Abstract
Background
Cancer immunotherapy has gained increasing popularity as a novel approach to treat cancer. A member of the B7 family, V-domain immunoglobulin suppressor of T-cell activation (VISTA) is a novel immune checkpoint that regulates a broad spectrum of immune responses. VISTA is an acidic pH-selective ligand for P-selectin glycoprotein ligand-1(PSGL-1). CA-170, a first-in-class small-molecule dual antagonist of VISTA/PD-L1, was collaboratively developed by Aurigene Discovery Technologies Limited and Curis, Inc. It is currently in Phase I clinical trial.
Results
In this study, we develop homology modeling for the VISTA 3D structure and subsequent virtual screening for VISTA small-molecule hit ligands. Visualization of the binding postures of docked ligands with the VISTA protein indicates that some small molecular compounds target VISTA. The ability of antagonist to disrupt immune checkpoint VISTA pathways was investigated though functional studies in vitro.
Conclusions
Affinity active molecule for VISTA was obtained through virtual screening, and the antagonist compound activity to VISTA was assayed in cellular level. We reported a small molecule with high VISTA affinity as antagonist, providing ideas for development VISTA-targeted small molecule compound in cancer immunotherapy.
Similar content being viewed by others
Background
Immune checkpoints protect peripheral tissues from damage during immune responses. Immune checkpoint blockade has become one of the most promising approaches to activating antitumor immunity [1, 2]. Immune checkpoint inhibitors may release the brake on the immune system, allowing it to target cancer cells. Antibodies targeting immune checkpoint cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) have been broadly applied in the clinic and have yielded promising results as immunotherapy for diseases such as late-stage metastatic melanoma and nonsmall cell lung cancer (NSCLC) [3,4,5,6,7,8]. Several ongoing trials with immune checkpoints inhibitors (PD-1/PD-L1) might bring new insights for role of immunotherapy for patients with stages I to III NSCLC [9].Vaccination with dendritic cells (DCs) or DCs/cytokine-induced killer (CIK) cells has shown limited success in the treatment of patients with advanced non-small-cell lung cancer [10]. Immunotherapy, mainly vaccine-based (aiming to activate the host immune system to induce human immune response to tumour-specific antigens) did not make people live longer. But it seemed that those who were given vaccine-based immunotherapy may have experienced, on average, more side effects. Therefore, reseachers are testing new, more promising immunotherapy drugs (e.g. checkpoint inhibitors) [11].
However, the clinical application of antibody-based immunotherapy has encountered a number of problems, including a low response rate (20–30%) [4], the inconvenience of intravenous administration, immune-related adverse events (irAEs) and high cost for patients. In recent years, scientists have been dedicated to identifying additional immune checkpoint pathways and orally available small molecules to overcome these flaws.
Among the expanding list of immune checkpoints, V-domain immunoglobulin suppressor of T-cell activation (VISTA) [12] has been indicated as an important regulator of the immune system. VISTA is a type I transmembrane protein and bears features of both the B7 and CD28 families of immunoregulatory molecules. Murine VISTA extracellular Ig-V domain with 136-aa linked to a 23-aa stalk segment has 76% homology with the human protein [13]. Within hematopoietic cells, CD11bhigh myeloid cells, e.g., monocytes, conventional dendritic cells (DCs), and macrophages can overexpress VISTA [14]. VISTA regulates a broad spectrum of immune responses [14,15,16,17,18,19,20]. VISTA is reported as an acidic pH-selective ligand for PSGL-1 recnetly [21]. Studies of multiple tumor models, autoimmune disease models and clinical samples have demonstrated a pivotal regulatory role of VISTA on the immune system and its potential as a therapeutic or combinational drug target.
The feasibility of targeting VISTA for cancer treatment is manifested by findings indicating high VISTA expression on tumor cells in approximately 20% of NSCLC specimens [22]. Clinical studies have shown that VISTA expression is upregulated in gastric and oral squamous carcinoma [23, 24]; it is also increased after ipilimumab therapy in patients with prostate cancer [25,26,27,28]. Furthermore, previous studies have shown that VISTA is highly expressed in immune cell subsets from human pancreatic cancer patients [28].
The VISTA antibody drug trial was terminated in the clinical phase [29]. Noelle et al. first identified a peptide of VISTA antagonists through phage display. The peptide antagonist enhanced T cell proliferation in vitro and significantly enhanced antitumor immunity [30]. A small-molecule inhibitor of VISTA/PD-L1 (CA-170, the compound structure has not yet been disclosed)-collaboratively developed by Aurigene Discovery Technologies Limited and Curis, Inc-can restore the IFN-γ production inhibited by soluble VISTA upon induction of human peripheral blood mononuclear cells (PBMCs) with anti-CD3 and anti-CD28 antibodies [31, 32]. Currently, compound CA-170 is undergoing Phase I clinical trial for advanced tumors and lymphoma (NCT02812875) [33]. CA-170 exhibits potent activity when tested in assays to rescue lymphocyte proliferation and effector functions inhibited by PD-L1, PD-L2, or VISTA/PD-1H proteins. In a panel of functional assays, CA-170 showed selectivity against other immune checkpoint pathways including CTLA4, LAG3, and BTLA. These non-clinical data provided a strong rationale for the clinical development of CA-170 [34,35,36].
In addition to being a promising target for cancer treatment, VISTA is also a key regulator of several types of autoimmune inflammatory diseases due to its multifaceted role in modulating both innate and adaptive immune responses. Antagonistic or agonistic agents can conceivably modulate VISTA and its interacting partners (VSIG8 and VSIG3) [37, 38], which will greatly benefit the treatment of autoimmune and inflammatory diseases.
In this study, we sought to discover and develop small-molecule antagonists targeting VISTA. Compounds were screened by molecular docking and virtual screening. Then, protein binding activity assays and a cell activity evaluation were performed to provide insights into the development of small-molecule antagonists of VISTA.
Methods
Homology modeling of the VISTA three-dimensional structure
Homology modeling of the VISTA 3D structure was as reported previously [39]. The extracellular domain without the signal peptide of human (162 amino acids, UniProt: Q9H7M9) and murine(159 amino acids, UniProt: Q9D659) VISTA protein were submitted to the I-TASSER online server [40,41,42] to generated the three-dimensional model [39].
Virtual screening of small-molecule ligands docked with VISTA
The construction of 3D models and methods for virtual screening are reported previously [39]. Firstly, the ligand-binding pockets was predicted,and the docked ligands with a binding energy lower than − 6.0 kcal/mol were considered as the candidate murine VISTA ligands. Then, the best 20 candidate ligands were selected to verify their protein–ligand interaction following the subsequent experiments (ELISA) in Additional file 1 (Additional file 1: Table S1). Lastly, the lowest Kd values of the hit ligand detected by ELISA was applied for the VISTA-targeted in vitro experiment.
Visualization of docked ligands with the VISTA protein
As reported previously [39], the amino acid residues of the murine VISTA proteins involved in the protein–ligand interaction were regarded as potential interactive residues if they close to the hit ligands (≤ 1 Å).
Microscale thermophoresis (MST) experiment
Following the manufacturer’s instructions provided in the Monolith NT™ Protein Labeling Kit RED-NHS (NanoTemper Technologies GmbH), the murine VISTA-ECD was labeled accordingly. A 16-step serial dilution of compound 6809-0223 in the repective binding buffer was prepared, equal volume of labeled protein was added and mixed by pipetting. Then, the mixtures were incubated at room temperature for 30 min in the dark and filled into standard capillaries.
Animals
Mice (C57BL/6) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. All animal work were followed by the Laboratory Animal Management Committee of Jiangsu Province and approved by the ethics committee of China Pharmaceutical University. Mice were kept on a 12-h light/dark cycle with food and water provided ad libitum.
Murine CD4+ and CD8+ T cell activation assays
According to the manufacturer’s instructions (Stem Cell), the murine CD4+ and CD8+ cells were isolated from the spleen of C57BL/6. Purified CD4+ and CD8+ cells were stimulated by murine CD3(clone 2C11) and either VISTA-Ig or control-Ig with different ratio which coated in 96-well flat-bottom plates. After 48 h, the cell supernatants was harvested for cytokine secretion assay. For T-cell proliferation assay,purified CD4+ and CD8+ cells were labeled by CFSE (5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester) dye (Biolegend) for 20 min at 37 °C and 5% CO2 according to manufacturer’s protocol. CFSE-labeled T cells were plated into the pre-coated 96-well flat-bottom plates described above.T cell proliferation was assessed at 72 h by flow cytometer.
Cytometric bead assay (CBA) for CD8+ T cells
CD8+ T cells were isolated as described above, and a density of 1 × 105 cells/well were plated into the 96-well flat-bottom plates coated with anti-mouse CD3 (clone 17A2, Biolegend) at 2.5 μg/mL together with 5 μg/mL of murine VISTA-ECD protein. The compound 6809-0223 diluted into indicated concentrations was added to the culture medium. The cytokine levels in the cell supernatant collected at 48 h were analyzed using a BD Cytometric Bead Array (CBA) kit according to the manufacturer’s instructions.
Statistical analysis
The data are analyzed and expressed as the mean ± SD unless indicated otherwise. Statistically significant differences was determined by unpaired Student’s t-test. A value of P < 0.05 was considered significant at the 95% confidence level.
Results
Virtual screening for VISTA small-molecule hit ligand
The criteria for selection of candidate compounds has been described previously [41]. The 20 candidate ligands were selected for the subsequent experimental protein–ligand interaction verification by ELISA. The binding rates of murine VISTA-ECD with the compounds selected by virtual screening were evaluated with the ELISA assay (Additional file 1: Table S1). Twenty candidate compounds (binding energy less than − 6 kcal/mol) were selected for experimental verification. The predicted binding energie for one hit ligand (6809-0223) showed good binding affinity for murine VISTA. The binding energie for the ligand (6809-0223) was − 9.4 kcal/mol. In the subsequent experiments, we verified its biological effect on VISTA.
The protein–ligand interaction visualized with the Pymol software was displayed (Fig. 1).The docked position of the ligand (6809-0223) (2 loop areas: 58–62 aa and 150–157 aa) was showed in Fig. 1. The overall docked parameter of this protein–ligand interaction was summarized in Table 1.
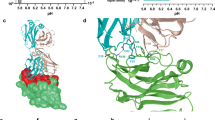
a Antagonist ligands (light blue) interacting with local residues of the murine VISTA protein (green). Residues on the VISTA protein interacting with antagonist ligands are highlighted in red. a Shows the whole VISTA protein extracellular domain and the docked positions of the ligand (6809-0223). b Structure of compound 6809-0223
The murine VISTA protein extracellular domain (159 amino acids, Fig. 2b) is slightly shorter than that of the human VISTA protein (162 amino acids, Fig. 2a). Both VISTA proteins had highly similar structures based on the 3D structural alignment (Fig. 2c). The antagonist-interacting residues for the murine VISTA proteins are located in close proximity in the 3D structure (Fig. 2d).
The 3D structural comparison between the human and murine VISTA proteins and their ligand-interacting residues. a The human VISTA protein extracellular domain (green); b The murine VISTA protein extracellular domain (light blue); c Aligned human VISTA protein (green) and murine VISTA protein (light blue); d Ligand interacting residues: murine VISTA antagonist interacting residues (orange), 3D structural alignment of the whole human/murine VISTA protein extracellular domain (bottom right)
The Kd value of the compound for the VISTA-ECD protein
The Kd value of compound 6809-0223 for murine VISTA-ECD was measured by MST and calculated as 0.647 ± 0.0387 μM (Fig. 3). Based on the Kd value, the binding affinity of the compounds for mVISTA was relatively weak and was at the micromolar level. The hits identified based on this approach will be a good starting point for further structural optimization in the future.
Murine VISTA antagonist 6809-0223 increases IL-2 secretion by CD4+ and CD8+ T cells
Murine T cells were plated in a 96-well plate (1 × 105 cells/well) and then stimulated with a CD3 antibody (2.5 μg/mL) with or without the murine VISTA-Fc protein (2.5 μg/mL, 5 μg/mL, or 10 μg/mL). As the concentration of VISTA increased, the inhibitory effect of murine VISTA-Fc on the secretion of IL-2 by CD4+ and CD8+ T cells was enhanced (Fig. 4a, b). Compound 6809-0223 was added to the cells, and IL-2 was measured by ELISA. The results showed that compound 6809-0223 increased IL-2 secretion by CD4+ and IFN-γ secretion by CD8+ T cells (Fig. 4c, d).
The murine VISTA protein inhibited cytokine production in T cells. a CD4+ T cells (1 × 105 cells/well) were stimulated with anti-CD3 (2C11) at 2.5 μg/mL and murine VISTA at ratios of 1:1, 1:2, and 1:4. Culture supernatants were collected at 48 h. IL-2 production was analyzed by ELISA. b CD8+ T cells (1 × 105 cells/well) were stimulated with anti-CD3 (2C11) at 2.5 μg/mL and murine VISTA at ratios of 1:1, 1:2, and 1:4. Culture supernatants were collected at 48 h. IL-2 production was analyzed by ELISA. c Purified CD4+ T cells (1 × 105 cells/well) were stimulated with anti-CD3 (2.5 μg/mL) and the murine VISTA protein as indicated. Culture supernatants were collected after 48 h. IL-2 production was analyzed by ELISA. d Bulk purified CD8+ T cells (1 × 105 cells/well) were stimulated with anti-CD3 (2.5 μg/mL) and the murine VISTA protein (5 μg/mL). Culture supernatants were collected at 48 h. IFN-γ production was analyzed by ELISA. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. All results were repeated three times with consistent results (n = 3)
Compound 6809-0223 increased murine CD4+ T cell proliferation
CD4+ T cell proliferation was analyzed at 72 h by examining the CFSE profiles. CD4+ T cell proliferation was increased after compound 6809-0223 administration (Fig. 5).
Compound 6809-0223 increases CD4+ T cell proliferation. CFSE-labeled cells were stimulated with anti-CD3 (2C11) at 2.5 μg/mL and murine VISTA-Fc at 5 μg/mL. Each well was seeded with 1 × 105 cells/well. Cell supernatants were collected for flow cytometry at 72 h. Compound 6809-0223 promoted CD4+ T cell proliferation. All results were repeated three times with consistent results (n = 3)
Compound 6809-0223 increased cytokine production in CD8+ T cells based on the CBA assay.
CD8+ T cells were stimulated with anti-mouse CD3 antibodies, and mouse VISTA-ECD was added as indicated. The CBA results showed that production of the IL-2, IL-4, IFN-γ and TNF-α cytokines was decreased by murine VISTA-Fc. Whereas, IL-2, IL-4, IFN-γ and TNF-α production was increased by the addition of compound 6809-0223 (Fig. 6). The results showed that compound 6809-0223 could increase the secretion of certain cytokines, although the mechanism is still under investigation.
The activity of compound 6809-0223 was detected by CBA in CD8+ T cells. Cells (1 × 105 cells/well) were incubated with an immobilized anti-mouse CD3 antibody (2.5 μg/mL) and mouse VISTA-ECD at 5 μg/mL, and compound 6809-0223 was added as indicated. The levels of a IL-2, b IL-4, c IFN-γ, d TNF-α in the cell culture supernatants were measured at 48 h with Cytometric Bead Array (CBA) Kits (n = 3)
Discussion
As reported, therapeutic antibodies of immune checkpoints possess high binding affinity for their target protein. However, its low response rate, immune-related toxicities and high cost remain unsolved problems that warrant effort to discover additional immune pathways or find alternatives to antibody-based treatment. As an alternative to monoclonal antibodies, small-molecule modulators are expected to overcome some of the aforementioned disadvantages. Discovery and development of small-molecule drugs for immune checkpoints is a promising and challenging prospect. At present, immunologic checkpoint inhibitor (ICI) have remarkable clinical efficacy in the treatment of cancers, however, the breakdown of immune escape causes a variety of immune-related adverse events, such as pneumonitis, rash and pruritus [43,44,45,46]. And this point need further investigation.
We homologously reconstructed the VISTA protein 3D structure using the protein amino acid sequence. Approximately 130,000 chemical compounds from our compound library were virtually screened using the VISTA protein 3D models. After the preliminary screening, potential compounds were tested for VISTA protein binding affinity by ELISA, and the compounds corresponding to the top three test results were selected. These selected compounds were used to adjust the predicted binding position on the VISTA protein for the second round of virtual compound screening, and the candidate molecules were repeatedly subjected to a protein affinity test until the screening results reached a plateau. The final obtained compounds are candidate compounds for verification. Although the amino acid sequence of human VISTA is slightly different from that of murine VISTA, their 3D models are very similar when structurally aligned (RMSD, root mean square deviation < 1 Å).
The predicted Mlog P value of the VISTA binding compound is 2.3–2.8. Combined with cellular bioassay, the compound 6809-0223 was considered lead compound for VISTA antagonist. ExpiCHO cells were used to express the murine VISTA extracellular domain proteins and obtain high purity murine VISTA proteins by affinity chromatography in Additional file 1 (Additional file 1: Figure S1 and S2). The ELISA screening model was established, and potential compounds from the virtual screen were tested for their murine VISTA protein binding affinity. To the best of our knowledge, this study is the first time to screen and identify VISTA modulators through a eukaryotic expression method. The affinity of the active compound for VISTA was further evaluated using MST experiments. We found that compound 6809-0223 had high affinity for the murine VISTA protein with Kd = 0.647 ± 0.0387 μM (Fig. 3). Compound 6809-0223 increased IL-2 secretion by CD4+ and CD8+ T cells and increased CD4+ T cell proliferation. Besides, the expression patterns of CD4 and CD8 in tumor tissues can be a potential option for further investigation. Their correlation with survival data need to be further studied.
In this study, the compound affinity for VISTA was relatively weak, and the activity of the molecule was in the submicromolar range. Therefore, the hit identified based on this approach is a good starting point for future optimization. The crystal structure of VISTA and the compound/VISTA complex are undergoing.
Conclusions
In summary, affinity active molecule for VISTA was obtained through virtual screening, and the antagonist compound activity to VISTA was assayed in cellular level (Fig. 7). We reported a small molecule with high VISTA affinity as antagonist, providing ideas for development VISTA-targeted small molecule compound in cancer immunotherapy.
Availability of data and materials
All data in the article can be requested from the corresponding author.
Abbreviations
- CBA:
-
cytometric bead assay
- CFSE:
-
5- (and 6)-carboxyfluorescein diacetate succinimidyl ester
- MST:
-
microscale thermophoresis
- VISTA:
-
V-domain immunoglobulin suppressor of T-cell activation
References
Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–77. https://doi.org/10.1038/nri2326.
Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. https://doi.org/10.1146/annurev.immunol.23.021704.115611.
Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–14. https://doi.org/10.1016/j.cell.2015.03.030.
Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61. https://doi.org/10.1016/j.ccell.2015.03.001.
Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: what’s here, what’s next? Curr Opin Immunol. 2015;33:23–35. https://doi.org/10.1016/j.coi.2015.01.006.
Hoos A. Development of Immuno-oncology drugs-from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov. 2016;15:235–47. https://doi.org/10.1038/nrd.2015.35.
Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, cars and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273–90. https://doi.org/10.1038/nrclinonc.2016.65.
Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14:561–84. https://doi.org/10.1038/nrd4591.
Li RJ, Tiselius E, et al. Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database Syst Rev. 2017;12(12):CD011300. https://doi.org/10.1002/14651858.CD011300.pub2.
Mohsenzadegan M, Peng RW, Roudi R. Dendritic cell/cytokine-induced killer cell-based immunotherapy in lung cancer: what we know and future landscape. J Cell Physiol. 2020;235(1):74–86. https://doi.org/10.1002/jcp.28977.
Zhu J, Li R, Tiselius E, et al. Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database Syst Rev. 2017;12(12):CD011300. https://doi.org/10.1002/14651858.CD011300.pub2.
Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, Lu LF, Gondek D, Wang Y, Fava RA, Fiser A, Almo S, Noelle RJ. VISTA, a novel murine Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208:577–92. https://doi.org/10.1084/jem.20100619.
Hosseinkhani N, Derakhshani A, Shadbad MA, et al. The role of V-domain Ig suppressor of T cell activation (VISTA) in cancer therapy: lessons learned and the road ahead. Front Immunol. 2021;12: 676181. https://doi.org/10.3389/fimmu.2021.676181.
Mehta N, Maddineni S, Mathews II, et al. Structure and functional binding epitope of V-domain Ig suppressor of T cell activation. Cell Rep. 2019;28:2509-2516.e5. https://doi.org/10.1016/j.celrep.2019.07.073.
Gavrieli M, Sedy J, Nelson CA, Murphy KM. BTLA and HVEM cross talkregulates inhibition and costimulation. Adv Immunol. 2006;92:157–85. https://doi.org/10.1016/S0065-2776(06)92004-5.
Yi KH, Chen L. Fine tuning the immune response through B7–H3 and B7–H4. Immunol Rev. 2009;229:145–51. https://doi.org/10.1111/j.1600-065X.2009.00768.x.
Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. https://doi.org/10.1016/j.immuni.2016.05.001.
Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276:80–96. https://doi.org/10.1111/imr.12519.
Lines JL, Sempere LF, Broughton T, Wang L, Noelle R. VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol Res. 2014;2:510–7. https://doi.org/10.1158/2326-6066.CIR-14-0072.
Janakiram M, Shah UA, Liu W, Zhao A, Schoenberg MP, Zang X. The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7–H3. Immunol Rev. 2017;276:26–39. https://doi.org/10.1111/imr.12521.
Johnston RJ, Su LJ, Pinckney J, et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature. 2019;574:565–70. https://doi.org/10.1038/s41586-019-1674-5.
Zielinski C, Knapp S, Mascaux C, Hirsch F. Rationale for targeting the immune system through checkpoint molecule blockade in the treatment of non-small-cell lung cancer. Ann Oncol. 2013;24(5):1170–9. https://doi.org/10.1093/annonc/mds647.
Böger C, Behrens HM, Krüger S, Röcken C. The novel negative checkpoint regulator VISTA is expressed in gastric carcinoma and associated with PD-L1/PD-1: a future perspective for a combined gastric cancer therapy? Oncoimmunology. 2017;6(4):e1293215. https://doi.org/10.1080/2162402X.2017.1293215.
Wu L, Deng WW, Huang CF, Bu LL, Yu GT, Mao L, Zhang WF, Liu B, Sun ZJ. Expression of VISTA correlated with immunosuppression and synergized with CD8 to predict survival in human oral squamous cell carcinoma. Cancer Immunol Immunother. 2017;66(5):627–36. https://doi.org/10.1007/s00262-017-1968-0.
Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, Zhao H, Chen J, Chen H, Efstathiou E, Troncoso P, Allison JP, Logothetis CJ, Wistuba II, Sepulveda MA, Sun J, Wargo J, Blando J, Sharma P. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med. 2017;23:551–5. https://doi.org/10.1038/nm.4308.
Villarroel-Espindola F, Yu X, Datar I, Mani N, Sanmamed M, Velcheti V, Syrigos K, Toki M, Zhao H, Chen L, Herbst RS, Schalper KA. Spatially resolved and quantitative analysis of VISTA/PD-1H as a novel immunotherapy target in human non-small cell lung cancer. Clin Cancer Res. 2018;24:1562–73. https://doi.org/10.1158/1078-0432.CCR-17-2542.
Kakavand H, Jackett LA, Menzies AM, Gide TN, Carlino MS, Saw RPM, Thompson JF, Wilmott JS, Long GV, Scolyer RA. Negative immune checkpoint regulation by VISTA: a mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Mod Pathol. 2017;30:1666–76. https://doi.org/10.1038/modpathol.2017.89.
Liu J, Xie X, Xuan C, Li T, Wang L, Teng L, Liu J. High-density infiltration of V-domain immunoglobulin suppressor of T-cell activation up-regulated immune cells in human pancreatic cancer. Pancreas. 2018;47(6):725–31. https://doi.org/10.1097/MPA.0000000000001059.
A study of safety, pharmacokinetics, pharmacodynamics of JNJ-61610588 in participants with advanced cancer. https://clinicaltrials.gov/. Cited 6 May 2018.
Spaller M.; Noelle R.; Ceeraz S.; Lemercier I.; Nowak E.; Lines J.et al. VISTA antagonist and methods of use[P].US: WO2015109340.2015–07–23.
Sasikumar PGN, Ramachandra M, Naremaddepalli SSS. VISTA signaling pathway inhibitory compounds useful as immunomodulators. IN: WO2018047143, 2018-03-15.
Sasikumar PG, Naremaddepalli SS, Ramachandra RK, Gowda N, Yerramsetti MR, Bandireddy SR, Adurthi S, Mani J, Nair R, Dhudashia AA, Dodheri SS, Gowda NM, Ramachandr M. Functional antagonism of VSIG8-mediated immune suppression by oral VISTA agents. Mol Cancer Ther. 2018;17:B006. https://doi.org/10.1158/1535-7163.
A study of CA-170 (Oral PD-L1, PD-L2 and VISTA check point antagonist) in patients with advanced tumors and lymphomas. https://clinicaltrials.gov/. Cited 19 Jun 2018.
Sasikumar PG, Ramachandra M. Small-molecule immune checkpoint inhibitors targeting PD-1/PD-L1 and other emerging checkpoint pathways. BioDrugs. 2018;32(5):481–97. https://doi.org/10.1007/s40259-018-0303-4.
Wang T, Wu X, Guo C, Zhang K, Xu J, Li Z, Jiang S. Development of inhibitors of the programmed cell death-1/programmed cell death-ligand 1 signaling pathway. J Med Chem. 2019;28:1715–30. https://doi.org/10.1021/acs.jmedchem.8b00990.
Blevins DJ, Hanley R, Bolduc T, et al. In vitro assessment of putative PD-1/PD-L1 inhibitors: suggestions of an alternative mode of action. ACS Med Chem Lett. 2019;10:1187–92. https://doi.org/10.1021/acsmedchemlett.9b00221.
Yang W, Padkjær SB, Wang J, Sun Z, Shan B, Yang L, Chen H, Kang L, Madsen D, Li X, Shen C, Yu B, Zhu H, Chao TY, Cao Z, Li D, Liu W, Du Y, Xu J, Hao D, Xu F, Peng L, Li T, Wang L, Li L, Xing H, Liu D, Liu Z, Guan Z, Wang W, Cheng H, Østergaard H, Chang C, Yang Z, Boel E, Su J. Construction of a versatile expression library for all human single-pass transmembrane proteins for receptor pairings by high throughput screening. J Biotechnol. 2017;260:18–30. https://doi.org/10.1016/j.jbiotec.2017.08.023.
Prodeus A, Abdul-Wahid A, Sparkes A, Fischer NW, Cydzik M, Chiang N, Alwash M, Ferzoco A, Vacaresse N, Julius M, Gorczysnki RM, Gariépy J. VISTA.COMP-an engineered checkpoint receptor agonist that potently suppresses T cell-mediated immune responses. JCI Insight. 2017;2:94308. https://doi.org/10.1172/jci.insight.94308.
Hu X, Qie C, Jiang J, et al. M351–0056 is a novel low MW compound modulating the actions of the immune-checkpoint protein VISTA. Br J Pharmacol. 2021. https://doi.org/10.1111/bph.15357.
Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. The I-TASSER suite: protein structure and function prediction. Nat Methods. 2015;12:7–8. https://doi.org/10.1038/nmeth.3213.
Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–38. https://doi.org/10.1038/nprot.2010.5.
Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008;9:40. https://doi.org/10.1186/1471-2105-9-40.
Sun X, Roudi R, Dai T, et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a PRISMA systematic review and meta-analysis. BMC Cancer. 2019;19(1):558. https://doi.org/10.1186/s12885-019-5701-6.
Alfredo T, Giandomenico R, Rosa L, et al. Anti-PD-1 versus anti-PD-L1 therapy in patients with pretreated advanced non-small-cell lung cancer: a meta-analysis. Future Oncol. 2019;15:2423–33. https://doi.org/10.2217/fon-2018-0868.
Sun X, Roudi R, Dai T, et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a PRISMA systematic review and meta-analysis. BMC Cancer. 2019;19(1):558. https://doi.org/10.1186/s12885-019-5701-6.
Tartarone A, Roviello G, Lerose R, et al. Anti-PD-1 versus anti-PD-L1 therapy in patients with pretreated advanced non-small-cell lung cancer: a meta-analysis. Future Oncol. 2019;15(20):2423–33. https://doi.org/10.2217/fon-2018-0868.
Acknowledgements
Not applicable.
Funding
This research was funded by the National Natural Science Foundation of China (No. 81973361) and Natural Science Foundation of Jiangsu Province (BK20202009).
Author information
Authors and Affiliations
Contributions
JL designed the research, contributed to the execution of the research, analyzed the data and wrote the manuscript. TTL and CXQ performed the research; JWJ performed the molecular docking and virtual screening, CXX and XLH provided the materials and protocols. WML and WTC performed the experiments and analyzed the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experiments were performed in accordance with the Laboratory Animal Management Committee of Jiangsu Province and approved by the ethics committee of China Pharmaceutical University.
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Figure S1. Murine VISTA-Fc expression and purification. (a) Protein expression in the cell supernatant gradually increased after transfection. (b) The protein was purified from the cell supernatant using Protein A resin. The murine VISTA-Fc protein was detected by Western blotting. (c) The purity of the recombinant VISTA-Fc protein was determined by SDS-PAGE. Figure S2. Murine VISTA-His expression and purification. (a) Protein expression in the cell supernatants as detected by Western blotting after transfection. (b) The protein was purified from the cell supernatant using Ni SepharoseTM 6 Fast Flow resin. The murine VISTA-his10 protein was detected by Western blotting. (c) The purity of the recombinant VISTA-His protein was determined by SDS-PAGE. Table S1. The binding rate of murine VISTA with compounds by ELISA assay.The absorbance was read at 450 nm (OD450) and 570nm(OD570).The absorbance at 570nm can be subtracted from the absorbance at 450nm. Binding rates(%) = (ODcompound-ODprotein)/ODprotein × 100%.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Tt., Jiang, Jw., Qie, Cx. et al. Identification of active small-molecule modulators targeting the novel immune checkpoint VISTA. BMC Immunol 22, 55 (2021). https://doi.org/10.1186/s12865-021-00446-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12865-021-00446-4