Abstract
Background
Probiotic bacteria are known to modulate host immune responses against various pathogens. Recently, extracellular vesicles (EVs) have emerged as potentially important mediators of host-pathogen interactions. In this study, we explored the role of L. plantarum derived EVs in modulating host responses to vancomycin-resistant Enterococcus faecium (VRE) using both Caenorhabditis elegans and human cells.
Results
Our previous work has shown that probiotic conditioning C. elegans with L. acidophilus NCFM prolongs the survival of nematodes exposed to VRE. Similarly, L. plantarum WCFS1 derived extracellular vesicles (LDEVs) also significantly protected the worms against VRE infection. To dissect the molecular mechanisms of this EV-induced protection, we found that treatment of C. elegans with LDEVs significantly increased the transcription of host defense genes, cpr-1 and clec-60. Both cpr-1 and clec-60 have been previously reported to have protective roles against bacterial infections. Incubating human colon-derived Caco-2 cells with fluorescent dye-labeled LDEVs confirmed that LDEVs could be transported into the mammalian cells. Furthermore, LDEV uptake was associated with significant upregulation of CTSB, a human homologous gene of cpr-1, and REG3G, a human gene that has similar functions to clec-60.
Conclusions
We have found that EVs produced from L. plantarum WCFS1 up-regulate the expression of host defense genes and provide protective effects on hosts. Using probiotic-derived EVs instead of probiotic bacteria themselves, this study provides a new direction to treat antimicrobial resistant pathogens, such as VRE.
Similar content being viewed by others
Background
Lactobacillus is a genus of Gram-positive facultative anaerobic bacteria [1]. Considered as non-pathogenic and generally regarded as safe, lactobacilli have been widely used for fermentation and food production for centuries [2, 3]. The beneficial or probiotic effects of lactobacilli are under intense investigation with both laboratory and clinical studies [4–8], suggesting that administration of lactobacilli inhibit cytokine-induced apoptosis [9] and decreases the pathogenicity of various pathogens, such as E. coli [10] and VRE [11]. However, the molecular mechanisms by which lactobacilli impact VRE are incompletely understood.
Lactobacilli may exert immunomodulatory effects using multiple mechanisms including binding directly to C-type lectin receptors (CLRs) or Toll-like receptors (TLRs), on the host cell surface [12, 13]. For example, administration of L. casei CRL 431 increased the expression of TLR2, TLR4, and TLR9 and improved the production and secretion of TNFα, IFNγ, and IL-10 in mice [12]. Alternatively, lactobacilli may produce antimicrobial substances to inhibit the growth of various pathogens. For example, a bacteriocin produced by lactobacilli formed pores in the membranes of pathogens and thus caused leaking of target cells [14, 15]. More recently, studies have revealed that extracellular vesicles (EVs) and associated proteins from lactobacilli can also modulate the activity of immune cells and affect host innate and adaptive immune responses [16–18]. For example, EVs from lactobacilli were found to enhance cellular TLR2/1 and TLR4 responses while suppressing TLR2/6 signaling [17].
Extracellular vesicles (EVs) are nanometer-scale membrane-contained vesicles released in an evolutionally conserved manner by a wide range of cells [19, 20]. By facilitating the transfer of proteins, nucleic acids, and other molecules between cells [21, 22], EVs are associated with molecular transport, mediation of stress response and biofilm formation thus influencing their hosts [23, 24]. This EV-mediated interaction is likely prevalent in the gut as a major method of communication between bacteria and hosts, since a layer of mucin prevents direct physical contact between bacteria and host tissues [25]. Another unique feature associated with EVs is their potential to mediate therapeutic molecule delivery without inducing adverse immune reactions [26].
In this study, we selected L. plantarum WCFS1, a leading probiotic strain found in the gastrointestinal tract, due to its potency to inducing immunomodulatory effects [27]. We found that L. plantarum WCFS1 produces EVs that are 30–200 nm in diameter. Proteomic analysis revealed that L. plantarum derived EV (LDEV) cargo was enriched with membrane-associated proteins. Using the experimental nematode C. elegans, LDEV treatment prolonged the survival rates of C. elegans under E. faecium (VRE) challenge. To investigate the underlying mechanisms, we found that the host defense genes, cpr-1 and clec-60, were significantly upregulated. LDEV treatment of human colonic cells lines also led to similar upregulation of CTSB (Cathepsin B) and REG3G (Regenerating islet-derived protein 3-gamma).
Results
L. plantarum produces EVs
We isolated EVs from the supernatant of L. plantarum WCFS1 using ExoQuick-TC kit (System Biosciences) [28]. The isolated particles were characterized by electron microscopy, nanoparticle tracking analysis (NTA, NanoSight) and proteomic identification. Electron microscopy showed typical EV-like size (30–200 nm) and morphologic appearance (enclosed by membranes) (Fig. 1a). NTA analysis, a measure of particle size, revealed that over 80% of isolated EVs ranged from 31 nm to 200 nm (Fig. 1b), within the range of previously described EV sizes between 30 and 1000 nm [23]. We also used liquid chromatography–mass spectrometry to profile EV protein content. 31 proteins were identified in the EV fraction (Additional file 1: Table S1). Notably, according to gene ontology analysis, over half of the proteins were found to be associated with membrane, where typical bacterial EVs originate (Fig. 1c) [29, 30]. In all, these results confirmed that L. plantarum WCFS1 produces and release EVs.
a Electron microscopy of L. plantarum WCFS1 EVs. Representative transmission electron micrograph shows EVs isolated from L. plantarum growth medium, magnification 92,000. EVs measure between 30 and 150 nm in diameter and have the morphologic appearance consistent with EVs. b NanoSight size analysis of L. plantarum WCFS1 EVs. The graph represents the size (X-axis) versus concentration (Y-axis) where the white line represents EV size distribution, and the gray line is the accumulated percent of EVs assayed. Over 80% of EVs are sized between 31 nm and 200 nm, while the highest enriched EVs are around 101 nm. c Gene ontology analysis of L. plantarum WCFS1 EV proteome. Eighteen out of thirty-one proteins were found to be part of membrane or associated with membrane, where typical bacterial EVs either get produced or exported
L. plantarum EVs are biofunctional and increase the survival of C. elegans
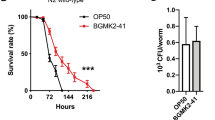
Our previous study showed that preconditioning C. elegans with L. acidophilus NCFM prolongs the survival of the nematode after infection with Enterococcus faecalis [31]. We asked if EV fractions of lactobacilli can also provide similar protective effects. Using an agar-based solid killing assay [32], C. elegans were pre-treated with L. plantarum bacteria, LDEVs or mock EV’s as described in the Methods section. The nematodes were then challenged with a clinically isolated vancomycin-resistant E. faecium C68. Compared to the control worms conditioned with mock EVs, C. elegans conditioned with L. plantarum WCFS1 bacteria survived significantly longer (~3 days) (Fig. 2). This result is similar to that previously obtained using L. acidophilus [31]. Notably, worms pre-treated with LDEVs also survived significantly longer (~4 days) than those treated with mock EVs. We did not observe significantly different survival between LDEV treated and L. plantarum treated groups (Fig. 2).
Conditioning with LDEVs prolonged the survival of C. elegans nematodes infected with VRE. Compared to the worms conditioned with mock EVs, significantly longer survival was found in the LDEV conditioned worms (~4 days, p < 0.01). L. plantarum WCFS1 conditioned worms also had significantly (~3 days, p < 0.01) longer survival than those conditioned with mock EVs. There was no statistical difference in survival between worms conditioned with LDEVs and with L. plantarum WCFS1 bacteria
L. plantarum EVs up-regulate host defense genes, clec-60 and cpr-1 in a C. elegans model
The protection induced by LDEVs prompted us to investigate the possible immunomodulatory effects of LDEVs on C. elegans. Our previous research had shown that five host defense genes (asp10, celec-60, cpr-1, cpr-5, and lys-5) were significantly up-regulated when C. elegans were conditioned with L. acidophilus NCFM (Fig. 3a) [31]. When L. plantarum bacteria were applied to C. elegans, a similar significant up-regulation of clec-60 (~6.2 fold), cpr-1(~2.4 fold) and lys-5(~2.3 fold) was observed (Fig. 3b). When C. elegans were treated with L. plantarum derived EVs, we observed a significant up-regulation of gene expression for the C-type lectin clec-60 (~9 fold) and the gut-specific cysteine protease cpr-1(~3 fold) (Fig. 3c).
Expression profiles of host defense genes when C. elegans were conditioned with lactobacilli and then LDEVs. a RNAs of five host defense genes (asp10, clec-60, cpr-1, cpr-5, and lys-5) were significantly (p < 0.01) up-regulated when C. elegans fer-15;fem-1 were conditioned with L. acidophilus NCFM (See reference [31]). Data are derived from qPCR with fold change in gene expression listed below each gene. b RNAs of clec-60, cpr-1 and lys-5 were significantly (**, p < 0.01) up-regulated when C. elegans fer-15;fem-1 were conditioned with L. plantarum WCFS1. c Significant (**, p < 0.01) up-regulation of clec-60 and cpr-1 was associated with LDEV conditioned C. elegans fer-15;fem-1
L. plantarum EVs incubation led to LDEV cargo delivery and up-regulation of host defense genes, CTSB and REG3G in Caco-2 cells
Having established the immunomodulatory effects of LDEVs in the nematode model, we next investigated the impact of LDEVs on Caco-2 cells as a model of human colonic epithelium [33]. LDEVs were fluorescently labeled with Exo-Green and then incubated with Caco-2 cells for 24 h. We observed ~25% of Caco-2 cells retained the fluorescent label after washing. No detectable fluorescence was observed from the mock EV control, which went through the same EV isolation and labeling procedures (Fig. 4a). Incubating LDEVs with Caco-2 cells did not impact the viability of the mammalian cells (Fig. 4b). Next, we tested if the two genes that showed significantly increased expression in C. elegans, clec-60, and cpr-1, translated to the mammalian model system. CTSB [34], the cysteine proteinase, is the human orthologue of cpr-1. There is no direct human orthologue of clec-60 based on sequence homology. We, therefore, investigated REG3G, an intestinally secreted C-type lectin that likely has functional similarity [35, 36]. Both CTSB and REG3G RNAs were significantly up-regulated in Caco-2 cells after the LDEV treatment (Fig. 4c). This upregulation of CTSB and REG3G confirmed the results obtained from C. elegans model.
a The incubation of LDEVs with Caco-2 cells led to cargo delivery from LDEVs to Caco-2 cells. Compared to mock EVs, only LDEVs treated Caco-2 group showed positive fluorescence. b The incubation of LDEVs did not cause any significant toxicity to Caco-2 cells. MTT assay was employed to examine the viability of Caco-2 cells 24 h after they were treated with mock EVs or LDEVs. c LDEVs increased gene expression of CTSB and REG3G in Caco-2 cells. At 24 h post-incubation, the RNA expression of CTSB (2.5-fold, p < 0.01) and REG3G (2-fold, p < 0.01) were significantly up-regulated in the LDEV treated group
Discussion
The importance of EVs has been increasingly recognized. Virtually all kinds of cell types studied so far secret EVs, and they are also found in various bio-fluids [19, 20]. This phenomenon indicates that EVs are evolutionarily conserved and likely functionally important [21, 22]. Indeed, numerous studies on mammalian cell derived EVs have shown that EVs play important roles in intercellular communication and mediation of immunomodulatory response [22]. However, EV-mediated interactions between host and bacterial pathogens are less explored. Limited studies suggest that pathogenic bacterial strains affect biofilm formation via EV pathways [23, 24]. A recent study on probiotic bacteria has also shown EVs from multiple Lactobacillus strains modulate host-microbe responses by regulating the TLR2 activity and phagocytosis [17]. Here, we focused on L. plantarum, a gut-associated commensal bacteria often used in probiotic nutritional supplements. We found that L. plantarum WCFS1 produces functional EVs that enhance host defense gene expression and directly augments protection against VRE infections. These findings suggest LDEVs, at least partially, mediate the immunomodulatory properties of probiotic lactobacilli.
It is interesting to note that L. plantarum derived EVs up-regulate clec-60 and cpr-1, while the L. plantarum bacteria promote the expression of both genes and cpr-5. The shared upregulation of clec-60 and cpr-1 suggest that L. plantarum derived EVs retain much of the immunomodulatory effects of L. plantarum. This is probably because EVs have similar cargo contents as their parental bacteria [23]. The different regulation observed with gene cpr-5, however, illustrates that bacterial EVs are not equal to the intact bacteria regarding the spectrum of induced immunomodulatory effects.
Our experiments using human Caco-2 cells confirmed biological activity of the LDEVs. Both REG3G [36], which is functionally similar to clec-6, and CTSB [37] (the human orthologue of cpr-1) are upregulated by LDEV treatment. REG3G is an intestinally secreted C-type lectin with potent bactericidal activity against Gram-positive bacteria [35]. It also promotes the spatial segregation of microbiota and host in the intestine [36], thus decreasing the chance of bacterial colonization on the intestinal epithelial surfaces [38, 39]. CTSB, a cysteine proteinase involved in cell death and inflammation [40], is associated with antibacterial activity [41, 42]. Although it may involve autophagy [43], the exact mechanism of CTSB on bacterial pathogens is unclear.
This study provided a mechanistic insight as to how LDEVs enhance host immune response via upregulation of the two host genes, REG3G and CTSB.
Conclusions
In summary, our study revealed that EVs produced from L. plantarum up-regulate the expression of host defense genes, clec-60 and cpr-1, and provide protection against VRE infection in a C. elegans model. LDEV treatment of human colonic cells lines also led to similar upregulation of CTSB and REG3G. The findings of this study could be harnessed to design a new therapeutic treatment of antimicrobial resistant infections by using EVs derived from probiotic strains rather than the bacteria themselves.
Methods
Preparation of probiotic bacteria
Single colony inoculated L. plantarum WCFS1 (BAA-793, ATCC) was grown in de Man, Rogosa, and Sharpe (MRS) medium (Difco Laboratories) at 37 °C for 24 h.
Isolation of extracellular vesicles
Extracellular vesicle fractions were independently enriched from culture supernatants of L. plantarum WCFS1 and medium control. Supernatants from overnight cultures were generated by first centrifuging cultures at 1000 g for 10 min. All supernatants were then passed through a 0.22 μm filter to remove large particles and possible contaminants. EVs were isolated using an ExoQuick-TC™ (System Biosciences) kit per the manufacturer’s directions. Briefly, five parts of supernatant were mixed with one mL of ExoQuick-TC solution. The mixtures were incubated overnight at 4 °C and followed by two centrifugations at 1500 × g for 30 min and then 5 min, respectively. The supernatants were discarded, and the resulting pellets were resuspended in PBS to use directly in downstream experiments or placed in a −80 °C freezer for long-term storage. Mock EVs were isolated from sterile, uninoculated L. plantarum WCFS1 culture broth using the same EV isolation procedures.
Electron microscopy
LDEVs were fixed with 3% glutaraldehyde in 0.15 M sodium cacodylate buffer and then post-fixed in 1% osmium tetroxide (Electron Microscopy Sciences). Fixed samples were cut into 1.5 mm cubes and covered with a 3% agar solution. Samples were dehydrated through a graded series of acetone and embedded in Spurr epoxy resin (Ladd Research Industries). Ultra-thin sections were then prepared, retrieved onto 300-mesh thin bar copper grids, and contrasted with uranyl acetate and lead citrate. Sections were examined using a Morgagni 268-transmission electron microscope and images collected with an AMT Advantage 542 CCD camera system.
Nanoparticle tracking analysis (NTA)
The NTA analysis was carried out using a NanoSight™ NS500 (Malvern) and an automatic syringe pump system. This instrument generates a detailed analysis of the size distribution and concentration of nanoparticles. The analysis was performed on EVs suspended in PBS at 22 °C. Thirty of 30-s videos were recorded for each sample with camera shutter at 33 ms. Videos recorded for each sample were analyzed with NTA software (version 2.3). For this analysis, auto settings were used for blur, minimum track length, and expected particle size; detection threshold was set at 4 Multi.
Proteomics
Proteomic characterization of LDEVs was performed by liquid chromatography-tandem mass spectrometry (LC-MS/MS, nano-LC from LC Packings/Dionex, and Qstar XL from Applied Biosystems). LDEV samples were suspended in Novex® (Thermo Scientific) reducing sample buffer and heated for 10 min at 70 °C. Samples were run on Novex® 4-20% Tris-Glycine gradient gels and stained with SimplyBlue® SafeStain (Life Technologies) for 1 h followed by destaining with water. Gel bands were excised and digested with modified Trypsin (Promega). Tryptic digests were fractionated with a reversed-phase column and the column eluate introduced onto a Qstar XL mass spectrometer via ESI. Protein identifications were performed with ProteinPilot (Applied Biosystems) using the L. plantarum WCFS1 reference sequence database from UniProt and NCBI. To increase confidence, a further manual inspection was carried out to select the proteins associated with at least two unique as the potential candidates [44–46].
Gene ontology (GO) analysis
Protein candidates listed in Additional file 1: Table S1 were searched against UniProt, EBI, and GO databases. Visualization of GO analysis results was carried out in Excel.
Nematode and pathogenic bacteria
C. elegans Bristol N2 or fer-15;fem-1 was used in this study. C. elegans strains were routinely maintained on nematode growth medium (NGM) plates seeded with E. coli OP50 or HB101 using standard procedures [47]. Clinically isolated Enterococcus faecium (vancomycin-resistant) C68 [48] was grown at 37 °C using brain heart infusion (BHI; Difco) broth.
C. elegans killing assays
Solid killing assays were performed using published methods, with slight modifications [47]. For positive control, 1x109 CFU L. plantarum bacteria were spread on an SK plate. LDEVs that were isolated from an equivalent number of L. plantarum, 1x109 CFU, were suspended in PBS and spread on an SK plate. For the negative control, the same volume of mock EVs was spread on SK plate. Each plate was dried for 3 h at room temperature before use. Forty to sixty C. elegans Bristol N2 were seeded into each plate after pre-incubating with L. plantarum WCFS1, LDEVs or controls for 24 h, followed by VRE challenge (a clinically isolated C68 E. faecium strain at 1x109 VRE/plate). After worms had been placed on the plates with the VRE, plates were then incubated at 25 °C and examined for viability at 24-h intervals for 15 days using a Nikon SMZ645 dissecting microscope. Worms were counted as alive or dead based on their response or lack of response to gentle touching with a platinum wire. For preventing hatching of examined adult worm, worms were treated with 5-fluorodeoxyuridine (50 μM) from L4 to end of assays.
Culture and EV treatment of cell lines
Caco-2 (HTB-37, ATCC), a human colon carcinoma cell line, was maintained in Eagle’s Minimum Essential Medium (EMEM) supplemented with 20% fetal bovine serum (FBS) and was used to test LDEV’s effect on mammalian cells. LDEVs were labeled by Exo-Green (System Biosciences) according to manufacturer’s instruction. Briefly, 500 μl of LDEVs suspended in PBS was mixed with 50 μl stain. After 10 min 37 °C incubation and precipitation by ExoQuick-TC, the labeled LDEVs was re-suspended in PBS and added to Caco-2 cells. At 24 h post-incubation, the culture wells were rinsed twice with PBS to remove residual fluorescent dyes. The cells were then examined by fluorescent microscopy (Olympus IX-70). A control experiment using mock EV was also carried out in parallel.
Caco-2 viability assay
(3-[4,5- dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) or MTT assay (Sigma) was used to measure Caco-2 cellular proliferation rate after LDEV treatment. All procedures were performed according to the manufacture’s instruction.
RNA isolation and qPCR
Total RNA from C. elegans and Caco-2 cells was extracted using TRIzol® (Thermo Scientific) by following standard protocols. The concentrations of all RNA samples were determined by spectrophotometry. 1 μg of total RNAs was used for reverse transcription and PCR, which was carried out on a Mastercycler® gradient 5331 (Eppendorf, Westbury, NY) by using Maxima® First Strand cDNA Synthesis Kit (Thermo Scientific). Primers were designed by using PrimerQuest online tools available at http://www.idtdna.com/Primerquest/Home/Index. Primer sequences are provided in Additional file 2: Table S2. Real-time PCR was performed on Mastercycler® ep realplex (Eppendorf). All reactions were performed in 96-well plates with the following reagents in a final volume of 20 μl: 1 μl of primers (5 μM each for forward and reverse) and 2X Maxima® SYBR Green qPCR Master Mix from Thermo Scientific. 10 ng of cDNA was added to this mixture. Triplicate reactions of the target and housekeeping genes were performed simultaneously for each cDNA template analyzed. The PCR reaction consisted of an initial enzyme activation step at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. A cycle threshold value (Ct) value was obtained for each sample, and triplicate sample values were averaged. The 2-ΔΔCt method was used to calculate relative expression of each target gene. The control genes snb-1 [49] and ACTB [50] were used to normalize the gene expression data from C. elegans or Caco-2 cells respectively.
Statistical analysis
The log-rank test was used to determine the difference in C. elegans survival rates. Differences in qPCR results were determined by using the Student’s t-test. A P < 0.05 in all experiments was considered statistically significant. Statistical analysis and graphing were performed with Prism version 6.05 (GraphPad).
Abbreviations
- CTSB:
-
Cathepsin B
- EVs:
-
Extracellular vesicles
- IFNγ:
-
Interferon gamma
- IL-10:
-
Interleukin 10
- LC-MS/MS:
-
Liquid chromatography–tandem mass spectrometry
- LDEVs:
-
L. plantarum derived extracellular vesicles
- qPCR:
-
Quantitative polymerase chain reaction
- REG3G:
-
Regenerating islet-derived protein 3 gamma
- TLR:
-
Toll-like receptor
- TNFα:
-
Tumor necrosis factor alpha
- VRE:
-
Vancomycin-resistant enterococci
References
Makarova K, Slesarev A, Wolf Y, Sorokin A, Mirkin B, Koonin E, Pavlov A, Pavlova N, Karamychev V, Polouchine N, et al. Comparative genomics of the lactic acid bacteria. Proc Natl Acad Sci U S A. 2006;103(42):15611–6.
Adams MR, Marteau P. On the safety of lactic acid bacteria from food. Int J Food Microbiol. 1995;27(2–3):263–4.
Stiles ME, Holzapfel WH. Lactic acid bacteria of foods and their current taxonomy. Int J Food Microbiol. 1997;36(1):1–29.
Dobrogosz WJ, Peacock TJ, Hassan HM. Evolution of the probiotic concept from conception to validation and acceptance in medical science. Adv Appl Microbiol. 2010;72:1–41.
Schlee M, Harder J, Koten B, Stange EF, Wehkamp J, Fellermann K. Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin Exp Immunol. 2008;151(3):528–35.
Bongaerts GP, Severijnen RS. The beneficial, antimicrobial effect of probiotics. Med Hypotheses. 2001;56(2):174–7.
Kaur IP, Chopra K, Saini A. Probiotics: potential pharmaceutical applications. Eur J Pharm Sci. 2002;15(1):1–9.
Kim YG, Ohta T, Takahashi T, Kushiro A, Nomoto K, Yokokura T, Okada N, Danbara H. Probiotic Lactobacillus casei activates innate immunity via NF-kappaB and p38 MAP kinase signaling pathways. Microbes Infect. 2006;8(4):994–1005.
Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277(52):50959–65.
Medellin-Pena MJ, Griffiths MW. Effect of molecules secreted by Lactobacillus acidophilus strain La-5 on Escherichia coli O157:H7 colonization. Appl Environ Microbiol. 2009;75(4):1165–72.
Manley KJ, Fraenkel MB, Mayall BC, Power DA. Probiotic treatment of vancomycin-resistant enterococci: a randomised controlled trial. Med J Aust. 2007;186(9):454–7.
Castillo NA, Perdigon G, de Moreno de Leblanc A. Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol. 2011;11:177.
Walker WA. Mechanisms of action of probiotics. Clin Infect Dis. 2008;46 Suppl 2:S87–91. discussion S144-151.
Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis. 2009;15(2):300–10.
Todorov SD. Bacteriocins from Lactobacillus plantarum - production, genetic organization and mode of action: producao, organizacao genetica e modo de acao. Braz J Microbiol. 2009;40(2):209–21.
Corthesy B, Gaskins HR, Mercenier A. Cross-talk between probiotic bacteria and the host immune system. J Nutr. 2007;137(3 Suppl 2):781S–90.
van Bergenhenegouwen J, Kraneveld AD, Rutten L, Kettelarij N, Garssen J, Vos AP. Extracellular vesicles modulate host-microbe responses by altering TLR2 activity and phagocytosis. PLoS One. 2014;9(2):e89121.
Ruiz L, Hevia A, Bernardo D, Margolles A, Sanchez B. Extracellular molecular effectors mediating probiotic attributes. FEMS Microbiol Lett. 2014;359(1):1–11.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83.
Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol. 2013;200(4):367–71.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9.
Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89.
Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066.
Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19(22):2645–55.
Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8(6):411–20.
Marcus ME, Leonard JN. FedExosomes: engineering therapeutic biological nanoparticles that truly deliver. Pharmaceuticals. 2013;6(5):659–80.
Paolillo R, Romano Carratelli C, Sorrentino S, Mazzola N, Rizzo A. Immunomodulatory effects of Lactobacillus plantarum on human colon cancer cells. Int Immunopharmacol. 2009;9(11):1265–71.
Caradec J, Kharmate G, Hosseini-Beheshti E, Adomat H, Gleave M, Guns E. Reproducibility and efficiency of serum-derived exosome extraction methods. Clin Biochem. 2014;47(13–14):1286–92.
Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15(6):375–87.
Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–84.
Kim Y, Mylonakis E. Caenorhabditis elegans immune conditioning with the probiotic bacterium Lactobacillus acidophilus strain NCFM enhances gram-positive immune responses. Infect Immun. 2012;80(7):2500–8.
Shivers RP, Pagano DJ, Kooistra T, Richardson CE, Reddy KC, Whitney JK, Kamanzi O, Matsumoto K, Hisamoto N, Kim DH. Phosphorylation of the conserved transcription factor ATF-7 by PMK-1 p38 MAPK regulates innate immunity in Caenorhabditis elegans. PLoS Genet. 2010;6(4):e1000892.
Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96(3):736–49.
Hentze H, Lin XY, Choi MS, Porter AG. Critical role for cathepsin B in mediating caspase-1-dependent interleukin-18 maturation and caspase-1-independent necrosis triggered by the microbial toxin nigericin. Cell Death Differ. 2003;10(9):956–68.
Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455(7214):804–7.
Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334(6053):255–8.
Mort JS, Buttle DJ. Cathepsin B. Int J Biochem Cell Biol. 1997;29(5):715–20.
Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12(7):503–16.
Mukherjee S, Hooper LV. Antimicrobial defense of the intestine. Immunity. 2015;42(1):28–39.
Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010;120(10):3421–31.
Lawrence CP, Kadioglu A, Yang AL, Coward WR, Chow SC. The cathepsin B inhibitor, z-FA-FMK, inhibits human T cell proliferation in vitro and modulates host response to pneumococcal infection in vivo. J Immunol. 2006;177(6):3827–36.
Flannagan RS, Cosio G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7(5):355–66.
Amer AO, Swanson MS. Autophagy is an immediate macrophage response to Legionella pneumophila. Cell Microbiol. 2005;7(6):765–78.
Gupta N, Bark SJ, Lu WD, Taupenot L, O’Connor DT, Pevzner P, Hook V. Mass spectrometry-based neuropeptidomics of secretory vesicles from human adrenal medullary pheochromocytoma reveals novel peptide products of prohormone processing. J Proteome Res. 2010;9(10):5065–75.
Li M, Aliotta JM, Asara JM, Tucker L, Quesenberry P, Lally M, Ramratnam B. Quantitative proteomic analysis of exosomes from HIV-1-infected lymphocytic cells. Proteomics. 2012;12(13):2203–11.
Li M, Ramratnam B. Proteomic characterization of exosomes from HIV-1-infected cells. Methods Mol Biol. 2016;1354:311–26.
Breger J, Fuchs BB, Aperis G, Moy TI, Ausubel FM, Mylonakis E. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007;3(2):e18.
Carias LL, Rudin SD, Donskey CJ, Rice LB. Genetic linkage and cotransfer of a novel, vanB-containing transposon (Tn5382) and a low-affinity penicillin-binding protein 5 gene in a clinical vancomycin-resistant Enterococcus faecium isolate. J Bacteriol. 1998;180(17):4426–34.
Irazoqui JE, Ng A, Xavier RJ, Ausubel FM. Role for beta-catenin and HOX transcription factors in Caenorhabditis elegans and mammalian host epithelial-pathogen interactions. Proc Natl Acad Sci U S A. 2008;105(45):17469–74.
Li M, Tucker LD, Asara JM, Cheruiyot CK, Lu H, Wu ZJ, Newstein MC, Dooner MS, Friedman J, Lally MA, et al. Stem-loop binding protein is a multifaceted cellular regulator of HIV-1 replication. J Clin Invest. 2016;126(8):3117–29.
Acknowledgments
We thank Carol A. Ayala at the Division of Core Research Laboratories of Rhode Island Hospital for helping with electron microscopy. We thank Mark S. Dooner and Yan Cheng at the Division of Hematology/Oncology of Rhode Island Hospital for helping with NanoSight analyses. We also thank Dr. Louis Rice for the kind gift of VRE C68.
Funding
This work was supported by a University Medicine Foundation Research Fund to M. Li and P30GM110759, R01HD072693, K24HD080539 to B. Ramratnam.
Availability of data and materials
All data generated during this study are included in this published article and its supplementary information files. Moreover, the reader can contact the corresponding author to get needed information.
Authors’ contributions
ML, GJN, EM and BR conceived and designed the study. ML, KL, and MH designed and performed the experiments. ML, KL, GJN, EM, and BR wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Table S1.
Proteomics data from L. plantarum derived extracellular vesicles. Protein name, GO term (cellular component), Accession number, Peptide sequence, Validation Score, Xcorr score, Change in mass (ppm), Isolated mass, Peak area, Charge state, and Number of peptides are given. (XLSX 28 kb)
Additional file 2: Table S2.
qPCR primers. Their names and sequences are given. (XLSX 8 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, M., Lee, K., Hsu, M. et al. Lactobacillus-derived extracellular vesicles enhance host immune responses against vancomycin-resistant enterococci. BMC Microbiol 17, 66 (2017). https://doi.org/10.1186/s12866-017-0977-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-017-0977-7