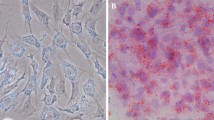
Abstract
Objectives
Serum/glucocorticoid-inducible kinase 1 (SGK1) gene encodes a serine/threonine protein kinase that plays an essential role in cellular stress response and regulation of multiple metabolic processes. However, its role in bovine adipogenesis remains unknown. In this study, we aimed to clarify the role of SGK1 in bovine lipid accumulation and improvement of meat quality.
Methods
Preadipocytes were induced to differentiation to detect the temporal expression pattern of SGK1. Heart, liver, lung, spleen, kidney, muscle and fat tissues were collected to detect its tissue expression profile. Recombinant adenovirus and the lentivirus were packaged for overexpression and knockdown. Oil Red O staining, quantitative real-time PCR, Western blot analysis, Yeast two-hybrid assay, luciferase assay and RNA-seq were performed to study the regulatory mechanism of SGK1.
Results
SGK1 showed significantly higher expression in adipose and significantly induced expression in differentiated adipocytes. Furthermore, overexpression of SGK1 greatly promoted adipogenesis and inhibited proliferation, which could be shown by the remarkable increasement of lipid droplet, and the expression levels of adipogenic marker genes and cell cycle-related genes. Inversely, its knockdown inhibited adipogenesis and facilitated proliferation. Mechanistically, SGK1 regulates the phosphorylation and expression of two critical proteins of FoxO family, FOXO1/FOXO3. Importantly, SGK1 attenuates the transcriptional repression role of FOXO1 for PPARγ via phosphorylating the site S256, then promoting the bovine fat deposition.
Conclusions
SGK1 is a required epigenetic regulatory factor for bovine preadipocyte proliferation and differentiation, which contributes to a better understanding of fat deposition and meat quality improvement in cattle.
Similar content being viewed by others
Introduction
Intramuscular fat (IMF) content is essential to the quality of beef as it determines sensory characteristics and palatability [1]. Meanwhile, it also contains fatty acids and proteins that cannot be synthesized in human [2]. Adipose tissue played a significant role in the regulation of energy storage [3], and the maintenance of homeostasis [4]. Adipogenesis included two processes, over-proliferation and hypertrophy, which was regulated by multi-tissues via paracrine and endocrine mechanisms. The development of adipose tissue depended on transcription factors, hormones, enzymes and non-coding RNAs [5,6,7,8]. Exploring the underlying genetic mechanism was beneficial for the improvement of beef quality.
SGK1, a member of the serine/threonine kinase AGC family, is a conserved protein in mammals [9]. It was first cloned in rat breast cancer cells stimulated by serum and glucocorticoi. Functionally, it is similar to the phosphorylated mammalian target of rapamycin (mTOR) [10]. SGK1 inhibited autophagy in muscle by acting on the upstream of ULK1 [11, 12]. SGK1 also controlled renal tubular transport by inhibiting the degradation of ENaC [13]. The inhibitory role of SGK1 in angiogenesis was regulated by BMP9, leading to endothelial cell proliferation [14]. SGK1 also played a key role in regulating adipocyte differentiation and maintaining glycolipid homeostasis. Both its mRNA and protein levels are high in white adipose tissue, which was relevant to obesity and type 2 diabetes [15]. Expression of SGK1 in subcutaneous and omental adipose tissue was stimulated by inflammatory signals and regulated by obesity-associated inflammation [16]. SGK1 was a mediator of glucocorticoids and high-fat feeding, and it induced insulin resistance in adipocytes by phosphorylating FOXO1 in db/db mice [17].
Multiple pathways participate in the regulation of adipogenesis [18], among which the PI3K/Akt signaling pathway plays a pivotal role in maintaining lipid metabolism and regulating insulin [19]. The family of forkhead box O (FOXO) transcription factors located downstream of the PI3K/Akt signaling was negatively regulated by phosphorylated AKT [20, 21]. Genetic mutations or abnormal expression of FOXO genes were associated with metabolic disease, cancer or altered lifespan in humans and animals [22]. FOXO1, a downstream target of Akt, regulated the cell cycle and lipogenesis in adipocytes through PPARγ [23]. Moreover, the FOXO1 antagonist increased adipocyte autophagy and inhibited obesity, indicating their potentiality in anti-obesity treatments [24]. FOXO3 knockdown significantly inhibited autophagy and lipid accumulation, reducing LPS-induced inflammation [25].
Previous RNA-seq data showed that SGK1 was differentially expressed in bovine subcutaneous adipocytes. Therefore, we explored the role of SGK1 in bovine adipogenesis, and found it could affect the transcriptional activity of FOXO1 further triggered the lipid accumulation in bovine subcutaneous adipocytes. The results contribute to expanding the network of candidates regulating bovine adipogenesis, and facilitate the study in promoting beef IMF. Our study also suggests that SGK1 may act as one of the key targets for improving the meat quality.
Materials and methods
Ethics statement
Three adult (~ 2.5 years old) Nanyang beef cattle (castrated) were slaughtered from Biyin cattle farm Nanyang city, Henan province. Subcutaneous adipose tissue was obtained from calves for isolation of preadipocytes. The Animal Ethics Committees of Ningxia University approved the experimental design and the animal sample collection for the present study (permit number NXUC20220625). Animal experiments complied with the requirements of the directory of the Ethical Treatment of Experimental Animals of China. Animal experiments were conducted strictly following the Regulations for the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China, 2004). All methods are in accordance with ARRIVE guidelines (https://arriveguidelines.org) for the reporting of animal experiments.
Isolation and induced differentiation of bovine preadipocytes
Bovine preadipocytes were isolated using collagenase digestion. Briefly, adipose tissue without blood vessels and connective tissues was minced into cubes with size ~ 1 mm3 and digested in 1 mg/mL collagenase type II (Sigma, C0130) in a water bath for 90 min at 37 ℃. Subsequently, preadipocytes were seeded and cultured on a 10 cm2 plate with a growth medium containing Dulbecco's modified eagle medium (DMEM), 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) at 37 ℃ with 5% CO2 for 24 h. Cells were then washed with PBS three times and fresh medium was replaced every two days.
When density reached 90%, cells were transferred to 6-well cell culture plates using trypsin. Subsequently, adipocytes were differentiated by feeding induction medium (IM) containing DMEM, 10% FBS, 10 µg/mL insulin, 1 µM rosiglitazone, 1 µM dexamethasone (DEXA, Sigma) and 0.5 mM 1-methyl-3-isobutylxanthine (IBMX, Sigma). Three days later, the medium was replaced with a maintenance medium which was changed every two days until maturation.
Sequence analysis of bovine SGK1
Primers were designed to amplify the CDS region of SGK1 (Genbank No. NM_001102033.1) (Supplementary Table 1) and interacting proteins were predicted using STRING.
Recombinant adenovirus packaging
Endonucleases Kpn I (Takara, Dalian, China) and Hind III (Takara) were added to forward and reverse primers, respectively. Subsequently, the CDS region was inserted into the shuttle vector pAd-tract-CMV and was transformed into competent cells BJ5183. The recombinant vector was transfected into HEK293A. Cells from culture medium (OE-SGK1) and negative control named (OE-NC) were collected eight days after transfection. pAd-track-CMV and HEK293A were preserved in our laboratory.
The 1 296 bp-long CDS region of full length bovine SGK1 gene was amplified without mutations. The recombinant plasmid was digested with Pac I enzyme, producing two expected fragments (Supplementary Fig. 1A). The digestion products were transfected into transformed HEK293A cells. HEK293A cells grew well 24 h after transfection (Supplementary Fig. 1B-1) and expressed green fluorescent protein (GFP) that appeared to form a scattered pattern (Supplementary Fig. 1B-2). GFP expression increased after day 5 (Supplementary Fig. 1B-3) and appeared as a comet-like shape tail (Supplementary Fig. 1B-4). GFP covered the whole plate and detached from plate after day 8. Subsequently, the collected cells were infected with HEK293A cells 4–5 times to obtain a high-titer virus solution. The virus titers of OE-SGK1 and OE-NC were 2.51*10^10 and 3.16*10^10 pfu/mL, respectively. Therefore, the overexpression virus was successfully packaged.
Dual-luciferase report analysis
Three short hairpin RNAs (shRNA1, shRNA2, and shRNA3) were designed using online software (http://rnaidesigner.thermofisher.com.rnaiexpress/) (Table 1) and were synthesized. They were connected to the pENTR/U6 plasmid and named pENTR/U6-shRNA1, pENTR/U6-shRNA2, and pENTR/U6-shRNA3. The recombinant plasmid was constructed with psicheck II and the CDS region of SGK1 (psicheck II-SGK1). Cell transfection was performed using Lipofectamine 3000 (Thermo, Waltham, MA, USA) according to the manufacturer’s instructions. pENTR/U6-shRNA or pENTR/U6-NC were dissolved using Opti-MEM (Sigma, St. Louis, Missouri, USA) and incubated with psicheckII-SGK1 to form a DNA-liposome mixture. Subsequently, the DNA-liposome mixture was added to the culture medium and incubated for 48 h at 37 °C and 5% CO2. The luciferase activity in 24 wells containing HEK293T cells was measured. Two plasmids, pGL3-basic and pcDNA3.1 co-transfected into 293T WT cell to perform the luciferase assay of transcriptional activity. For transcriptional activity assay, we seed 293T WT cells in 24 wells. Two plasmids, pGL3-basic with pFOXO1-luciferase and pcDNA3.1-SGK1 with pFOXO1-luciferase co-transfected into 293T WT cells, respectively. The luciferase activity was measured after 48 h.
Short hairpin RNA lentivirus packagin
The selected shRNA1 for single target lentivirus packaging and the negative control were named sh-SGK1 and sh-NC, respectively. Recombinant vector c-shRNA1 and helper vectors psPAX2 and pMD2G were co-transfected with Lipofectamine 3000 into HEK293T cells according to the manufacturer’s instructions. Fluorescence was detected with an inverted microscope after 24 h. The supernatant was collected at 48 h and 72 h to concentrate and purify the virus.
Three 61 bp shRNAs were inserted in pENTR/U6 (Supplementary Fig. 2A) and the coding region of SGK1 was inserted in psicheck II (Supplementary Fig. 2B). A dual-luciferase reporter assay identified shRNA1 as the best interfering sequence (Supplementary Fig. 2C). Thus, shRNA1 was used to package lentivirus and the recombinant plasmid c-shRNA1 was constructed (Supplementary Fig. 2D). The titers of sh-SGK1 and sh-NC were 108 and 3 × 108 TU/mL, respectively.
Cell infection
Preadipocytes were seeded onto 6-well plates overnight to reach 70% confluence. The OE-SGK1 and sh-SGK1 virus solutions were added to adipocyte culture plates (n = 3) with an optimal MOI (multiplicity of infection) value determined in a preliminary experiment, using OE-NC and sh-NC, respectively, as controls. After 48 h incubation at 37 °C and 5% CO2, fluorescence was detected with an inverted microscope after 24 h and the medium was replaced by high glucose medium containing 10% FBS.
Total RNA extraction and RT-qPCR
Total RNA was extracted from adipocytes or tissues using Trizol reagent. cDNA was synthesized by using a Primescript™ RT reagent kit (Takara) and SYBR premix Ex Taq II kit (Takara) was used to perform the RT-qPCR reaction on a Bio-Red CFX 96 Touch instrument (Bio-Rad, Hercules, CA, USA). The 2−ΔΔCt method was used to analyze the data. Primers of adipogenic and cell cycle genes were designed by Primer Premier 5 according to the primer designing criteria (Supplementary Table 1). Relative expression levels were normalized with the internal control GAPDH [26].
Total protein extraction and immunoblotting
Total protein was extracted using whole cell Lysis Assay (KeyGEN BioTECH, Nanjing, China) and quantified using a BCA protein assay kit (KeyGEN BioTECH, Nanjing, China). Equal amounts of protein (10 μg per lane) were run on 5% and 12% or 8% SDS-PAGE and were transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore Corporation, Bedford, MA, USA). The membranes were cut near the purpose bands before hybridisation with primary antibodies. The cropped membranes were blocked with 3% bovine serum albumin (Sigma, WXBD4881V) for 2 h at room temperature and incubated with the primary antibody overnight at 4 °C. Primary antibodies were: anti-CDK2 (abbexa 1:500, cat. no. abx009457), anti-PPARγ (abbexa, 1:500, cat. no. abx104516), anti-C/EBPα (abbexa, 1:500, cat. no. abx009679), anti-Cyclin D2 (Abmart, 1:500, cat. no. TA5410), anti-FOXO1 (Abmart, WB: 1:500, cat. no. PA1431; pS256: 1:500, cat. no. PA5293), anti-FOXO3 (Abmart, WB: 1:500, cat. no. PA5311; pS322 + S325/pS318 + S321: 1:500, cat. no. PA5855) and anti-GAPDH (ZSGB-BIO, 1:500, cat. no. ZB-2301). Then membranes were incubated with anti-rabbit IgG (ZSGB-BIO, 1:5000, ZB-2301) secondary antibodies for 2 h at room temperature. Finally, blots were visualized by ECL reagent and captured using the Tanon-5200 imaging system (Shanghai, China).
Yeast two-hybrid assay
The Y2H interaction mating assays were described as previously published [27, 28]. Briefly, constructing bait protein named pGBKT7-SGK1 as well as prey proteins named pGADT7-FOXO1 and pGADT7-FOXO3. Then, detecting self-activation toxic effect of bait proteins. For interaction mating, bait protein and prey proteins were co-transformed into yeast strains Y2H Gold applying to YPDA plate incubation for ~ 3 days at 30°C to preform preliminary screening. After primary screening, the blue positive clones on the primary screening plate were transferred to the secondary screening medium SD/-Ade/-His/-Leu/-Trp/X-α-Gal/AbA to screen positive clones and sequence. Finally, prey plasmids and bait plasmid performed one to one verification coating on SD/-Leu/-Trp plate and SD/-Ade/-His/-Leu/-Trp/X-α-Gal/AbA plate incubation for 3–5 days at 30°C.
RNA-seq analysis
Adipocytes infected by OE-SGK1 or OE-NC and induced differentiation for 6 days. Subsequently, cells were used to perform deep sequencing (n = 4) using Illumina xten completed by BioMarker Co (Qingdao, China). RNA-seq data was analyzed in R (R × 64 4.1.2) using the R package (such as pheatmap and GOplot) and visual analysis was performed in Cytoscape (cytoscape_3.9.0).
Statistical analysis
For each group at least three independent experiments were performed and data was expressed as mean ± standard deviation (SD). GraphPad Prism v8.0.2 (GraphPad Software, Inc., La Jolla, CA, USA) was used to analyze the experimental data. One-way analysis of variance (ANOVA) and T test were used to analysis the significance of qPCR. P < 0.05 was considered to indicate a statistically significant difference, and P < 0.01 was considered to indicate that the difference was extremely significant.
Results
Establishment of bovine preadipocytes-induced differentiation system
Preadipocytes were isolated from bovine subcutaneous adipose tissue using collagenase Type II digestion, and induced to differentiate into mature adipocytes using insulin when the confluence reached ~ 90%-100%. The accumulation process of lipid droplets was observed during the induction of differentiation from day 2 to 6 (Fig. 1A). Oil Red O staining showed that the lipid content of mature adipocytes was significantly higher than that of preadipocytes (Fig. 1B), as evidenced by the dramatically increased absorbance (Fig. 1C). In addition, the relative expression level of adipocyte-specific genes, e.g., peroxisome proliferative activated receptor gamma (PPARγ), CCAAT enhancer binding protein alpha (C/EBPα), Lipoprotein Lipase (LPL) and fatty acid-binding protein 4 (FABP4), also increased with adipocyte differentiation (Fig. 1D-G). These results indicated a successful induction of preadipocytes into mature adipocytes, which could be used for the subsequent study.
Bovine preadipocytes induced differentiation into mature adipocytes. A Lipid droplet accumulation during differentiation 0 d, 2 d, 4 d, 6 d (40 ×). B Oil Red O staining of bovine preadipocyte (above, 100 ×) and differentiated adipocyte (below, 100 ×). C OD value measured of lipid droplets in preadipocytes and Mature adipocytes. D-G detected the relative mRNA expression level of PPARγ, C/EBPα, FABP4 and LPL, respectively (compare with 0 day). ** indicated the difference is extremely significant (P < 0.01) and * indicated significant (P < 0.05)
SGK1 is highly expressed in bovine differentiated adipocytes
It has been reported that SGK1 mainly expressed in kidney to regulate multiple renal ion channels and in adipose tissue to affect adipogenesis in mice [29]. The expression pattern and function of SGK1 in cattle have not been reported. We examined the expression levels of SGK1 in heart, liver, lung, spleen, kidney, muscle and fat tissues, and expression patterns of preadipocytes during the induction and differentiation using Nanyang cattle. Results showed the expression of SGK1 was significantly higher in kidney and adipose tissue compared with heart (Fig. 2A). Moreover, the relative expression level of SGK1 peaked at day 6 during adipocyte differentiation (Fig. 2B). Thus, through analysis of the expression pattern of bovine SGK1 gene in different tissues and during adipocyte differentiation, we propose that SGK1 might have potential regulatory effect during adipogenesis in cattle.
Overexpression of SGK1 promotes bovine adipogenesis
Bovine preadipocytes were infected with OE-SGK1 and OE-NC for overexpression study. Adipocytes grew well, reaching ~ 90% confluence and GFP positive adipocyte covered the visual field after infected 48 h (Fig. 3A). In OE-SGK1, the mRNA and protein of SGK1 were both significantly higher than OE-NC (Fig. 3B, C). The lipid content of OE-SGK1 was significantly higher than that of OE-NC (Fig. 3D). The integrity of RNA for qPCR was proved by electrophoresis on agarose gel (Supplementary Fig. 1C). The expression of adipocyte-specific genes, e.g., PPARG and C/EBPA, increased in OE-SGK1 compared with OE-NC (Fig. 3E), whereas cell cycle-related genes decreased (Fig. 3F). Western blots showed no differences in PPARγ and C/EBPα, whereas CDK2 and CCND2 were down-regulated when SGK1 was over-expressed (Fig. 3G and H). Thus, overexpression of SGK1 might promote bovine adipogenesis mainly via inhibiting the cell cycle in preadipocytes. Moreover, we also used EDU assay to detect the effect of over-expression of SGK1 on cell cycle in adipocyte. From the result, we can find that SGK1 significantly inhibited the cell proliferation compared to negative control (Fig. 3I and J).
Overexpression of SGK1 promotes bovine adipogenesis. A cell growth status and expression in OE-SGK1 and OE-NC 48 h after infection. B In OE-SGK1 and OE-NC: mRNA levels of SGK1 48 h after infection. C protein expression of SGK1 in OE-SGK1 and OE-NC 48 h after infection. D oil red O staining 6 d after induced differentiation. E mRNA expression of adipocyte differentiation-related genes 6 d after induced differentiation. F mRNA expression of cell cycles related genes 6 d after induced differentiation. G expression of adipogenic-differentiation proteins (PPARγ and C/EBPα) and proliferation-related proteins CDK2 and CCND2 (the samples derive from the same experiment and those blots were processed in parallel) 6 d after induced differentiation. H protein quantitative. ** indicated the difference is extremely significant (P < 0.01), * indicated significant (P < 0.05) and ns indicated isn’t significant (P > 0.05). I EDU assay to detect the cell proliferation under over-expression of SGK1. J quantification of Fig. 3I. ** indicated the difference is extremely significant (P < 0.01)
Knockdown of SGK1 inhibits bovine adipogenesis
Bovine preadipocytes infected with sh-SGK1 and sh-NC were used for knockdown study. Adipocytes grew well and confluence reached ~ 90% after 72 h, with GFP covering the whole visual field (Fig. 4A). In sh-SGK1, reduction of mRNA (Fig. 4B), SGK1 protein expression (Fig. 4C) and lipid droplets (Fig. 4D) were observed, compared with sh-NC. RNA integrity was measured by electrophoresis on agarose gel (Supplementary Fig. 2E). Compared to sh-NC, it revealed that the expression of four adipocyte-specific genes (C/EBPα, C/EBPβ, SREBP-1c, and FABP4) reduced (Fig. 4E), and the expression of two cell cycle related genes (CCND2 and MCM6) increased in sh-SGK1 (Fig. 4F), which was opposite to the results observed under SGK1 overexpression. Furthermore, western blotting results revealed the protein of PPARγ and C/EBPα were significantly down-regulated, but there was no statistic difference in CDK2 and CCND2 (Fig. 4G and H). Hence, it was speculated that knockdown of SGK1 repressed bovine adipogenesis mainly through repressing adipocytes differentiation.
Knockdown of SGK1 inhibits bovine adipogenesis. A cell growth status and expression in sh-SGK1 and sh-NC 72 h after infection. B In sh-SGK1 and sh-NC: mRNA levels of SGK1 72 h after infection. C protein expression of SGK1 in sh-SGK1 and sh-NC 72 h after infection. D oil red O staining 6 d after induced differentiation. E mRNA expression of adipocyte differentiation-related genes 6 d after induced differentiation. F mRNA expression of cell cycle related genes 6 d after induced differentiation. G expression of adipogenic-differentiation proteins (PPARγ and C/EBPα) and proliferation-related proteins CDK2 and CCND2 (the samples derive from the same experiment and those blots were processed in parallel) 6 d after induced differentiation. H protein quantitative. ** indicated the difference is extremely significant (P < 0.01), * indicated significant (P < 0.05) and ns indicated isn’t significant (P > 0.05)
SGK1 affects the transcriptional activity of FOXO1 via up-regulating its phosphorylation level
Lots of evidences indicated that SGK1 could exert regulatory functions by influencing the expression and phosphorylation of FOXO family in human and mice [30]. Indeed, the expression of FOXO1 and FOXO3 were decreased after the overexpression of SGK1, and their expression was increased after SGK1 knockdown (Fig. 5A). Furthermore, protein expression and phosphorylation of FOXO1 and FOXO3 were detected by western blot after SGK1 was over-expressed. The results showed that overexpression of SGK1 significantly increased the phosphorylation of FOXO1 without affecting the total protein. For FOXO3, the total protein was significantly decreased without altering the phosphorylation level (Fig. 5B, C). Then we want to know whether the interaction between SGK1 and FOXO family is directly, so yeast two hybrid was used to detect this point. The result of SD/-Ade/-His/-Trp/-Leu/X-α-Gal/AbA (QDO/X/A) screening revealed that SGK1 couldn’t interact with FOXO1 or FOXO3 directly (Fig. 5D, E). Nextly, we wondered, as a transcription factor, whether the changes of phosphorylation level of FOXO1 altered its transcriptional activity, so we performed the luciferase assay to detect this point. We found that SGK1 significantly upregulated the transcriptional activity of FOXO1 comparing with control (Fig. 5F). Therefore, it could be inferred that SGK1 attenuates the transcription repression of FOXO1 via regulating its phosphorylation then promotes bovine adipogenesis.
SGK1 affects the transcriptional activity of FOXO1 via changing its phosphorylation level. A mRNA expression of FOXO1 and FOXO3 after overexpression and interference of SGK1 on 6th d of induced differentiation. B, C expression and phosphorylation of FOXO1 (pS256) and FOXO3 (pS322 + pS325/pS318 + pS321) in OE-SGK1 and OE-NC on 6th d of induced differentiation (the samples derive from the same experiment and those blots were processed in parallel). D, E Y2H between SGK1 and FOXO1/FOXO3. ** indicated the difference is extremely significant (P < 0.01), * indicated significant (P < 0.05) and ns indicated isn’t significant (P > 0.05). F transcriptional activity of FOXO1 promoter using luciferase assay. Luciferase activity was quantified in two groups via triplicate results. ** indicated the difference is extremely significant (P < 0.01)
SGK1 regulates adipogenesis through related to energy and lipid metabolism pathways
RNA-seq was used to further investigate the effect of SGK1 in the lipid metabolism of bovine adipocytes. Six up-regulated genes and four down-regulated genes were randomly selected to verify the reliability by qRT-PCR (primers shown in Supplementary Table 2). It showed that the expression trend of the ten randomly selected genes was consistent with RNA-seq results (Fig. 6A).
SGK1 regulates lipid metabolism in bovine adipocytes. A comparison of expression of DEGs by qRT-PCR and RNA-seq. B heatmap depicting expression of DEGs (red and blue indicate up-regulation and down-regulation, respectively). C volcano plots of the DEGs; D KEGG signaling pathway analysis of the DEGs. E visual analysis of signaling pathway and DEGs of interest, where red and green indicate up-regulated and down-regulated genes, respectively. Node size indicates fold changes and larger circles means larger fold change. F GO chord graph terms for DEGs of interests
Subsequently, the sequencing data was shown in Supplementary Table 3. A total of 1 704 differentially expressed genes (DEGs) were detected. The differential expression heatmap showed that it clustered well between the SGK1 overexpression and control groups (Fig. 6B). Among all the DEGs, 1 126 of which were up-regulated and 578 were down-regulated (Fig. 6C). KEGG enrichment analysis revealed that DEGs were significantly enriched in PI3K/Akt, MAPK and lipid acid metabolism signaling pathways, which played an important role in adipocyte development and lipid metabolism (Fig. 6D). As the most significant enrichment signaling pathway, the genes enriched in PI3K/Akt pathway was shown (Fig. 6E). The genes in the GO terms related to metabolism and development, positive regulation of MAPK cascade, triglyceride biosynthetic process and cellular response to cAMP were displayed in Fig. 6F. It showed that SGK1 was involved in the regulation of cell proliferation and growth, which was consistent with the above results (Fig. 3F-G; Fig. 4F-G). Thus, SGK1 might regulate bovine adipogenesis by affecting some signaling pathways related to energy and lipid metabolism.
SGK1 may be a key epigenetic regulatory factor for bovine adipogenesis
Based on the above results, we uncovered the critical role of SGK1 in regulating bovine adipogenesis. SGK1, as a kinase, phosphorylated FOXO1 in the site S256, which significantly attenuated the transcriptional repression role FOXO1 for PPARγ. So we drew a regulatory diagram of SGK1 regulating the lipid accumulation shown in Fig. 7.
Discussion
SGK1 is a ubiquitously expressed protein kinase with significant function
Serum and glucocorticoid-induced protein kinase 1 (SGK1) is a protein kinase belonged to the ‘AGC’ subfamily, which contains protein kinases A, G and C. Particularly, SGK1 shows about 50% identity in its catalytic domain with protein kinase B (PKB, also called Akt), indicating the similarity in their functions [31]. SGK1 is widely expressed, and its function cover a variety of physiological aspects (e.g., cell proliferation and apoptosis, transport, and hormone release) and a multitude of pathophysiological conditions (e.g., fibrosing disease, tumour growth, and the sequelae of ischaemia) [32, 33]. We found that it was also ubiquitously expressed in bovine heart, liver, lung, spleen, kidney, muscle and fat tissues, especially a higher expression in kidney and fat tissues (Fig. 2A). Interestingly, extensive research has shown that SGK1 was closely related to the development and progression of obesity, metabolic syndrome, diabetes and hypertension [15, 34]. Meanwhile, increased expression of SGK1 has been observed in bovine differentiated adipocytes (Fig. 2B). Particularly, SGK1 overexpression displayed accelerated adipogenesis, and its knockdown decreased the adipogenesis, supporting its role in bovine adipocytes. However, the underlying mechanism is not clearly understood.
SGK1 regulates adipocyte differentiation and proliferation in cattle
Exploring the genes and mechanism of adipogenesis is beneficial for understanding adipocyte differentiation and lipid deposition. It has been reported that transgene of SGK1 increased the lipid droplets formation in 3T3-L1 cells compared with wild type cells [34]. Cell proliferation and differentiation are important processes in development. PCNA, CCND2 and MCM6, have proved to be marker genes regulating cell proliferation [35, 36]. As a key regulator of adipogenesis, PPARγ controlled the transcription of numerous genes related to adipocyte differentiation and lipid accumulation [37, 38], and a high level of the C/EBPα maintained adipocytes in a fully differentiated state [39, 40]. To study the function of SGK1 during adipogenesis, overexpression and knockdown were conducted. Results showed that overexpression of SGK1 produced more lipid droplets than the control. Meanwhile, it also led to the increased expression of adipocyte differentiation-related genes, e.g., PPARγ and C/EBPα, and decreased expression of proliferation-related genes e.g., PCNA, CCND2 and MCM6. Consistently, knockdown of SGK1 produced the opposite results.
Further study indicated that overexpression of SGK1 did not affect the protein levels of PPARγ and C/EBPα, but decreased that of proliferation-related proteins (CCDN2 and CDK2). This suggested that it might positively regulates bovine adipogenesis mainly via inhibition of adipocyte proliferation. The excessive SGK1 expression might contribute to the development of obesity, diabetes and metabolic syndrome in vitro [15]. In vivo, SGK1 inhibitor led to the significant decrease in insulin resistance in db/db mouse, and the inhibition of insulin signaling by dexamethasone and oleic acid was reversed by lv-shSGK1. SGK1 could promote fat deposition by regulating the phosphorylation and transcription level of FOXO1 to increase the expression of PPARγ in mouse [17, 41]. In cattle, it was found that knockdown of SGK1 decreased the proteins related to differentiation (PPARγ and C/EBPα), and didn’t affect the proteins related to proliferation (CCDN2 and CDK2). Therefore, SGK1 promotes bovine adipogenesis through facilitating preadipocytes differentiation and repressing proliferation.
SGK1 affects the phosphorylation and expression of FOXO1 and FOXO3
SGK1 affected multiple physiologic processes via phosphorylation of downstream proteins [42,43,44]. SGK1 overexpression improved the hypercholesterolemic induced by diet or genetically obese due to disorder of lipid metabolism in mice [45]. Previous studies also showed that it regulated adipocyte proliferation and differentiation by altering the phosphorylation level of FOXO1 and FOXO3, respectively [46, 47]. Overexpression of FOXO1 inhibited bovine adipogenesis and induced apoptosis [48]. FOXO1 could act on the promoter of PPARγ to inhibit its transcription and expression, resulting in the suppression of adipogenesis [49]; [23, 50]. In addition, there existed interaction between SGK1 and FOXO1 and FOXO3 by prediction in cattle.
In our study, mRNA expression level of FOXO1 and FOXO3 were down-regulated after SGK1 overexpression, and its inhibition led to the opposite results. Meanwhile, overexpression of SGK1 elevated the phosphorylation of FOXO1, but didn’t affect the phosphorylation of FOXO3 and the protein expression of FOXO1. FOXO1 is located in the nucleus where it might control the cell cycle [51]. Phosphorylation of FOXO1 by SGK1 might lead to the loss of its transcriptional activity by nuclear exclusion [52]. However, shRNA was synthesized in the cell’s nucleus and transported to the cytoplasm [53]. This explained the poor interference efficiency of sh-SGK1, which have resulted in unchanged phosphorylation level of FOXO1. Moreover, SGK1 overexpression significantly inhibited the protein expression of FOXO3, and SGK1 knockdown promoted its protein expression. Although the H2Y results showed that SGK1 couldn’t directly interact on FOXO1 (Fig. 5F) and FOXO3 (Fig. 5G), SGK1 significantly up-regulated the phosphorylation of FOXO1 then attenuated the transcriptional repression role of FOXO1 for PPARγ. Phosphorylation of FOXO1 transferred in cytoplasm which is also the reason reduced the transcriptional repression of FOXO1 [53].
SGK1 functions by regulating genes in energy and lipid metabolism signaling pathway
1 704 DEGs were found when SGK1 was over-expressed. The DEGs were related to metabolic process, development process and biological process activation, and significantly enriched in the PI3K/Akt signaling pathway, MAPK signaling pathway and lipic acid metabolism signaling pathway.
The MAPK/PI3K/Akt signaling pathway is important in regulating lipid metabolism, energy homeostasis and cell proliferation [54,55,56]. The expression of numerous gene has been affected in PI3K/Akt signaling pathway after the overexpression of SGK1. For instance, FGF23, a key factor in the MAPK and Akt signaling pathway, was up-regulated. It has been proved to be a key endocrine factor involved in the regulation of systemic homeostasis and lipid metabolism [57]. The FOXO signaling pathway is essential in cellular energy production, oxidative stress resistance, and cell viability and proliferation [58]. FOXO family proteins are located downstream of this signaling pathway and regulated by phosphorylated Akt [21]. SGK1 down-regulated PCK1, which located in the FOXO and PI3K/Akt signaling pathway. Tissue-specific knockout of PCK1 in mice produced a phenotype of obesity, lipodystrophy, fatty liver, and death [59]. The DEG, colony-stimulating factor 3 (CSF-3), was enriched in the PI3K/Akt signaling pathway. Previous study has revealed its function in adipose tissue, whole-body insulin sensitivity and glucose tolerance in human [60]. Furthermore, SGK1 also affected the expression of genes enriched in the lipid metabolism signaling pathway. In conclusion, SGK1 might regulate the expression of PCK1 in the PI3K/Akt signaling pathway and up-regulates FGF23, FGFR3, promoting mitosis, cell growth and adipogenesis in cattle.
In addition, the SGK1/Akt signaling pathway affected adipogenesis in mouse white adipose tissue via phosphorylation of FOXO1 [41, 61]. The interaction between SGK1 and FOXO3 has been described in several disease contexts [62, 63]. Our study showed that SGK1 could also affect the enrichment related to energy and lipid metabolism signaling pathways, such as PI3K/Akt signaling pathways, MAPK signaling pathways and lipoic acid metabolism pathway.
Conclusion
Overall, SGK1 was a positive regulatory factor during bovine adipogenesis. It affected the transcriptional repression role of FOXO1 via the attenuates its phosphorylation level, eventually promoted the fat deposition in bovine adipocytes. Our study provides clues for the epigenetic regulatory role of SGK1 during bovine adipogenesis, which lay molecular foundation for the improvement of beef quality.
Availability of data and materials
All data are available within the article or Supplementary Information. The RNA-seq data reported in this paper are available in NCBI and BioProject ID: PRJNA78643. Source data are provided with this paper.
References
Xiong Y, Wang Y, Xu Q, Li A, Yue Y, Ma Y, Lin Y. LKB1 regulates goat intramuscular adipogenesis through focal adhesion pathway. Front Physiol. 2021;12:755598–766611.
Gotoh T, Nishimura T, Kuchida K, Mannen H. The Japanese Wagyu beef industry: current situation and future prospects - a review. Asian-Australas J Anim Sci. 2018;31:933–50.
Schoettl T, Fischer IP, Ussar S. Heterogeneity of adipose tissue in development and metabolic function. J Exp Biol. 2018;7:221–38.
Kaisanlahti A, Glumoff T. Browning of white fat: agents and implications for beige adipose tissue to type 2 diabetes. J Physiol Biochem. 2019;75:1–10.
Yang W, Yang C, Luo J, Wei Y, Wang W, Zhong Y. Adiponectin promotes preadipocyte differentiation via the PPARgamma pathway. Mol Med Rep. 2018;17:428–35.
Lei Z, Wu H, Xiong Y, Wei D, Wang X, Luoreng Z, Cai X, Ma Y. ncRNAs regulate bovine adipose tissue deposition. Mol Cell Biochem. 2021;476:2837–45.
Birsoy K, Chen Z, Friedman J. Transcriptional regulation of adipogenesis by KLF4. Cell Metab. 2008;7:339–47.
Hua Y, Yue Y, Zhao D, Ma Y, Xiong Y, Xiong X, Li J. Ablation of KDM2A inhibits preadipocyte proliferation and promotes adipogenic differentiation. Int J Mol Sci. 2021;22:9759.
Arencibia JM, Pastor-Flores D, Bauer AF, Schulze JO, Biondi RM. AGC protein kinases: From structural mechanism of regulation to allosteric drug development for the treatment of human diseases. Biochim Biophys Acta Proteins Proteom. 2013;1834:1302–21.
Alessi DR, Pearce LR, Komander D. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22.
Zuleger T, Heinzelbecker J, Takacs Z, Hunter C, Voelkl J, Lang F, Proikas-Cezanne T, Francisco JR, Romero FJ. SGK1 inhibits autophagy in murine muscle tissue. Oxid Med Cell Longev. 2018;2018:4043712–26.
Lin MG, Hurley JH. Structure and function of the ULK1 complex in autophagy. Curr Opin Cell Biol. 2016;39:61–8.
Satoh N, Nakamura M, Suzuki M, Suzuki A, Seki G, Horita S, Ghosh Choudhury G. Roles of Akt and SGK1 in the regulation of renal tubular transport. Biomed Res Int. 2015;2015:971697–8.
Medina Jover F, Gendrau Sanclemente N, Viñals F. SGK1 is a signalling hub that controls protein synthesis and proliferation in endothelial cells. Febs Lett. 2020;594:3200–15.
Li P, Pan F, Hao Y, Feng W, Song H, Zhu D. SGK1 is regulated by metabolic-related factors in 3T3-L1 adipocytes and overexpressed in the adipose tissue of subjects with obesity and diabetes. Diabetes Res Clin Pr. 2013;102:35–42.
Schernthaner-Reiter MH, Kiefer F, Zeyda M, Stulnig TM, Luger A, Vila G. Strong association of serum- and glucocorticoid-regulated kinase 1 with peripheral and adipose tissue inflammation in obesity. Int J Obes. 2005;2015(39):1143–50.
Zhang M, Chen H, Liu MS, Zhu KY, Hao Y, Zhu DL, Li P. Serum- and glucocorticoid-inducible kinase 1 promotes insulin resistance in adipocytes via degradation of insulin receptor substrate 1. Diabetes Metab Res Rev. 2021;37:e3451–e3459.
Lowe CE, O’Rahilly S, Rochford JJ. Adipogenesis at a glance. J Cell Sci. 2011;124:2681–6.
Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806.
Burgering BM, Medema RH. isions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003;73:689–701.
Essafi A, Gomes AR, Pomeranz KM, Zwolinska AK, Varshochi R, McGovern UB, Lam EW. Studying the subcellular localization and DNA-binding activity of FoxO transcription factors, downstream effectors of PI3K/Akt. Methods Mol Biol. 2009;462:201–11.
Lee S, Dong HH. FoxO integration of insulin signaling with glucose and lipid metabolism. J Endocrinol. 2017;233:R67–79.
Fan W, Imamura T, Sonoda N, Sears DD, Patsouris D, Kim JJ, Olefsky JM. FOXO1 transrepresses peroxisome proliferator-activated receptor γ transactivation, coordinating an insulin-induced feed-forward response in adipocytes. J Biol Chem. 2009;284:12188–97.
Liu L, Zheng LD, Zou P, Brooke J, Smith C, Long YC, Almeida FA, Liu D, Cheng Z. FoxO1 antagonist suppresses autophagy and lipid droplet growth in adipocytes. Cell Cycle (Georgetown, Tex). 2016;15:2033–41.
Zhang X, Liu Q, Zhang X, Guo K, Zhang X, Zhou Z. FOXO3a regulates lipid accumulation and adipocyte inflammation in adipocytes through autophagy : Role of FOXO3a in obesity. Inflamm Res. 2021;70:591–603.
Zhang J, Zhang F, Hong C, Giuliano AE, Cui X, Zhou G, Zhang G, Cui Y. Critical protein GAPDH and its regulatory mechanisms in cancer cells. Cancer Biol Med. 2015;12:10–22.
Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, Stroedicke M, Zenkner M, Schoenherr A, Koeppen S, et al. A human protein-protein interaction network: a resource for annotating the proteome. Cell. 2005;122:957–68.
Klockmeier K, Silva RE, Rasko T, Marti PA, Wanker EE. Schizophrenia risk candidate protein ZNF804A interacts with STAT2 and influences interferon-mediated gene transcription in mammalian cells. J Mol Biol. 2021;17:167184–201.
Valinsky WC, Touyz RM, Shrier A. Aldosterone, SGK1, and ion channels in the kidney. Clin Sci. 1979;2018(132):173–83.
Tao GZ, Lehwald N, Jang KY, Baek J, Xu B, Omary MB, Sylvester KG. Wnt/β-catenin signaling protects mouse liver against oxidative stress-induced apoptosis through the inhibition of forkhead transcription factor FoxO3. J Biol Chem. 2013;288(24):17214–24.
Kobayashi T, Deak M, Morrice N, Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J. 1999;344(Pt 1):189–97.
Anacker C, Cattaneo A, Musaelyan K, Zunszain PA, Horowitz M, Molteni R, Luoni A, Calabrese F, Tansey K, Gennarelli M, et al. Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2013;110:8708–13.
Sang Y, Kong P, Zhang S, Zhang L, Cao Y, Duan X, Sun T, Tao Z, Liu W. SGK1 in human cancer: emerging roles and mechanisms. Front Oncol. 2021;10:608722–39.
Sierra-Ramos C, Velazquez-Garcia S, Vastola-Mascolo A, Hernandez G, Faresse N, Alvarez DLRD. SGK1 activation exacerbates diet-induced obesity, metabolic syndrome and hypertension. J Endocrinol. 2020;244:149–62.
Baddela VS, Sharma A, Michaelis M, Vanselow J. HIF1 driven transcriptional activity regulates steroidogenesis and proliferation of bovine granulosa cells. Sci Rep. 2020;10:3906–18.
Wei Y, Cui YF, Tong HL, Zhang WW, Yan YQ. MicroRNA-2400 promotes bovine preadipocyte proliferation. Biochem Biophys Res Commun. 2016;478:1054–9.
Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–95.
Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–7.
Lin F, Macdougald OA, Diehl AM, Lane MD. A 30-kDa alternative translation product of the CCAAT/Enhancer binding protein α message: transcriptional activator lacking antimitotic activity. Proc Natl Acad Sci - PNAS. 1993;90:9606–10.
Legraverend C, Antonson P, Flodby P, Xanthopoulos KG. High level activity of the mouse CCAAT/enhancer binding protein (C/EBP alpha) gene promoter involves autoregulation and several ubiquitous transcription factors. Nucleic Acids Res. 1993;21:1735–42.
Ding L, Zhang L, Biswas S, Schugar RC, Brown JM, Byzova T, Podrez E. Akt3 inhibits adipogenesis and protects from diet-induced obesity via WNK1/SGK1 signaling. JCI Insight. 2017;2:e95687–e95702.
Bernard M, Yang B, Migneault F, Turgeon J, Dieude M, Olivier MA, Cardin GB, El-Diwany M, Underwood K, Rodier F, Hebert MJ. Autophagy drives fibroblast senescence through MTORC2 regulation. Autophagy. 2020;16:2004–16.
Voelkl J, Castor T, Musculus K, Viereck R, Mia S, Feger M, Alesutan I, Lang F. SGK1-sensitive regulation of cyclin-dependent kinase inhibitor 1B (p27) in cardiomyocyte hypertrophy. Cell Physiol Biochem. 2015;37:603–14.
Yang C, Li J, Sun F, Zhou H, Yang J, Yang C. The functional duality of SGK1 in the regulation of hyperglycemia. Endocr Connect. 2020;9:R187–94.
Tao R, Xiong X, DePinho RA, Deng CX, Dong XC. Hepatic SREBP-2 and cholesterol biosynthesis are regulated by FoxO3 and Sirt6. J Lipid Res. 2013;54:2745–53.
Mori S, Nada S, Kimura H, Tajima S, Takahashi Y, Kitamura A, Oneyama C, Okada M. The mTOR pathway controls cell proliferation by regulating the FoxO3a transcription factor via SGK1 kinase. PLoS One. 2014;9:e88891–e88903.
Liu W, Wang X, Liu Z, Wang Y, Yin B, Yu P, Duan X, Liao Z, Chen Y, Liu C, et al. SGK1 inhibition induces autophagy-dependent apoptosis via the mTOR-Foxo3a pathway. Br J Cancer. 2017;117:1139–53.
Liu X, Zhao H, Jin Q, You W, Cheng H, Liu Y, Song E, Liu G, Tan X, Zhang X, Wan F. Resveratrol induces apoptosis and inhibits adipogenesis by stimulating the SIRT1-AMPKα-FOXO1 signalling pathway in bovine intramuscular adipocytes. Mol Cell Biochem. 2018;439:213–23.
Ioannilli L, Ciccarone F, Ciriolo MR. Adipose tissue and FoxO1: bridging physiology and mechanisms. Cells-Basel. 2020;9:849–63.
Armoni M, Harel C, Karni S, Chen H, Bar-Yoseph F, Ver MR, Quon MJ, Karnieli E. FOXO1 represses peroxisome proliferator-activated receptor-gamma1 and -gamma2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivity. J Biol Chem. 2006;281:19881–91.
Chen J, Lu Y, Tian M, Huang Q. Molecular mechanisms of FOXO1 in adipocyte differentiation. J Mol Endocrinol. 2019;62:R239–53.
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–68.
Cullen BR. RNAi the natural way. Nat Genet. 2005;37:1163–5.
Sun H, Liu X, Long SR, Teng W, Ge H, Wang Y, Yu S, Xue Y, Zhang Y, Li X, Li W. Antidiabetic effects of pterostilbene through PI3K/Akt signal pathway in high fat diet and STZ-induced diabetic rats. Eur J Pharmacol. 2019;859.
Xiao J, Bai X, Liao L, Zhou M, Peng J, Xiang Q, Ren Z, Wen H, Jiang Z, Tang Z, et al. Hydrogen sulfide inhibits PCSK9 expression through the PI3K/Akt-SREBP-2 signaling pathway to influence lipid metabolism in HepG2 cells. Int J Mol Med. 2019;43:2055–63.
Cui X, Qian D, Jiang S, Shang E, Zhu Z, Duan J. Scutellariae radix and coptidis rhizoma improve glucose and lipid metabolism in T2DM rats via regulation of the metabolic profiling and MAPK/PI3K/Akt signaling pathway. Int J Mol Sci. 2018;19:3634.
Degirolamo C, Sabbà C, Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discovery. 2016;15:51–69.
Link W: Introduction to FOXO Biology. In|,. New York, NY: Springer, New York. 1890;2018:1–9.
Beale EG, Harvey BJ, Forest C. PCK1 and PCK2 as candidate diabetes and obesity genes. Cell Biochem Biophys. 2007;48:89–95.
Ordelheide AM, Gommer N, Bohm A, Hermann C, Thielker I, Machicao F, Fritsche A, Stefan N, Haring HU, Staiger H. Granulocyte colony-stimulating factor (G-CSF): a saturated fatty acid-induced myokine with insulin-desensitizing properties in humans. Mol Metab. 2016;5:305–16.
Di Pietro N, Panel V, Hayes S, Bagattin A, Meruvu S, Pandolfi A, Hugendubler L, Fejes-Tóth G, Naray-Fejes-Tóth A, Mueller E. Serum- and glucocorticoid-inducible kinase 1 (SGK1) regulates adipocyte differentiation via forkhead box O1. Mol Endocrinol (Baltimore, Md). 2010;24:370–90.
Xie Y, Jiang D, Xiao J, Fu C, Zhang Z, Ye Z, Zhang X. Ischemic preconditioning attenuates ischemia/reperfusion-induced kidney injury by activating autophagy via the SGK1 signaling pathway. Cell Death Dis. 2018;9:314–38.
Han X, Sun Z. Epigenetic Regulation of KL (Klotho) via H3K27me3 (Histone 3 Lysine 27 Trimethylation) in Renal Tubule Cells. Hypertension. 2020;75:1233–41.
Funding
This work was funded by the National Natural Science Foundation of China (U22A2050632072720, 32072720), the Key R & D projects in Ningxia Hui Autonomous Region (2023BCF01006, 2021BEF01002), Leading Talents Fund in Science and Technology Innovation in Ningxia Hui Autonomous Region (2020GKLRLX02).
Author information
Authors and Affiliations
Contributions
Z.L.: Conceived and designed the research, writing-original draft preparation, Software, and data analysis. Y.M.: Conceived and designed the research, supervision, and modified manuscript. D.W.: Methodology, modified manuscript. C.P. and F.L.: modified manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lei, Z., Pan, C., Li, F. et al. SGK1 promotes the lipid accumulation via regulating the transcriptional activity of FOXO1 in bovine. BMC Genomics 25, 737 (2024). https://doi.org/10.1186/s12864-024-10644-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-024-10644-0