild-type and ΔbfmS mutant strains to form biofilms on a polystyrene surface was determined. Bacterial growth at OD600 and biofilm cells at OD570 were significantly different between the wild-type and ΔbfmS mutant strains, respectively, but biofilm cells relative to planktonic and sessile cells (OD570/600) were not different between the two strains (Fig. 4a). Complementation of the bfmS gene deletion restored the wild-type biofilm formation phenotype. The expression of csuC and csuD, which are required for pili assembly and biofilm formation [24], was not different between the wild-type and ΔbfmS mutant strains (Fig. 4b). Next, to investigate the involvement of bfmS in adherence and invasion of host cells, A549 cells were infected with A. baumannii strains at multiplicity of infection (MOI) 100 for 3 h, and the number of bacteria adhered to and invading A549 cells was counted. No significant differences in numbers of bacteria were observed between wild-type (2.57 × 105 colony forming units [CFUs]), ΔbfmS mutant (3.47 × 105 CFUs), and bfmS-complemented (7.09 × 105 CFUs) strains (Fig. 4c). The CFUs of ΔompA mutant HKD14 were significantly decreased compared to the wild-type strain, as observed in a previous study [31].
Biofilm formation, expression of the csuCD genes, and interactions with host cells in A. baumannii strains. a Biofilms formed on 5 ml polystyrene tubes were stained with crystal violet. The amount of crystal violet eluted from the biofilms with ethanol was quantified as the OD570 normalized to total bacterial growth (OD600). The data are presented as mean ± SD of three independent experiments. ** p < 0.01 compared to wild-type ATCC 17978. b Transcription levels of csuC and csuD in the three A. baumannii strains were determined using qPCR. Data are mean ± SD expression levels of the target genes in each strain relative to expression of these genes in A. baumannii ATCC 17978. The data were obtained from three independent experiments. c Adherence to and invasion of A549 cells by A. baumannii strains. A549 cells were infected with the A. baumannii strains at MOI 100 for 3 h, and then the cell monolayers were lysed with Triton X-100. Dilutions of the bacterial lysates were plated on LB agar, and CFUs were counted. The data are presented as mean ± SD of three independent experiments
Effect of bfmS on the antimicrobial susceptibility of A. baumannii
Minimum inhibitory concentrations (MICs) of antimicrobial agents for the wild-type, ΔbfmS mutant, and bfmS-complemented strains were determined. The ΔbfmS mutant was more resistant to aztreonam (2.67-fold) and colistin (2.63-fold) than the wild-type strain (Table 2). The remaining antimicrobial agents tested showed the same or a < 2-fold difference in MICs for the ΔbfmS mutant. MICs of all antimicrobial agents determined for the bfmS-complemented strain were the same as, or similar to, those for the wild-type strain.
Effect of bfmS on OMV production
We determined OMV production in the ΔbfmS mutant, because a large amount of OmpA in the culture supernatants was packaged in A. baumannii OMVs [13]. A. baumannii strains were cultured in Luria-Bertani (LB) broth to reach late exponential phase and then OMVs were isolated from the culture supernatants. The sizes of OMVs from the wild-type, ΔbfmS mutant, and bfmS-complemented strains were 193.7 ± 11.9 nm, 186.8 ± 1.6 nm, and 174.8 ± 1.3 nm, respectively (Fig. 5a). OMV samples obtained from 1 L culture of the wild-type, ΔbfmS mutant, and bfmS-complemented strains contained 5.1 × 1012, 1.2 × 1013, and 8.4 × 1012 particles, respectively. The ΔbfmS mutant produced 4.46 (233.3 ± 38.7 μg/L) times more OMV proteins than the wild-type strain (52.3 ± 8.7 μg/L) (Fig. 5b). Further, SDS-PAGE analysis exhibited that protein profiles were very similar among the three different OMVs, but the intensity of several protein bands was different between OMVs from the wild-type and ΔbfmS mutant strains (Fig. 5c). Western blot analysis showed that OMVs derived from the ΔbfmS mutant contained more OmpA than those from the wild-type strain.
OMV production and its protein profile in A. baumannii strains. (a and b) Production of OMVs from A. baumannii strains. OMVs were isolated from A. baumannii cultured in LB broth. a The size and number of OMV particles isolated from three A. baumannii strains were determined using nanoparticle tracking analysis. The data are representative of three independent experiments with similar results. b The protein concentration of OMVs isolated from 1 L of bacterial culture was measured using a modified BCA assay. The data are presented as mean ± SD of two independent experiments. ** p < 0.01 compared to wild-type ATCC 17978. c SDS-PAGE and western blot analyses of OMV proteins. Protein samples were resolved by SDS-PAGE in 12% gels, transferred to nitrocellulose membranes, and immunoblotted with a polyclonal anti-rabbit OmpA immune sera. Lane MW, molecular weight marker; 1, A. baumannii ATCC 17978; 2, ΔbfmS mutant OH0790; 3, ΔbfmS-complemented OH0883
Effect of bfmS on OMV-mediated pathogenesis of A. baumannii
To determine whether OMVs derived from the wild-type and ΔbfmS mutant strains played a different role in biofilm formation, OMVs (5 μg/ml) isolated from the wild-type, OH0790, and OH0883 strains were added to each bacterial culture after A. baumannii strains were inoculated in a polystyrene tube. Biofilm formation (OD570/600) was not significantly different between the wild-type and ΔbfmS mutant strains regarding the treatment of different OMVs (Fig. 6a). Next, we determined host cell cytotoxicity induced by OMVs isolated from the three A. baumannii strains, because OmpA in A. baumannii OMVs was responsible for the cytotoxicity of epithelial cells [13]. A549 cells were treated with various concentrations (0.625–20 μg/ml protein concentrations) of OMVs isolated from three A. baumannii strains for 24 h, and cell viability was assessed using the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT) assay. Cytotoxicity was induced in A549 cells treated with 20 μg/ml of the wild-type and bfmS-complemented strain OMVs, whereas cytotoxicity was induced in A549 cells treated with ≤0.625 μg/ml of the ΔbfmS mutant OMVs (Fig. 6b). Cytotoxicity significantly differed between the wild-type and ΔbfmS mutant OMVs at concentrations ≥0.625 μg/ml.
Pathogenic effect of OMVs derived from three A. baumannii strains. a Biofilm formation of A. baumannii strains cultured with OMVs from different A. baumannii strains. A. baumannii was inoculated in polystyrene tubes and then OMVs (5 μg/ml) obtained from different A. baumannii strains were added to bacterial culture media. Biofilms formed on polystyrene tubes were stained with crystal violet. The amount of crystal violet eluted from the biofilms with ethanol was quantified as the OD570 normalized to total bacterial growth (OD600). The data are presented as mean ± SD of three independent experiments. b Cytotoxicity of A549 cells treated with OMVs from A. baumannii strains. Cells were treated with various concentrations of A. baumannii OMVs for 24 h. Cell viability was determined using the MTT assay. Data are presented as mean ± SD of three independent experiments. + p < 0.05 compared to untreated control cells. * p < 0.05 comparing the same concentration of OMVs from A. baumannii ATCC 17978
Discussion
The response regulator BfmR contributes to the pathogenesis of A. baumannii infections regarding biofilm formation, antimicrobial resistance, and bacterial survival and disease process in animal models, whereas sensor kinase BfmS negatively regulates BfmR and plays a less relevant role [22,23,24,25,26,27]. The present study demonstrated that BfmS controlled OMV production. Moreover, BfmS negatively regulated resistance to aztreonam and colistin and OMV-mediated host cell cytotoxicity.
Two research groups characterized the role of BfmS in the pathogenicity of A. baumannii ATCC 17978 using BfmS-deficient mutant strains [22, 23, 30]. Liou et al. [30] showed that insertional inactivation of the bfmS gene in A. baumannii 17,978 resulted in reduction in biofilm formation, adherence to host cells, and survival in human serum. However, other studies conducted by Geisinger et al. [22, 23] demonstrated that the ΔbfmS mutant of A. baumannii 17,978 constructed by allelic replacements with the aacC1 gene was resistant to killing by rabbit serum and was more virulent than the wild-type strain in a murine model of systemic infection. The discrepancy in virulence of A. baumannii ATCC 17978 mutants lacking BfmS between the two study groups was possibly due to different methods of mutant construction. We therefore constructed the ΔbfmS mutant of A. baumannii ATCC 17978 by markerless, in-frame deletions. Phenotypes of the ΔbfmS mutant constructed in this study, regarding bacterial growth under shaking culture conditions and hyperproduction of exopolysaccharides, were consistent with the previous studies [22, 23]. However, the ΔbfmS mutant constructed in this study exhibited growth retardation under static culture conditions. The ability to form biofilms (OD570/600) was not different between the wild-type and ΔbfmS mutant strains. The mutant of A. baumannii ATCC 17978 with bfmS::Tn showed a significant reduction in biofilm formation, which only measured biofilm cells by staining with crystal violet at OD595 [30], whereas the mutant derivative of A. baumannii ATCC 19606 with bfmS::Tn displayed no drastic defect in biofilm formation, which measured the biofilm cells relative to bacterial growth (OD580/600) [24]. The low ability to form biofilms in the A. baumannii ATCC 17978 mutant with bfmS::Tn conducted by Liou et al. [30] possibly resulted from growth retardation of this mutant strain under static culture conditions.
BfmS negatively regulates the production of capsular exopolysaccharides via phosphorylation of the cognate regulator BfmR [22]. In the present study, the ΔbfmS mutant showed hypermucoid phenotype as compared to the wild-type strain, but bfmR gene expression was not different between the two strains. The expression of csuC and csuD genes was not different between the wild-type and ΔbfmS mutant strains. RNA-sequencing analysis also showed that deletion of the bfmS gene did not significantly alter expression of the bfmR gene and csuA/BABCDE operon in A. baumannii ATCC 17978 [23]. The csuA/BABCDE operon plays a role in biofilm formation, but not in adherence to bronchial epithelial cells [24]. OmpA contributes to both biofilm formation and adherence to host cells [10, 31]. Adherence and invasion of A. baumannii in host cells were not different between the wild-type and ΔbfmS mutant strains, although the ΔbfmS mutant produced less OmpA in the outer membrane than the wild-type strain. Other bacterial molecules such as poly-β-(1,6)-N-acetyl glucosamine [34], a homolog of the staphylococcal biofilm-associated protein (BAP) [35], BAP-like proteins [36], and the products of LHp2_11085 gene [37] may compensate biofilm formation and host cell adherence of the ΔbfmS mutant. Taken together, our results suggest that deletion of bfmS increases the production of capsular exopolysaccharides but does not affect biofilm formation and adherence and invasion of A. baumannii ATCC 17978 in host cells.
The ΔbfmS mutant produced more OMV particles than the wild-type strain. Moreover, the ΔbfmS mutant released more proteins, including OmpA, via OMVs in the supernatants. Instead, the ΔbfmS mutant produced less OmpA in the outer membrane than the wild-type strain. Although the biogenesis of OMVs was not fully understood, several models of OMV biogenesis were proposed, such as a reduction in cross-linking between the outer membrane and peptidoglycans [38], accumulation of phospholipids in the outer leaflet of the outer membrane [39], and deacylation of lipopolysaccharides [40]. We previously showed that the ΔompA mutant of A. baumannii ATCC 19606 produced 13.2 times more OMV proteins and 7.30 times more OMV lipopolysaccharides than the wild-type strain [41]. These results suggest that OmpA directly or indirectly contributes to the production of A. baumannii OMVs. OmpA interacts with other membrane proteins in the outer and inner membranes and peptidoglycans [42, 43]. The C-terminal OmpA-like domain of OmpA interacts with diaminopimelate of peptidoglycan [43]. Therefore, low localization of OmpA in the outer membrane reduces interaction of the outer membrane with peptidoglycan, which may increase OMV production. The association of bacterial extracellular vesicle production with TCSs was reported in Streptococcus pyogenes [44]. Inactivating mutations in sensor kinase (CovS) of control of virulence regulator-sensor (CovRS) increased extracellular vesicle production in S. pyogenes. Moreover, mutant strains expressing truncated and inactive CovS produced a significantly higher number of extracellular vesicles relative to the wild-type strain. Although the association of OMV biogenesis with TCSs, especially in sensor kinases, has not been characterized in gram-negative bacteria, genes under the control of BfmS or BfmRS may regulate OMV biogenesis. The exact mechanisms by which BfmS controls OMV production should be determined in further studies.
OMVs derived from the ΔbfmS mutant were more cytotoxic in cultured epithelial cells than OMVs from the wild-type strain. We previously showed that several virulence factors, including OmpA, β-lactamases, and tissue-degrading enzymes, were associated with OMVs of A. baumannii ATCC 19606 [13, 45]. The OMVs derived from A. baumannii ATCC 19606 induced host cell death, whereas OMVs from the ΔompA mutant did not [13], thus suggesting that OmpA in OMVs is directly responsible for host cell cytotoxicity. In this study, the ΔbfmS mutant rather than wild-type strain showed a reduced susceptibility to colistin and aztreonam. Hyperproduction of exopolysaccharides in the ΔbfmS mutant may explain reduced susceptibility to colistin [26]. The ΔbfmS mutant of A. baumannii 17,978 constructed by allelic replacements with the aacC1 gene also showed a reduced susceptibility to aminoglycosides (amikacin) and β-lactams (mecillinam, ampicillin, carbenicillin, cephalexin, aztreonam, ceftazidime, and sulbactam), whereas the ΔbfmRS mutant showed hypersensitivity to several classes of antimicrobial agents, including aminoglycosides and β-lactams [23]. The BfmRS system controls antimicrobial resistance via cell wall homeostasis, and BfmS negatively regulates the resistance activity of BfmR.
Conclusions
The BfmRS system regulates the physiology and pathogenic traits of A. baumannii. However, the role of BfmS in the pathogenic traits of A. baumannii is still poorly understood. Here, we demonstrate that BfmS controls production of OMVs and regulates antimicrobial resistance and OMV-mediated host cell cytotoxicity. Understanding of the BfmRS-mediated regulatory system is expected to provide insights into A. baumannii pathogenicity. Controlling the BfmS may represent a strategy to combat this notorious pathogen, because overproduction of OmpA in A. baumannii is a risk factor for nosocomial pneumonia, bacteremia, and high mortality rate [46].
Methods
Bacterial strains, plasmids, and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 1. A. baumannii ATCC 17978 was purchased from American Type Culture Collection (ATCC). Escherichia coli DH5α (Catalogue number 18258012) was purchased from Invitrogen (Grand Island, NY, USA). Bacteria were grown in LB medium at 37 °C. A. baumannii strains were cultured in blood agar plates containing 5% sheep red blood cells for the analysis of viscosity of bacterial colonies. Chloramphenicol (20 μg/ml) or kanamycin (50 μg/ml) was added to the growth medium to maintain plasmids in E. coli. A. baumannii merodiploids were selected on medium supplemented with kanamycin (30 μg/ml) and ampicillin (100 μg/ml).
Random transposon mutagenesis
A mutant library of A. baumannii was constructed by random transposon mutagenesis. A. baumannii ATCC 17978 was mutagenized using the S17–1 λ pir tra strain [32] containing pRL27, a suicide vector carrying the transposable mini-Tn5 element [33]. Tn-inserted colonies were selected by plating on LB agar plates containing 50 μg/ml kanamycin and stored at − 80 °C until use. To determine transposon insertion sites on the bacterial genome, bacterial genomic DNA was digested by BamHI. The digested DNA was ligated with BamHI-digested pBR322 (Catalogue number N3033 L, New England Biolabs, Ipswich, MA, USA) and then introduced into E. coli DH5α. The transposon insertion site was analyzed by DNA sequencing.
Construction of the ΔbfmS mutant strain
The bfmS (A1S_0749) gene of A. baumannii ATCC 17978 was deleted by an overlap extension polymerase chain reaction (PCR) method as previously described [31]. The genomic DNAs purified from A. baumannii strains and pUC4K (Catalogue number 27–4958-01, Amersham Pharmacia Biotech, Piscataway, NJ, USA) for amplification of the kanamycin resistance cassette were used as templates for the PCR. In brief, a mutated DNA fragment, in which upstream and downstream regions of the bfmS gene were combined with nptI conferring kanamycin resistance by overlap extension PCR using specific primers (Table 3), was ligated into FspI-digested pHKD01 to generate pOH786 (Table 1). E. coli S17–1 λ pir strain containing pOH786 was used as a conjugal donor to A. baumannii ATCC 17978. Conjugation and isolation of the transconjugants were performed as previously described [31]. Deletion of the bfmS gene in A. baumannii ATCC 17978 was confirmed by PCR analysis and the ΔbfmS mutant was named OH0790 (Table 1).
Complementation of the bfmS gene in the ΔbfmS mutant strain
To complement the bfmS mutation, the bfmS coding region with its native promoter was inserted into the attTn7 site located downstream of the glmS gene in the genome of A. baumannii ATCC 17978 using the modified markerless gene deletion method [31]. A DNA fragment, in which the bfmS coding region with its native promoter and the upstream and downstream regions of the attTn7 site were fused with nptI by overlap extension PCR using specifi14primers (Table 3), was cloned into FspI-digested pHKD01 to generate pOH875 (Table 1). The chimeric plasmid was integrated into the chromosome of the ΔbfmS mutant by conjugation-based gene transfer and homologous recombination. Insertion of the bfmS coding region with its native promoter was confirmed by PCR analysis. The bfmS-complemented strain was named OH0883 (Table 1).
Isolation of OMVs
OMVs of A. baumannii strains were prepared from bacterial culture supernatants as previously described [13, 47]. Bacteria were cultured with 500 ml of LB broth with shaking at 37 °C until to reach late exponential phase (OD600 of 1.5). Bacterial cells were harvested by centrifugation at 8000 g for 15 min, and supernatants were filtered using a bottle-top filter with a 0.22 μm membrane. The filtered supernatants were concentrated using a QuixStand Benchtop System (GE Healthcare, Amersham, UK) with a 500 kDa hollow fiber membrane (GE Healthcare). OMV samples were collected by ultracentrifugation at 150,000 g at 4 °C for 3 h and then washed in phosphate-buffered saline (PBS) followed by another ultracentrifugation. The OMV fractions were then resuspended in PBS. The protein concentration of OMVs was determined using a modified bicinchoninic acid (BCA) assay (Thermo Scientific, Waltham, MA, USA). The purified OMVs were streaked on blood agar plates to check for sterility and then stored at -80 °C until use.
SDS-PAGE and western blotting
Bacteria were cultured in LB broth with shaking at 37 °C until to reach 1.5 at OD600. Cultured bacterial cells were harvested and lysed by sonication (Branson Ultrasonics Corp., Danbury, CT, USA). After centrifugation at 1700 g for 20 min, the supernatant was centrifuged at 100,000 g for 1 h at 4 °C. The pellet containing cell envelope was resuspended in 10 mM HEPES buffer with 2% sodium lauryl sarcosine and incubated for 30 min at room temperature to solubilize the inner membrane. Then the suspension was centrifuged at 100,000 g for 1 h at 4 °C and outer membrane fractions were resuspended in PBS. Proteins in the culture supernatants (200 ml) were precipitated with 80% ammonium sulfate and then 10% trichloroacetic acid, and the samples were resuspended in 200 μl of PBS. The bacterial lysate, outer membrane fractions, and purified OMVs corresponding to 10 μg of protein were resuspended in SDS-PAGE sample buffer (1 M Tris HCl [pH 6.8], 10% SDS, 1% bromophenol blue, glycerol, and β-mercaptoethanol) and boiled for 10 min. Precipitated proteins (15 μl) in the culture supernatants were resuspended in SDS-PAGE sample buffer. The proteins were separated on a 12% SDS-PAGE gel, and gels were stained with Coomassie brilliant blue R-250 (Bio-Rad, Hercules, CA, USA). Western blot analysis was performed following SDS-PAGE. Proteins were electroblotted onto nitrocellulose membrane. Membranes were incubated with a polyclonal anti-rabbit OmpA immune serum. The membrane was incubated with a secondary antibody coupled to horseradish peroxidase and developed using an enhanced chemiluminescence system (Amersham Pharmacia Biotech).
Nanoparticle tracking analysis (NTA)
OMV size and concentration were measured using a NanoSight NS500 instrument with a 488 nm laser module and sCMOS camera module (Malvern Instruments, Worcestershire, UK) [48]. Briefly, OMV samples were diluted in MilliQ water to a concentration of approximately 8–9 × 108 particles/ml; the NTA measurement yielded 50–100 particles per frame. Samples were loaded in the sample chamber and videos were recorded for 30s three times. The captured data were analysed using NTA 3.1 software build 3.1.46. All measurements were performed in triplicate at room temperature.
Bacterial growth studies
Overnight cultures of A. baumannii strains were diluted 1:20 in LB broth and cultured under shaking or static conditions for 36 and 60 h at 37 °C, respectively. Bacteria were sampled at the indicated times, and OD600 was determined. Bacterial growth was determined in triplicate.
Biofilm assay
A biofilm formation assay was performed as previously described [14]. Overnight cultures were adjusted to an OD600 of 2.0, and diluted 200-fold in LB medium without sodium chloride. Aliquots (2 ml) of the bacterial suspension were inoculated into 5 ml polystyrene tubes and incubated without shaking at 37 °C for 24 h. Planktonic cells were removed, and the tubes were washed twice with 1 ml of PBS. Biofilm cells on the tube wall were stained with 0.1% w/v crystal violet solution for 15 min at room temperature. Then, biofilm formation was quantified using a biofilm cell-associated dye, which was eluted with 100% ethanol, as the absorbance at OD570, which was normalized to bacterial growth at OD600. To evaluate whether OMVs derived from A. baumannii strains affected biofilm formation, OMVs (5 μg/ml) were added to the bacterial culture after inoculation of bacteria in the tubes. Biofilm formation ability of the Tn-inserted A. baumannii mutants was determined using 96-well cell culture plates. A total of 200 μl of the bacterial suspension was incubated in U-bottomed 96-well microtiter plates at 37 °C for 24 h. In each plate, the wild-type strain was included as a control. Biofilm assays were performed in duplicate and repeated three times.
Antimicrobial susceptibility test
MICs were determined by the Etest method according to the manufacturer’s instructions. Antimicrobial agents included aztreonam, ceftazidime, ciprofloxacin, colistin, gentamicin, imipenem, nalidixic acid, tetracycline, tigecycline, tobramycin, and trimethoprim (bioMe’rieux, Marcy-l’_Etoile, France). E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as quality control strains. Interpretation of antimicrobial susceptibility was based on guidelines of the Clinical Laboratory Standards Institute (CLSI) [49].
RNA isolation and quantitative PCR
The mRNA expression levels of bfmR, bfmS, ompA, csuC, and csuD genes were analyzed. Bacteria were cultured to an OD600 of 1.5 in LB broth with shaking at 37 °C. Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Complementary DNA was generated by reverse transcription of 2 μg of total RNA using oligo dT primers and M-MLV reverse transcriptase in a total reaction volume of 20 μl (Enzynomics, Daejeon, Korea). The specific primers for csuC and csuD genes were described in previous studies [14]. The primer sequences were 5′-GTT TAA CCG TTT GTC GTG-3′ and 5′-GTG GTT GAA CTG GTT TCG-3′ for bfmR, 5′-TTG AAC TTA TTC ACC GCC TTT-3′ and 5′-GCC CGT AAT CCG AAC TTT GTT-3′ for bfmS, and 5′- TTG CAC TTG CTA CTA TGC TTG TTG-3′ and 5′- TGG CTG TCT TGG AAA GTG TAA CC-3′ for ompA. Gene transcripts were quantified using TOPreal™ qPCR 2X PreMIX (SYBR Green with high ROX) (Enzynomics) with an ABI PRISM 7500 Real-Time System (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. The amplification specificity was evaluated using melting curve analysis. Gene expression was normalized to 16S rRNA expression in each sample, and the fold change was determined using the ΔΔCt method. Gene expression assays were performed in three independent experiments.
Cell culture
Human lung epithelial A549 cells were used to analyze interactions with bacteria or OMVs. A549 cells were obtained from the Korean Cell Line Bank (Seoul, Korea). A549 cells were grown in RPMI 1640 medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (HyClone), 2.0 mM L-glutamine, 100 U/ml penicillin, and 50 mg/ml streptomycin at 37 °C in 5% CO2. Confluent cells were seeded in 24- and 96-well plates for bacterial adherence and cell viability assays, respectively.
Adherence and invasion assays
Adherence and invasion of A549 cells by A. baumannii strains were determined as previously described [10]. A549 cells were seeded at a density of 6 × 104 cells in 24-well culture dishes. Cells were infected with A. baumannii strains at MOI 100 for 3 h. The infected monolayers were washed five times with PBS and then lysed with 0.1% Triton X-100 at 37 °C for 20 min. Dilutions of the lysates were plated on LB agar, and colonies were enumerated after 20 h of incubation. CFUs of the ΔbfmS mutant were compared with those of the wild-type and ΔompA mutant strain (HKD14) of A. baumannii ATCC 17978 as the positive and negative controls, respectively. Adherence and invasion assays were performed in three independent experiments.
Cell viability test
The viability of A549 cells was measured using the MTT assay (Abcam, Cambridge, UK). Cells were seeded at a concentration of 2 × 104/well in a 96-well microplate. After treatment with different concentrations of A. baumannii OMVs for 24 h, cell viability was measured 3 h after treatment with MTT reagent at 600 nm. The cell viability assay was performed in three independent experiments.
Statistical analysis
Data were analyzed using R 3.3.4 (https://www.r-project.org/). One-way analysis of variance (ANOVA) and Student’s t-tests were performed and post-hoc tests were applied when needed. Differences of p < 0.05 were considered statistically significant.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- Apr :
-
Ampicillin-resistant
- CFUs:
-
Colony forming units
- Cmr :
-
Chloramphenicol-resistant
- Kmr :
-
Kanamycin-resistant
- MICs:
-
Minimum inhibitory concentrations
- MOI:
-
Multiplicity of infection
- OmpA:
-
Outer membrane protein A;
- OMVs:
-
Outer membrane vesicles
- Smr :
-
Streptomycin-resistant
- Tcr :
-
Tetracycline-resistant
- TCSs:
-
Two-component systems
- Tpr :
-
Trimethoprim-resistant
References
Martín-Aspas A, Guerrero-Sánchez FM, García-Colchero F, Rodríguez-Roca S, Girón-González JA. Differential characteristics of Acinetobacter baumannii colonization and infection: risk factors, clinical picture, and mortality. Infect Drug Resist. 2018;11:861–72.
Antunes LCS, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis. 2014;71:292–301.
Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–82.
Piperaki ET, Tzouvelekis LS, Miriagou V, Daikos GL. Carbapenem-resistant Acinetobacter baumannii: in pursuit of an effective treatment. Clin Microbiol Infect. 2019;25:951–7.
Asif M, Alvi IA, Rehman SU. Insight into Acinetobacter baumannii: pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect Drug Resist. 2018;11:1249–60.
Pendleton JN, Gorman SP, Gilmore BF. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti-Infect Ther. 2013;11:297–308.
Morris FC, Dexter C, Kostoulias X, Uddin MI, Peleg AY. The mechanisms of disease caused by Acinetobacter baumannii. Front Microbiol. 2019;10:1601.
Lee JS, Choi CH, Kim JW, Lee JC. Acinetobacter baumannii outer membrane protein a induces dendritic cell death through mitochondrial targeting. J Microbiol. 2010;48:387–92.
Kim SW, Choi CH, Moon DC, Jin JS, Lee JH, Shin JH, et al. Serum resistance of Acinetobacter baumannii through the binding of factor H to outer membrane proteins. FEMS Microbiol Lett. 2009;301:224–31.
Choi CH, Lee JS, Lee YC, Park TI, Lee JC. Acinetobacter baumannii invades epithelial cells and outer membrane protein a mediates interactions with epithelial cells. BMC Microbiol. 2008;8:216.
Choi CH, Hyun SH, Kim J, Lee YC, Seol SY, Cho DT, et al. Nuclear translocation and DNAse I-like enzymatic activity of Acinetobacter baumannii outer membrane protein a. FEMS Microbiol Lett. 2008;288:62–7.
Choi CH, Hyun SH, Lee JY, Lee JS, Lee YS, Kim SA, et al. Acinetobacter baumannii outer membrane protein a targets the nucleus and induces cytotoxicity. Cell Microbiol. 2008;10:309–19.
Jin JS, Kwon SO, Moon DC, Gurung M, Lee JH, Kim SI, et al. Acinetobacter baumannii secretes cytotoxic outer membrane protein a via outer membrane vesicles. PLoS One. 2011;6:e17027.
Selasi GN, Nicholas A, Jeon H, Na SH, Kwon HI, Kim YJ, et al. Differences in biofilm mass, expression of biofilm-associated genes, and resistance to desiccation between epidemic and sporadic clones of carbapenem-resistant Acinetobacter baumannii sequence type 191. PLoS One. 2016;11:e0162576.
Jun SH, Lee JH, Kim BR, Kim SI, Park TI, Lee JC, et al. Acinetobacter baumannii outer membrane vesicles elicit a potent innate immune response via membrane proteins. PLoS One. 2013;8:e71751.
Smith SG, Mahon V, Lambert MA, Fagan RP. A molecular Swiss army knife: OmpA structure, function and expression. FEMS Microbiol Lett. 2007;273:1–11.
Lugtenberg B, Peters R, Bernheimer H, Berendsen W. Influence of cultural conditions and mutations on the composition of the outer membrane proteins of Escherichia coli. Mol Gen Genet. 1976;147:251–62.
Nilsson G, Belasco JG, Cohen SN, Von Gabain A. Growth-rate dependent regulation of mRNA stability in Escherichia coli. Nature. 1984;312:75–7.
Zschiedrich CP, Keidel V, Szurmant H. Molecular mechanisms of two-component signal transduction. J Mol Biol. 2016;428:3752–75.
Groisman EA. Feedback control of two-component regulatory systems. Annu Rev Microbiol. 2016;70:103–24.
Choi J, Groisman EA. Acidic pH sensing in the bacterial cytoplasm is required for Salmonella virulence. Mol Microbiol. 2016;101:1024–38.
Geisinger E, Isberg RR. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog. 2015;11:e1004691.
Geisinger E, Mortman NJ, Vargas-Cuebas G, Tai AK, Isberg RR. A global regulatory system links virulence and antibiotic resistance to envelope homeostasis in Acinetobacter baumannii. PLoS Pathog. 2018;14:e1007030.
Tomaras AP, Flagler MJ, Dorsey CW, Gaddy JA, Actis LA. Characterization of a two-component regulatory system from Acinetobacter baumannii that controls biofilm formation and cellular morphology. Microbiology. 2008;154(Pt 11):3398–409.
Umland TC, Schultz LW, MacDonald U, Beanan JM, Olson R, Russo TA. In vivo-validated essential genes identified in Acinetobacter baumannii by using human ascites overlap poorly with essential genes detected on laboratory media. MBio. 2012;3:e00113-12.
Russo TA, Manohar A, Beanan JM, Olson R, MacDonald U, Graham J, et al. The response regulator BfmR Is a potential drug target for Acinetobacter baumannii. mSphere. 2016;1:e00082-16.
Wang N, Ozer EA, Mandel MJ, Hauser AR. Genome-wide identification of Acinetobacter baumannii genes necessary for persistence in the lung. MBio. 2014;5:e01163–14.
Clemmer KM, Bonomo RA, Rather PN. Genetic analysis of surface motility in Acinetobacter baumannii. Microbiology. 2011;157(Pt 9):2534–44.
Gebhardt MJ, Gallagher LA, Jacobson RK, Usacheva EA, Peterson LR, Zurawski DV, et al. Joint transcriptional control of virulence and resistance to antibiotic and environmental stress in Acinetobacter baumannii. MBio. 2015;6:e01660–15.
Liou ML, Soo PC, Ling SR, Kuo HY, Tang CY, Chang KC. The sensor kinase BfmS mediates virulence in Acinetobacter baumannii. J Microbiol Immunol Infect. 2014;47:275–81.
Oh MH, Lee JC, Kim J, Choi CH, Han K. Simple method for markerless gene deletion in multidrug-resistant Acinetobacter baumannii. Appl Environ Microbiol. 2015;81:3357–68.
Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering transposon mutagenesis in gram negative bacteria. Nat Biotechnol. 1983;1:784–91.
Larsen RA, Wilson MM, Guss AM, Metcalf WW. Genetic analysis of pigment biosynthesis in Xanthobacter autotrophicus Py2 using a new, highly efficient transposon mutagenesis system that is functional in a wide variety of bacteria. Arch Microbiol. 2002;178:193–201.
Choi AH, Slamti L, Avci FY, Pier GB, Maira-Litrán T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-β-1-6-N-acetylglucosamine, which is critical for biofilm formation. J Bacteriol. 2009;191:5953–63.
Loehfelm TW, Luke NR, Campagnari AA. Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J Bacteriol. 2008;190:1036–44.
De Gregorio E, Del Franco M, Martinucci M, Roscetto E, Zarrilli R, Di Nocera PP. Biofilm-associated proteins: news from Acinetobacter. BMC Genomics. 2015;16:933.
Álvarez-Fraga L, Pérez A, Rumbo-Feal S, Merino M, Vallejo JA, Ohneck EJ, et al. Analysis of the role of the LH92_11085 gene of a biofilm hyper-producing Acinetobacter baumannii strain on biofilm formation and attachment to eukaryotic cells. Virulence. 2016;7:443–55.
Jan AT. Outer membrane vesicles (OMVs) of gram-negative bacteria: a perspective update. Front Microbiol. 2017;8:1053.
Roier S, Zingl FG, Cakar F, Durakovic S, Kohl P, Eichmann TO, et al. A novel mechanism for the biogenesis of outer membrane vesicles in gram-negative bacteria. Nat Commun. 2016;7:10515.
Elhenawy W, Bording-Jorgensen M, Valguarnera E, Haurat MF, Wine E, Feldman MF. LPS remodeling triggers formation of outer membrane vesicles in Salmonella. MBio. 2016;7:e00940-16.
Moon DC, Choi CH, Lee JH, Choi CW, Kim HY, Park JS, et al. Acinetobacter baumannii outer membrane protein a modulates the biogenesis of outer membrane vesicles. J Microbiol. 2012;50:155–60.
Wu X, Chavez JD, Schweppe DK, Zheng C, Weisbrod CR, Eng JK, et al. In vivo protein interaction network analysis reveals porin-localized antibiotic inactivation in Acinetobacter baumannii strain AB5075. Nat Commun. 2016;7:13414.
Park JS, Lee WC, Yeo KJ, Ryu KS, Kumarasiri M, Hesek D, et al. Mechanism of anchoring of OmpA protein to the cell wall peptidoglycan of the gram-negative bacterial outer membrane. FASEB J. 2012;26:219–28.
Resch U, Tsatsaronis JA, Le Rhun A, Stübiger G, Rohde M, Kasvandik S, et al. A two-component regulatory system impacts extracellular membrane-derived vesicle production in group A Streptococcus. MBio. 2016;7:e00207-16.
Yun SH, Choi CW, Park SH, Lee JC, Lee SH, Choi JS, et al. Proteomic analysis of outer membrane proteins from Acinetobacter baumannii DU202 in tetracycline stress condition. J Microbiol. 2008;46:720–7.
Sánchez-Encinales V, Álvarez-Marín R, Pachón-Ibáñez ME, Fernández-Cuenca F, Pascual A, Garnacho-Montero J, et al. Overproduction of outer membrane protein a by Acinetobacter baumannii as a risk factor for nosocomial pneumonia, bacteremia, and mortality rate increase. J Infect Dis. 2017;215:966–74.
Yun SH, Park EC, Lee SY, Lee H, Choi CW, Yi YS, et al. Antibiotic treatment modulates protein components of cytotoxic outer membrane vesicles of multidrug-resistant clinical strain, Acinetobacter baumannii DU202. Clin Proteomics. 2018;15:28.
Gerritzen MJH, Martens DE, Wijffels RH, Stork M. High throughput nanoparticle tracking analysis for monitoring outer membrane vesicle production. J Extracell Vesicles. 2017;6:1333883.
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-third Informational Supplement M100-S25. Wayne: CLSI; 2015.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF-2017R1A2A2A05001014). This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI17C1657). The funding agencies had no role in the design of the study, collection, analysis or interpretation of data, or in the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: MHO, JCL; Performed the experiments: SYK, MHK, JHS, SK; Analyzed the data: SYK, SIK, MS, YCL, MHO, JCL; Wrote the paper: SYK, MHO, JCL. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kim, S.Y., Kim, M.H., Kim, S.I. et al. The sensor kinase BfmS controls production of outer membrane vesicles in Acinetobacter baumannii. BMC Microbiol 19, 301 (2019). https://doi.org/10.1186/s12866-019-1679-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-019-1679-0