Abstract
Background
Pseudomonas syringae pv. glycinea PG4180 causes bacterial blight on soybean plants and enters the leaf tissue through stomata or open wounds, where it encounters a sucrose-rich milieu. Sucrose is utilized by invading bacteria via the secreted enzyme, levansucrase (Lsc), liberating glucose and forming the polyfructan levan. P. syringae PG4180 possesses two functional lsc alleles transcribed at virulence-promoting low temperatures.
Results
We hypothesized that transcription of lsc is controlled by the hexose metabolism repressor, HexR, since potential HexR binding sites were identified upstream of both lsc genes. A hexR mutant of PG4180 was significantly growth-impaired when incubated with sucrose or glucose as sole carbon source, but exhibited wild type growth when arabinose was provided. Analyses of lsc expression resulted in higher transcript and protein levels in the hexR mutant as compared to the wild type. The hexR mutant’s ability to multiply in planta was reduced. HexR did not seem to impact hrp gene expression as evidenced by the hexR mutant’s unaltered hypersensitive response in tobacco and its unmodified protein secretion pattern as compared to the wild type under hrp-inducing conditions.
Conclusions
Our data suggested a co-regulation of genes involved in extra-cellular sugar acquisition with those involved in intra-cellular energy-providing metabolic pathways in P. syringae.
Similar content being viewed by others
Background
Fructan or glucan polymers are formed wherever microbes encounter sucrose-rich conditions, would it be in association with plants, in the oral cavity, in food manufacturing, or during bio-fuel production processes [1]. When plant-borne sucrose is present, the soybean-infecting bacterial blight pathogen, Pseudomonas syringae pv. glycinea, uses levansucrase (EC 2.4.1.10, Lsc) to synthesize the extra-cellular high-molecular fructofuranan, levan, thereby releasing glucose for primary metabolism. Three levansucrase-encoding genes, lscA, lscB, and lscC, were identified in P. syringae pv. glycinea PG4180, from which only lscB and lscC are expressed, as a mutant lacking lscB and lscC but possessing lscA is levan-deficient [2]. Furthermore quantitative expression analysis of lsc genes by quantitative Reverse Transcriptase (qRT)-PCR showed that lscB and lscC are actively expressed. However lscA is not being expressed due to an altered upstream region of lscA which does not seem to promote lsc expression [3]. Both enzymes are synthesized maximally at 18°C in vitro and in planta and their expression is optimal at the early logarithmic growth stage [4,5].
Bacterial communities growing epiphytically on plants are primarily affected by carbon availability as supported by the finding that very low sugar concentrations are sufficient to support the growth of 107 to 108 cells per leaf [6]. Stomatal openings and wounds provide the site of entry for P. syringae. Under favorable micro-environmental conditions, the bacterial cells live endophytically and subsequently initiate the infection process via production of the phytotoxin coronatine [7,8] and attachment to plant cell surfaces. The infection process is fostered by low environmental temperatures such as 18-20°C as opposed to the optimal growth temperature of P. syringae, 28°C [9,10]. A complex sequence of events mediated by injection of bacterial hypersensitive reaction and pathogenicity (Hrp) effector proteins into plant cells [11] ultimately activates plant-borne K+ efflux and H+ influx, which increases the apoplastic pH from 5.5 to 7.5 [12]. Subsequently, this high extra-cellular pH induces efflux of the dominant photo assimilate, sucrose, from plant cells [12]. Apoplastic sucrose ranging in concentrations from 20 μM to 1–5 mM is hydrolyzed by either plant-borne invertases or by extra-cellular microbial enzymes, e.g. Lsc [13,14].
For glucose metabolism, metabolic pathway structures vary among bacterial species with different ecological niches [15]. In contrast to enterobacteria [16], pseudomonads utilize the Entner-Doudoroff (ED) pathway due to lack of 6-phosphofructokinase and hence do not catabolize sugars via the Embden-Meyerhof-Parnas pathway [17,18]. The ED pathway can be linear, alternative, modified with non-phosphorylated intermediates, or cyclic [19] and was first described in Pseudomonas saccharophila, which now belongs to the order Burkholderiales [17,20].
The genes required for glucose metabolism in Pseudomonas putida KT2440 are organized in several operons [21]. The first operon consists of the zwf, pgl, and eda genes coding for glucose-6-phosphate dehydrogenase, 6-phosphogluconolactonase (both enzymes of the glucose phosphorylative pathway), and Eda (an enzyme of the Entner-Doudoroff pathway) [22,23]. Divergently directed to this operon is the gene encoding for the hexose metabolic repressor, hexR [21]. The second operon consists of the edd and glk genes that encode 6-phosphogluconate dehydratase (the first enzyme of the Entner-Doudoroff pathway) and glucokinase (an enzyme of the glucose phosphorylative pathway), respectively [22,23]. Divergently directed to this operon is the gene gap1 which codes for glyceraldehydes 3-phosphoste dehydrogenase [21]. The transcription of these genes and operons is negatively regulated by HexR. Two monomers of HexR bind to the promoter regions of edd, zwf, and gap-1 genes by recognizing a palindromic sequence TTGTN7–8ACAA [21,23-25]. In the current study, it was hypothesized that in P. syringae not only genes involved in cellular glucose metabolism but also genes encoding extra-cellular Lsc were controlled by HexR. In turn, this might have consequences for our understanding about what determines bacterial in planta fitness and potentially virulence.
In order to address this hypothesis, a hexR mutant was generated in PG4180 and tested for its growth in minimal medium containing glucose, sucrose, or arabinose as sole carbon source. Analyses of lsc expression by qRT-PCR and Western blotting were conducted for the PG4180 wild type and its hexR mutant. The mutant was compared to the wild type in terms of its in planta fitness. Furthermore, hypersensitive response (HR) reactions and profiles of secreted proteins were compared for the wild type and the hexR mutant when grown under hrp-inducing conditions.
Methods
Bacterial strains, plasmids and growth conditions
Bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli was maintained at 37°C on Luria-Bertani (LB) medium [26]. P. syringae was routinely maintained at 28°C on mannitol-glutamate (MG) medium [27]. For liquid cultures at 18°C, bacteria were grown in 200 ml of Hoitink-Sinden (HS) medium [28] supplemented with various carbon sources in 1-l Erlenmeyer flasks. 113 mM of glucose (HS + glucose) was replaced by 57 mM of sucrose in HS + sucrose medium while HS + arabinose medium had the following constituents: 0.8 mM MgSO4, 30 mM KH2PO4, 16 mM K2HPO4, 16 mM KNO3, 20 μM FeCl3, 133 mM L-arabinose. Bacterial growth was continuously monitored by measuring the optical density at 600 nm (OD600). Antibiotics were added to media at the following concentrations (μg/ml): ampicillin, 50; spectinomycin, 25; kanamycin, 25; tetracycline, 25; gentamicin, 2.
Generation of hexR mutant in PG4180
A P. syringae pv. glycinea PG4180 hexR mutant was generated using the broad-host-range Flp-FRT recombination system [33]. Two fragments flanking the hexR gene were amplified from PG4180 genomic DNA using two pairs of primers: HexR_1f/HexR_1r and Hex_2f/HexR_2r (Table 2). PCR products were cloned into pGEM-T Easy (Promega) yielding plasmids pGEM.HexR1 and pGEM.HexR2 (Table 1). A 1,230-bp KpnI fragment containing a KmR cassette flanked with FRT sequences was removed from plasmid pFKm [34] and ligated into KpnI-digested pGEM.HexR1, yielding pGEM.HexR1-Km. A 360-bp SpeI-BamHI fragment digested from pGEM.HexR2 was ligated into SpeI-BamHI-digested pGEM.HexR1-Km, yielding plasmid pGEM.HexR-Km. Finally, a 2,046-bp EcoRI fragment was removed from pGEM.HexR-Km and ligated into EcoRI-digested plasmid pEX18Ap [33], yielding the hexR gene replacement plasmid pEX.HexR-Km. This plasmid was mobilized into P. syringae PG4180 by tri-parental mating. Putative mutants were screened on MG medium agar plates supplemented with kanamycin and were subsequently confirmed for the genotype by PCR using primers hex_up_fwd and hex_down_rev (Table 2).
Verification of the hexR mutant’s phenotype by Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR)
Template-specific primers were designed for hexR and lscB/C genes of P. syringae PG4180. Bacterial cells were grown in HS + arabinose medium and harvested at an OD600 of 0.5. RNA was extracted by acid phenol/chloroform extraction method [5]. RT-PCR was performed on total RNA using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Schwerte, Germany). The hexR_fwd, hexR_rev, hexR-verif_fwd, hexR_verif_rev and lscB/C primers were used to check for presence of a hexR and lscB/C mRNA by PCR using cDNA as template. Regular PCR with the same primer-pairs and genomic DNA as template were used as control. The thermocycler program was as follows: 1 cycle of 95°C for 60 s; 30 cycles of 95°C for 10 s, 60°C for 30 s, 72°C for 30 s; 1 cycle of 72°C for 5 min. The results were analyzed by 1% agarose gel electrophoresis.
Analysis of lsc expression by quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
Bacterial cells were grown in HS + arabinose medium at 18°C. When cultures reached respective OD600 values, total RNA was isolated by acid phenol/chloroform extraction, and samples were normalized by multiplexed fluorescent Northern hybridization and 23S rRNA transcript amount comparison as described previously [5]. Yield and purity of RNA were determined by measuring absorption at 260 and 280 nm. Total RNA samples were treated with TURBO DNA-free (Applied Biosystems, Darmstadt, Germany) to remove remaining traces of genomic DNA as described by the manufacturer’s recommendation.
SYBR green-based qRT-PCR was performed with 1 ng normalized RNA template and 200 nM primers (lscBC_RT_fwd, lscBC_RT_rev) using the QuantiTect SYBR Green one-step RT-PCR Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The thermocycler program comprised an initial step of 95°C for 15 min followed by 40 cycles of 95°C for 30 s, 55°C for 30 s, 72°C for 30 s. Reactions were performed in technical duplicates and biological triplicates with a Mastercycler® realplex2 real-time PCR system (Eppendorf, Hamburg, Germany) as described by the manufacturer using their universal program. Reactions with no addition of reverse transcriptase served as negative controls and proved lack of DNA contamination. Specificity of amplification was assessed by analyzing the melting curve of the amplification product. Due to very high sequence identity between lscB and lscC it was not possible to design primers discriminating between these two mRNAs, thereby expression profile of lsc is always referred as a combination of both genes.
Immunological detection of Lsc
Generation and concentration of cell-free supernatants of P. syringae cells and the use of polyclonal antibodies were carried out as described previously [4]. Cultures were grown in HS+ arabinose medium. Equal aliquots of protein fractions were loaded (0.5, 2.5 and 5 µg/lane) and separated by 10% SDS-PAGE. Electrophoresis, electro-blotting on nitrocellulose membranes, and immunodetection were conducted by standard procedures [26].
Growth of PG4180 wild type and hexR mutant in planta
In planta growth of PG4180 and its hexR mutant was evaluated on soybean plants. Soybean seedlings were germinated and grown in an environmentally controlled chamber for approximately three weeks prior to the growth assays. PG4180 wild type and hexR mutant were incubated for 48 h at 28°C on MG agar plates. Cells were suspended in distilled water, adjusted to an OD600 of 0.1 (corresponding to approximately 107 CFU/ml) and applied to the leaves with an airbrush (~8 psi) until the leaf surfaces were uniformly wet. Subsequently, humid environment was achieved by enclosing the inoculated plants with a clear plastic bag for overnight. Inoculated plants were grown in a plant growth chamber (19-21°C) with a 12-hr light period. At days 1, 3, 5, 7, 9, 11 and 14 after inoculations, two individual leaves were randomly excised from plants corresponding to each inoculums and their weight was measured. Epiphytic bacteria were isolated by placing leaves in a 50-ml falcon tubes containing 20 ml of external wash buffer (0.1 M potassium phosphate, 0.1% bactopeptone and pH = 7.0) and the tubes were sonicated for 7 minutes [35]. Leaves were then removed and macerated in 20 ml of isotonic solution (0.9% NaCl) using sterile mortar and pestle. Bacterial counts (CFU/g fresh weight) were determined by plating dilutions from external wash buffer and leaf homogenate onto MG agar and counting of fluorescent colonies after incubation at 28°C for 96 h.
Hypersensitive response assay on tobacco
Tobacco plants (Nicotiana tabacum cv. Petit Havana SR1) were grown in a light chamber at 20 to 25°C, 60% humidity, with a 12-h photoperiod (15,000 lux). PG4180 wild type and hexR mutant were incubated for 48 h at 28°C on MG agar. Cells were suspended in sterile 0.9% NaCl, adjusted to an OD600 of 0.1 (corresponding to approximately 107 CFU/ml). Tobacco plants were inoculated with bacterial suspensions by syringe injection of leaf veins of the third and fourth leaf. As negative control sterile 0.9% NaCl was used. Infiltrated areas were monitored for development of the hypersensitive reaction in form of necrosis after 24 and 48 h.
Extra-cellular protein pool preparation and SDS-PAGE
For sample preparation of secreted proteins, bacteria were grown in two consecutive overnight pre-cultures in King’s B broth [36] at 28°C. From the first pre-culture, the cell suspension was adjusted to an OD600 of 0.1 with 50 ml of fresh King’s B broth and incubated at 28°C. Bacterial cultures were harvested at an OD600 of 1.0 and centrifuged at 4,000 rpm for 30 min. The bacterial cells were washed two times with Hrp-inducing medium [37,38] or with HS+ glucose medium [28], respectively. Subsequently, the cell pellets were resusp ended in 50 ml of Hrp-inducing medium or HS+ glucose medium at 28°C and incubated shaking at 250 rpm for 4 h. Next, 25 ml of the bacterial cultures were centrifuged at 4,000 rpm for 30 min. Cell-free supernatant samples were prepared by filter-sterilization through 0.2-μm pore size membrane filters and concentrated 50-fold using 10-kDa millipore filters (Amicon, Billerica, USA). Extra-cellular protein samples were stabilized in 50 mM Tris–HCl (pH 8.8). Total protein amounts were determined using a Nanodrop apparatus (Thermo Fisher Scientific, Langenselbold, Germany), 10 μl of protein samples were separated by 12.5% SDS-PAGE and subsequently visualized by silver staining according to established procedures [39].
Results
Generation and genotypic characterization of the P. syringae hexR mutant
T del Castillo, E Duque and JL Ramos [23] and A Daddaoua, T Krell and JL Ramos [22] had previously shown that genes encoding enzymes of the phosphorylative branch and the ED pathway of glucose catabolism in P. putida were regulated by the hexose metabolism repressor, HexR. In P. syringae, extra-cellular Lsc releases glucose by sucrose hydrolysis. In order to investigate the impact of HexR on expression of lscB and lscC genes, a hexR-deficient mutant of P. syringae PG4180 was generated by insertion of a kanamycin resistance cassette in the hexR gene as a result of homologous recombination. The resulting mutant genotype was verified by growth on kanamycin-containing medium and RT-PCR analysis (Additional file 1: Figure S1).
In silico analysis of HexR binding site upstream of lsc genes
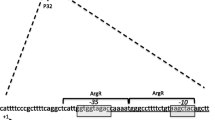
Analysis of the nucleotide sequences upstream of lscB and lscC, which are almost identical at nucleotide level [2,40], revealed the presence of a sequence (nucleotides +113 to +127 with respect to the corresponding translational start site) with high similarity to the conserved motif TTGTN7–8ACAA previously shown to be the DNA binding site of HexR in P. putida [22-24] (Figure 1). This finding suggested a putative binding of HexR of P. syringae to the upstream sequence of both lsc genes.
Nucleotide sequence of the upstream region of lscB . Sequence contains a putative HexR binding site (underlined) similar to that described by A Daddaoua, T Krell and JL Ramos [22]. +1 represents the translational start site of lscB.
Comparison of different HexR protein sequences
Multiple sequence alignments of several HexR protein sequences derived from Lsc-producing P. syringae strains revealed that those proteins have a high pair-wise identity scores of >97% (data not shown). PFAM analysis [41] of these proteins showed that there is a DNA binding domain (PFAM01418) and a sugar isomerase (SIS) binding domain (PFAM 01380) as described for P. putida KT2440 by A Daddaoua, T Krell and JL Ramos [22] (Additional file 2: Figure S2) suggesting a similar way of enzymatic activity. A consensus sequence obtained from multiple alignments of HexR sequences from several P. syringae strains revealed 91% identity at the amino acid sequence level to that of P. putida (Additional file 3: Figure S3).
In vitro and in planta growth of P. syringae PG4180 and its hexR mutant
To compare the growth of PG4180 wild type and its hexR mutant were grown in minimal media containing different carbon sources at 18°C (Figure 2). In contrast to the wild type, the hexR mutant did not grow significantly in HS medium containing 20 g/ml (113 mM) of glucose. Replacing glucose by 10 g/ml (57 mM) of sucrose, thereby providing an equal total number of carbon atoms did not change the weak growth phenotype of the hexR mutant. However, growth of the wild type was unaffected by this change of carbon source.
When glucose was replaced by an equal amount of 20 g/ml (133 mM) arabinose as the sole carbon source, growth of the hexR mutant was not distinguishable from that of the wild type indicating that HexR might not be involved in regulation of pathways utilizing arabinose, and that the hexR mutant growth phenotype was restricted to glucose utilization (Figure 2). For the wild type, sucrose apparently allowed for a faster adaptation as sucrose-supplemented cultures grew faster during early logarithmic growth. No significant difference was observed for the hexR mutant grown on glucose- or sucrose-supplemented medium. Arabinose allowed for the most efficient growth independent of the genotypes studied. These results indicated a potential HexR-mediated regulatory link of intra-cellular hexose metabolism with synthesis of Lsc since sucrose is the source of glucose via the enzymatic activity of the later enzyme.
To analyze the effect of a hexR mutation on growth in planta, PG4180 wild type and its hexR mutant were spray-inoculated onto soybean leaves with suspensions adjusted to 1 × 107 CFU/ml. Subsequently, the plants were kept in a plant growth chamber and bacteria were recovered between days 1 to 14 post inoculation (Figure 3). Results showed that the total population number and percentage of internalized bacteria were not significantly different between plants inoculated with PG4180 wild type or its hexR mutant. However, a clear trend was observed for the percentage of bacteria that entered the interior of the leaf tissue. Except for days 1 and 9, plants inoculated with PG4180 wild type showed a higher percentage of internalized bacterial population when compared to plants subjected to the hexR mutant. These results might indicate an important role of HexR for the in planta fitness of P. syringae.
Total population of PG4180 wild type (
 ) and its hexR mutant (
) and its hexR mutant (
 ) in soybean leaves. Columns represent the percentage of internalized PG4180 wild type (Black) and its hexR mutant (Black stripes). Bacterial suspensions were spray-inoculated on leaves of soybean plants grown in a greenhouse at 19-21°C. Data represent the mean values from five independent experiments with each two leaf samples. Error bars represent standard deviation of the mean of five biological replicates (n = 5).
) in soybean leaves. Columns represent the percentage of internalized PG4180 wild type (Black) and its hexR mutant (Black stripes). Bacterial suspensions were spray-inoculated on leaves of soybean plants grown in a greenhouse at 19-21°C. Data represent the mean values from five independent experiments with each two leaf samples. Error bars represent standard deviation of the mean of five biological replicates (n = 5).
Transcriptional analyses of lsc genes
To analyze the effect of the hexR mutation on lsc gene expression, a growth-phase dependent transcriptional analysis was conducted using qRT-PCR. Cells of PG4180 wild type and the hexR mutant were grown in minimal medium containing arabinose at 18°C since levan production was shown to be maximal at this temperature [4]. Transcription of lsc genes in wild type and hexR mutant was highest during the early exponential growth phase and significantly decreased during further growth (Figure 4). Expression of lsc in the hexR mutant showed a tendency of being higher than the wild type in the early and mid-logarithmic growth phase. Interestingly, a significantly higher expression (p < 0.01) of lsc was observed in the hexR mutant as compared to that of the wild type at an OD600 of 2.0 referring to late-logarithmic to stationary phase.
Quantitative Reverse Transcriptase PCR analysis of growth phase-dependent lsc gene expression. PG4180 and its hexR mutant were grown at 18°C in HS + arabinose. Relative mRNA levels were related to the mean value determined for the signals of PG4180 wild type at an OD600 of 0.5, which was defined as 100%. Data show the means and standard errors of three biological replicates (n = 3) (* = P < 0,005).
Abundance of Lsc in PG4180 wild type and hexR mutant
To qualitatively assess levan formation, PG4180 wild type and its hexR mutant were grown on sucrose-containing MG agar plates resulting in indistinguishable levels of levan formation for both (Data not shown). The accumulation of Lsc in extracellular fractions of the wild type and the hexR mutant of PG4180 was tested using immunological detection. Protein samples were obtained from cell-free culture supernatants of bacterial cultures grown with arabinose as sole carbon source to late exponential phase at 18°C. Comparison of wild type and hexR mutant showed a slightly higher Lsc accumulation in the mutant’s culture supernatant (Figure 5). These results further supported the hypothesis that HexR might repress lsc gene expression, resulting in more extra-cellular accumulation of its gene product in the hexR mutant.
Qualitative Western blot analysis of extra-cellular Lsc in cell-free supernatant of PG4180 wild type (WT) and its hexR mutant. Cultures are grown in HS medium supplemented with arabinose as sole carbon source at 18°C. 0.5, 2.5, 5 μg of protein samples per lane were electrophoretically separated, transferred to a polyvinylidene fluoride membrane, and hybridized with Lsc-specific polyclonal antibodies.
Lack of an effect of HexR on protein secretion and hypersensitive response
To further investigate the decreased in planta fitness of the hexR mutant of P. syringae as compared to its wild type, hypersensitive response (HR) assays were performed for both on the tobacco plant Nicotiana tabacum (Figure 6). The resulting HR after 24 hours on leaves inoculated with the mutant was indistinguishable from that induced by the wild type. In addition, extra-cellular protein profiles were determined for PG4180 wild type and its hexR mutant incubated in hrp gene-inducing IM medium or in HS+ glucose medium (Figure 7). After electrophoretic separation of extra-cellular proteins, nearly identical proteins profiles were observed for PG4180 wild type and its hexR mutant. In summary, these results indicated that a mutation of hexR does not influence the ability of P. syringae to induce an HR or alter its protein secretion pattern.
Discussion
This study revealed that expression of genes encoding for an extra-cellular protein appear to be co-regulated with genes required for central hexose metabolism in a Gram-negative bacterium. Complementing previous studies on the global hexose metabolism repressor, HexR, in P. putida [21-24], our results suggested that involvement of HexR in regulation of lsc expression might be a selective adaptation of the plant pathogen, P. syringae, to its well-studied infection cycle [42,43]. Once Lsc is secreted, cellular resources needed for its synthesis such as amino acyl residues are not available for the cell anymore. Consequently, P. syringae might repress Lsc synthesis in coordination with hexose utilization when sufficient levels of intra-cellular glucose are available to balance the cell’s energy demands.
In close proximity and upstream of the translation start site (TSS) of P. syringae lsc genes, palindromic sequences were identified, which resemble HexR binding sites previously predicted for P. putida [22]. Repressors such as HexR were suggested to bind to inverted repeats that partially or fully overlap RNA-polymerase binding sites [44].
Sucrose is the most abundant plant storage sugar [45]. Bacterial in planta and in vitro growth analyses indicated that the substrate of Lsc, sucrose, and in consequence its enzymatic product, glucose, seem to be major nutrient sources for P. syringae during in planta growth. This is in line with previous findings reporting high molecular abundances of sucrose in bean plant’s apoplastic fluids [12]. Consequently, expression and secretion of Lsc might be a fitness factor for the in planta life of P. syringae. Along the course of the experiment, the total populations of PG4180 wild type and its hexR mutant varied but were not significantly different from each other. This variation reflects a feature that is usually observed during assessment of bacterial populations [45]. LL Kinkel, M Wilson and SE Lindow [46] showed that two leaves of the same plant species might vary by over 10-fold in their total epiphytic bacteria. Moreover, a study by SS Hirano and CD Upper [47] also demonstrated that regardless of the geographic area, plant species, or time scale tested, variations in population sizes of P. syringae are common. Many factors such as availability of nutrients, bacterial immigration [46], and ability of bacteria to tolerate environmental stresses on leaf surfaces [48] contribute to the observed variations.
A tendency is seen regarding the percentage of internalized bacteria in plants, although it is not significantly different. PG4180 wild type shows higher percentage of internalized bacteria as opposed to those inoculated with the hexR mutant. On days 3, 5, and 7 post inoculation, the wild type had 20, 23 and 12% more internalized bacteria, respectively, than the hexR mutant. The high local abundance of sugars, mainly sucrose and glucose, in the apoplast of leave tissue [49] could potentially be responsible for the weaker multiplication of the hexR mutant inside the plant tissue. Consequently, it is hypothesized that HexR might play an important role in survival of PG4180 inside soybean leaves. In turn, a higher percentage of external hexR mutant cells were surviving on the leaf surface although not significantly different (data not shown). Although our study did not provide direct evidence for this, an increase in levan formation by the hexR mutation might have protected the mutant from damage by UV light or desiccation leading to higher survival rates on the leaf surface.
Finally, one may hypothesize that production of the levan exopolymer would rather be a ‘shunt’ product during the release of glucose from sucrose which, in turn, could be the actual major function of Lsc in the sugar metabolic pathway (Figure 8). Genes encoding Lsc might be part of the HexR regulon of P. syringae in contrast to the situation in P. putida [23], which is neither phytopathogenic nor harboring any lsc genes. However, other bacterial species, which possess similar enzymes for cleavage of sucrose to obtain readily usable glucose, could show a similar HexR-mediated regulation. Therefore, it is suggestive to screen the most important oral cavity inhabiting bacterial species [50] as well as bacteria, which cause mucus formation in sucrose-based food manufacturing [51] or bio-fuel production [52] for presence of this regulatory linkage.
Schematic presentation of putative sucrose utilization pathway in P. syringae PG4180. Enzymes shown in blocks are presumed to be repressed by HexR in P. putida [22,23] or in P. syringae (present study). Lsc, levansucrase; Glk, glucose kinase; Zwf, glucose-6-phosphate dehydrogenase; Pgl, 6-phosphogluconolactonase; Edd, 6-phosphogluconate dehydratase; Eda, 2-keto-3-deoxy-6-phosphogluconate aldolase; Gap, glyceraldehyde 3-phosphate-dehydrogenase; TCA, tricarboxylic acid cycle.
The use of arabinose as sole carbon source had no effect on the growth phenotype of the hexR mutant. This was not surprising since the assimilatory pathways of glucose and arabinose are independent. L-arabinose is converted to α–keto-glutarate in Pseudomonas which can directly be utilized in the Tricarboxylic acid cycle (TCA cycle) independent of HexR regulation [53,54].
Currently, there is no plausible explanation for the lack of significant growth of the hexR mutant when supplemented with glucose or sucrose as sole carbon source. However, one might speculate on an up-regulation of genes normally repressed by HexR such as glk, zwf-1, pgl, edd, eda, and gap-1 as previously shown in P. putida [23]. The effect could be potentiated in P. syringae by an increased expression of lsc, whose gene product in turn provides even more glucose. Derepressed glucose consumption in the hexR mutant might cause a surplus production of NADPH, NADH, and ATP (Figure 8). In turn, this may lead to an imbalance of cellular redox homeostasis thus alleviating cellular ‘reductive stress’ or even inducing ‘energy spilling’, respectively.
In E. coli, the redox potential influences the synthesis of fermentation products, which are formed to recycle and reoxidize NADH [55]. When E. coli cells are aerobically challenged with high glucose concentrations, they undergo a so-called ‘acetate switch’, which decelerates growth [56]. A metabolic flux analysis of E. coli predicted that excess of carbon and energy might cause over-flow metabolism, which results in less efficient carbon utilization and decreased growth [57]. In Streptococcus bovis, excess ATP generation can cause ‘energy spilling’ by futile cycling of protons through the membrane, which leads to lesser biomass production [58,59]. Whether reductive stress or ‘energy spilling’ take place in a glucose-exposed hexR mutant of P. syringae remains to be analyzed in future studies.
Expression of lsc genes was higher in the hexR mutant as compared to the wild type during late logarithmic growth. This result is in accordance with previous results of micro-array analyses of glucose metabolic genes in P. putida KT2440 where genes glk and zwf-1 showed a ~ two-fold increased expression while genes pgl, edd, eda, and gap-1 exhibited a four- to six-fold increased expression in a respective hexR mutant [23]. Why lsc genes were only moderately up-regulated in the hexR mutant of P. syringae might be explained by the peripheral role of these genes in glucose metabolism.
The HR test is a classical assay to qualitatively show pathogenicity of a plant-associated microbe. The extracellular protein profiles and HR assay conducted with the wild type and the hexR mutant, respectively, suggested that HexR does not influence P. syringae’s ability to cause a HR on non-host plants. Furthermore, our results suggested that the secretion of hrp-associated proteins was not affected when hexR was mutated. It is therefore tempting to speculate that the reduced ability of the hexR mutant to survive inside of the plant is not due to altered hrp gene expression but is rather due to a distorted sugar metabolism.
Conclusions
Data of this study prompt the question whether HexR-controlled genes such as edd, eda, glk, pgl, zwf-1, or gap-1 [23] are indeed co-regulated with lsc genes in P. syringae. The current study revealed exciting options for an in-depth analysis of intra-cellular and extra-cellular hexose metabolism in the plant pathogen P. syringae and may allow us to better understand the potentially complex interplay of factors and parameters contributing to epiphytic or pathogenic behavior of this organism, respectively.
References
Srivastava A, Zhurina D, Ullrich MS. Levansucrase and levan formation in pseudomonas syringae and related organisms. Bacterial Polysaccharides: Current Innovations and Future Trends. Caister Academic Press 2009;213-222
Li H, Ullrich MS. Characterization and mutational analysis of three allelic lsc genes encoding levansucrase in Pseudomonas syringae. J Bacteriol. 2001;183(11):3282–92.
Khandekar S, Srivastava A, Pletzer D, Stahl A, Ullrich MS. The conserved upstream region of lscB/C determines expression of different levansucrase genes in plant pathogen Pseudomonas syringae. BMC Microbiol. 2014;14:1471–2180. (Electronic)):79.
Li H, Schenk A, Srivastava A, Zhurina D, Ullrich MS. Thermo-responsive expression and differential secretion of the extracellular enzyme levansucrase in the plant pathogenic bacterium Pseudomonas syringae pv. glycinea. FEMS Microbiol Lett. 2006;265(2):178–85.
Schenk A, Weingart H, Ullrich MS. Extraction of high-quality bacterial RNA from infected leaf tissue for bacterial in planta gene expression analysis by multiplexed fluorescent Northern hybridization. Mol Plant Pathol. 2008;9(2):227–35.
Lindow SE, Brandl MT. Microbiology of the phyllosphere. Appl Environ Microbiol. 2003;69(4):1875–83.
Budde IP, Ullrich MS. Interactions of Pseudomonas syringae pv. glycinea with host and nonhost plants in relation to temperature and phytotoxin synthesis. Mol Plant Microbe Interact. 2000;13(9):951–61.
Melotto M, Underwood W, He SY. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu Rev Phytopathol. 2008;46:101–22.
Dunleavy JM. Bacterial, fungal, and viral diseases affecting soybean leaves. In Soybean Diseases of the North Central Region (Wyllie TD & Scott DH, eds). American Phytopathological Society 1988;40–46
Smirnova A, Li H, Weingart H, Aufhammer S, Burse A, Finis K, et al. Thermoregulated expression of virulence factors in plant-associated bacteria. Arch Microbiol. 2001;176(6):393–9.
Mansfield JW. From bacterial avirulence genes to effector functions via the hrp delivery system: an overview of 25 years of progress in our understanding of plant innate immunity. Mol Plant Pathol. 2009;10(6):721–34.
Atkinson MM, Baker CJ. Alteration of plasmalemma sucrose transport in Phaseolus vulgaris by Pseudomonas syringae pv. syringae and its association with K+/H+ exchange. Phytopathology. 1987;77:1573–8.
Roitsch T, Gonzalez MC. Function and regulation of plant invertases: sweet sensations. Trends Plant Sci. 2004;9(12):606–13.
Biemelt S, Sonnewald U. Plant-microbe interactions to probe regulation of plant carbon metabolism. J Plant Physiol. 2006;163(3):307–18.
Papp B, Teusink B, Notebaart RA. A critical view of metabolic network adaptations. HFSP J. 2009;3(1):24–35.
Lessie TG, Phibbs PV. Alternative Pathways of Carbohydrate Utilization in Pseudomonads. Annu Rev Microbiol. 1984;38(1):359–88.
Entner N, Doudoroff M. Glucose and gluconic acid oxidation of Pseudomonas saccharophila. J Biol Chem. 1952;196(2):853–62.
Portais JC, Delort AM. Carbohydrate cycling in micro-organisms: what can (13)C-NMR tell us? FEMS Microbiol Rev. 2002;26(4):375–402.
Conway T. The Entner-Doudoroff pathway: history, physiology and molecular biology. FEMS Microbiol Rev. 1992;9(1):1–27.
Xie CH, Yokota A. Reclassification of Alcaligenes latus strains IAM 12599 T and IAM 12664 and Pseudomonas saccharophila as Azohydromonas lata gen. nov., comb. nov., Azohydromonas australica sp. nov. and Pelomonas saccharophila gen. nov., comb. nov., respectively. Int J Syst Evol Microbiol. 2005;55:1466–5026. Print).
Kim J, Jeon CO, Park W. Dual regulation of zwf-1 by both 2-keto-3-deoxy-6-phosphogluconate and oxidative stress in Pseudomonas putida. Microbiology. 2008;154(Pt 12):3905–16.
Daddaoua A, Krell T, Ramos JL. Regulation of glucose metabolism in Pseudomonas: the phosphorylative branch and entner-doudoroff enzymes are regulated by a repressor containing a sugar isomerase domain. J Biol Chem. 2009;284(32):21360–8.
del Castillo T, Duque E, Ramos JL. A set of activators and repressors control peripheral glucose pathways in Pseudomonas putida to yield a common central intermediate. J Bacteriol. 2008;190(7):2331–9.
Petruschka L, Adolf K, Burchhardt G, Dernedde J, Jurgensen J, Herrmann H. Analysis of the zwf-pgl-eda-operon in Pseudomonas putida strains H and KT2440. FEMS Microbiol Lett. 2002;215(1):89–95.
Leyn SA, Li X, Zheng Q, Novichkov PS, Reed S, Romine MF, et al. Control of proteobacterial central carbon metabolism by the HexR transcriptional regulator: a case study in Shewanella oneidensis. J Biol Chem. 2011;286(41):35782–94.
Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual (2nd ed). New York: Cold Spring Harbor Laboratory Press; 1989.
Keane P, Kerr A, New P. Crown Gall of Stone Fruit II. Identification and Nomenclature of Agrobacterium Isolates. Aust J Biol Sci. 1970;23(3):585–96.
Palmer DA, Bender CL. Effects of Environmental and Nutritional Factors on Production of the Polyketide Phytotoxin Coronatine by Pseudomonas syringae pv. Glycinea. Appl Environ Microbiol. 1993;59(5):1619–26.
Bender CL, Liyanage H, Palmer D, Ullrich M, Young S, Mitchell R. Characterization of the genes controlling the biosynthesis of the polyketide phytotoxin coronatine including conjugation between coronafacic and coronamic acid. Gene. 1993;133(1):31–8.
Figurski DH, Helinski DR. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979;76(4):1648–52.
Kovach ME, Phillips RW, Elzer PH, Roop 2nd RM, Peterson KM. pBBR1MCS: a broad-host-range cloning vector. Biotechniques. 1994;16(5):800–2.
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop 2nd RM, et al. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166(1):175–6.
Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212(1):77–86.
Choi KH, Mima T, Casart Y, Rholl D, Kumar A, Beacham IR, et al. Genetic tools for select-agent-compliant manipulation of Burkholderia pseudomallei. Appl Environ Microbiol. 2008;74(4):1064–75.
O’Brien RD, Lindow SE. Effect of Plant Species and Environmental Conditions on Ice Nucleation Activity of Pseudomonas syringae on Leaves. Appl Environ Microbiol. 1988;54(9):2281–6.
King EO, Ward MK, Raney DE. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954;44(2):301–7.
He SY, Huang H-C, Collmer A. Pseudomonas syringae pv. syringae harpinPss: A protein that is secreted via the hrp pathway and elicits the hypersensitive response in plants. Cell. 1993;73(7):1255–66.
Huynh T, Dahlbeck D, Staskawicz B. Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science. 1989;245(4924):1374–7.
Sambrook J, Russell DW. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press; 2001.
Hettwer U, Jaeckel FR, Boch J, Meyer M, Rudolph K, Ullrich MS. Cloning, nucleotide sequence, and expression in Escherichia coli of levansucrase genes from the plant pathogens Pseudomonas syringae pv. glycinea and P. syringae pv. phaseolicola. Appl Environ Microbiol. 1998;64(9):3180–7.
Finn RD, Mistry J, Tate J, Coggill P, Heger A, Pollington JE, et al. The Pfam protein families database. Nucleic Acids Res. 2010;38(Database issue):D211–222.
Dulla G, Marco M, Quinones B, Lindow S. A closer look at Pseudomonas syringae as a leaf colonist—the pathogen P. syringae thrives on healthy plants by employing quorum sensing, virulence factors, and other traits. ASM News. 2005;71:469–75.
Morris CE, Sands DC, Vinatzer BA, Glaux C, Guilbaud C, Buffiere A, et al. The life history of the plant pathogen Pseudomonas syringae is linked to the water cycle. ISME J. 2008;2(3):321–34.
Rojo F. Repression of transcription initiation in bacteria. J Bacteriol. 1999;181(10):2987–91.
Mercier J, Lindow SE. Role of leaf surface sugars in colonization of plants by bacterial epiphytes. Appl Environ Microbiol. 2000;66(1):369–74.
Kinkel LL, Wilson M, Lindow SE. Effect of sampling scale on the assessment of epiphytic bacterial populations. Microb Ecol. 1995;29(3):283–97.
Hirano SS, Upper CD. Population Biology and Epidemiology of Pseudomonas Syringae. Annu Rev Phytopathol. 1990;28(1):155–77.
Beattie GA, Lindow SE. The secret life of foliar bacterial pathogens on leaves. Annu Rev Phytopathol. 1995;33:145–72.
Benkeblia N, Shinano T, Osaki M. Metabolite profiling and assessment of metabolome compartmentation of soybean leaves using non-aqueous fractionation and GC-MS analysis. Metabolomics. 2007;3(3):297–305.
Bergeron LJ, Burne RA. Roles of fructosyltransferase and levanase-sucrase of Actinomyces naeslundii in fructan and sucrose metabolism. Infect Immun. 2001;69(9):5395–402.
Bekers M, Upite D, Kaminska E, Grube M, Laukevics J, Vina I, et al. Fructan Biosynthesis by Intra- and Extracellular Zymomonas mobilis Levansucrase after Simultaneous Production of Ethanol and Levan. Acta Biotechnol. 2003;23(1):85–93.
Lee KJ, Lefebvre M, Tribe DE, Rogers PL. High productivity ethanol fermentations with Zymomonas mobilis using continuous cell recycle. Biotechnol Lett. 1980;2(11):487–92.
Weimberg R, Doudoroff M. The oxidation of L-arabinose by Pseudomonas saccharophila. J Biol Chem. 1955;217(2):607–24.
Palleroni NJ, Contopoulou R, Doudoroff M. Metabolism of carbohydrates by Pseudomonas saccharophila. II. Nature of the kinase reaction involving fructose. J Bacteriol. 1956;71(2):202–7.
Berrios-Rivera SJ, Bennett GN, San KY. Metabolic engineering of Escherichia coli: increase of NADH availability by overexpressing an NAD(+)-dependent formate dehydrogenase. Metab Eng. 2002;4(3):217–29.
Wolfe AJ. The acetate switch. Microbiol Mol Biol Rev. 2005;69(1):12–50.
Schuetz R, Kuepfer L, Sauer U. Systematic evaluation of objective functions for predicting intracellular fluxes in Escherichia coli. Mol Syst Biol. 2007;3:119.
Bond DR, Russell JB. Protonmotive force regulates the membrane conductance of Streptococcus bovis in a non-ohmic fashion. Microbiology. 2000;146(3):687–94.
Russell JB. The energy spilling reactions of bacteria and other organisms. J Mol Microbiol Biotechnol. 2007;13(1–3):1–11.
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–8.
Acknowledgements
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (UL 169/5-1) and doctoral stipends from the Deutscher Akademischer Austauschdienst for AM and KA. The authors thank Helge Weingart for valuable scientific discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AM designed experiments, conducted qRT-PCR and RT-PCR, and prepared the manuscript; KA designed experiments, conducted in planta experiments, and prepared the manuscript; SK conducted growth curve experiments and Western blots; AS and DP conducted the in silico analysis lsc upstream sequences; DZ and GA mutagenized the hexR gene; NK conducted the hypersensitive response assay; MU designed the study, supervised the work, and finalized manuscript writing. All authors read and approved the final version.
Amna Mehmood and Khaled Abdallah contributed equally to this work.
Additional files
Additional file 1: Figure S1.
Verification of hexR null mutant phenotype by PCR amplification. The total RNA was extracted by phenol chloroform method followed by cDNA generation. PCR amplification of hexR fragment on total cDNA and genomic DNA (gDNA) using hexR specific primers. The quality of total cDNA and genomic DNA were checked by performing PCR amplification of lscB/C gene which signified correct amplicon. H = hexR mutant, WT = PG4180 wild type.
Additional file 3: Figure S3.
Multiple sequence alignment of HexR amino acid sequences from levan-producing Pseudomonas syringae strains using ClustalW [60]. PFAM comparison revealed two domains: Helix-turn-helix domain from residues 6–108 (PFAM PF01418) shown in pale grey and SIS domain from residues 128–256 (PFAM PF01380) shown in dark grey. Mismatched residues are marked in blue. Residues marked in red are the predicted DNA recognition (Q46, K49, E52, R57, R60) and effector recognition (S143, S187) residues of HexR, respectively [22].
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Mehmood, A., Abdallah, K., Khandekar, S. et al. Expression of extra-cellular levansucrase in Pseudomonas syringae is controlled by the in planta fitness-promoting metabolic repressor HexR. BMC Microbiol 15, 48 (2015). https://doi.org/10.1186/s12866-015-0349-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-015-0349-0