Abstract
Background
Hox gene family is an important transcription factor that regulates cell process, and plays a role in the process of adipocytes differentiation and fat deposition. Previous transcriptome sequencing studies have indicated that the Homeobox A9 gene (HOXA9) is a candidate gene for regulating the process of bovine lipid metabolism, but the function and specific mechanism of action remain unclear. Therefore, this study aims to explore the role of HOXA9 in the proliferation, differentiation and apoptosis of bovine preadipocytes through gain-of-function and lose-of-function.
Result
It found HOXA9 highly expressed in bovine adipose tissue, and its expression level changed significantly during adipocytes differentiation process. It gave a hint that HOXA9 may be involved in the process of bovine lipid metabolism. The results of HOXA9 gain-of-function experiments indicated that HOXA9 appeared to act as a negative regulator not only in the differentiation but also in the proliferation of bovine preadipocytes, which is mainly reflected that overexpression of HOXA9 down-regulate the mRNA and protein expression level of PPARγ, CEBPα and FABP4 (P < 0.05). The mRNA expression level of CDK1, CDK2, PCNA, CCNA2, CCNB1, CCND1 and CCNE2, as well as the protein expression of CDK2 also significantly decreased. The decrease of lipid droplets content was the main characteristic of the phenotype (P < 0.01), which further supported the evidence that HOXA9 was a negative regulator of preadipocytes differentiation. The decrease of cell proliferation rate and EdU positive rate, as well as the limitation of transition of preadipocytes from G0/G1 phase to S phase also provided evidence for the inhibition of proliferation. Apart from this above, we noted an interesting phenomenon that overexpression of HOXA9 showed in a significant upregulation of both mRNA and protein level of apoptosis markers, accompanied by a significant increase in cell apoptosis rate. These data led us not to refute the fact that HOXA9 played an active regulatory role in apoptosis. HOXA9 loss-of-function experiments, however, yielded the opposite results. Considering that HOXA9 acts as a transcription factor, we predicted its target genes. Dual luciferase reporter assay system indicated that overexpression of HOXA9 inhibits activity of PCNA promoter.
Conclusion
Taken together, we demonstrated for the first time that HOXA9 played a role as a negative regulatory factor in the differentiation and proliferation of preadipocytes, but played a positive regulatory role in apoptosis, and it may play a regulatory role by targeting PCNA. This study provides basic data for further exploring the regulatory network of intramuscular fat deposition in bovine.
Similar content being viewed by others
Introduction
Intramuscular fat (IMF), or marbling, one of the indexes of meat quality, is essential to improve the flavor, juiciness, palatability and color of beef. Exploring the mechanism of IMF deposition is the premise of studying fat deposition [1]. There are two primary mechanisms for the expansion of adipose tissue, one is increasing the number of adipocytes (proliferation), the other is increasing the volume of adipocytes (differentiation) [2]. Fat formation includes two stages. The first stage is the differentiation of embryonic stem cells into mesenchymal stem cells with multiple differentiation potentials and the second stage is the terminal differentiation stage [3]. And these are regulated by the sequential activation of various transcription factors [3]. Peroxisome-proliferator-activated receptor γ (PPARγ), one of the core regulators factors of adipogenesis [4, 5], cooperates with transcription factors such as CCAAT-enhancer binding protein α (C/EBPα) and CCAAT-enhancer binding protein β (C/EBPβ) to induce the expression of Lipoprotein Lipase (LPL), Fatty acid binding protein 4 (FABP4), Perilipin 1 (PLIN1) and other downstream transcription factors, thus to regulate the differentiation of adipocytes [6]. Hox gene family is a highly conserved subgroup of homeobox superfamily, includes 39 genes, which are divided into four gene clusters: Hoxa, Hoxb, Hoxc and Hoxd. There are highly conserved gene sequences and similar gene functions among different species and genera [7]. Hox gene family not only participates in the browning of white adipose tissue and the thermogenesis of brown adipose tissue [8, 9], but also regulates the development of adipose tissue in different parts of the body [10, 11], which is an important transcription factor family for regulating adipose production [12,13,14]. HOXA9, a member of homeobox gene of cluster A, is a basic leucine-zipper transcription factor located on the short arm of the chromosome 7, located at the 5’ end of the Hoxa family and consisting of three exons (IAB, ICD, and II). It was first discovered as a partial transcript in the fetal liver [15], which is well known for its key role in lineage commitment of hematopoietic cells [16]. It not only plays a role in regulatory of cell proliferation, differentiation, apoptosis, tissue and organ formation and individual growth and development [17,18,19], but also participates in the occurrence and poor prognosis of various malignant tumors in human beings [20,21,22]. Existing reports show that HOXA9 differentially expressed in female abdominal and gluteal subcutaneous adipose tissue, which regulated the deposition of fat in different parts of body [23]. Sadkowski et al. also showed that HOXA9 was a key candidate gene for regulating IMF development in pigs and cattle through joint analysis of ATAC-seq and RNA-seq [24, 25], but the specific function and mechanism are not clear. Gain-of-function and lose-of-function are the main way to verify gene function in the field of life science [26]. Overexpression and RNA interference are the most common and direct way to explore gene function [27]. The purpose of this study is to investigate the effects of HOXA9 on proliferation, differentiation and apoptosis of bovine preadipocytes through overexpression and RNA interference experiments, and provide basic data for further study on the regulatory network of IMF deposition.
Materials and methods
Animal and cell culture
The samples were three 7-day-old Guyuan yellow calves from Guyuan Fumin Agricultural Technology Development Co., Ltd. in Yuanzhou District, Guyuan City, Ningxia Hui Autonomous Region. Animals were killed in a painless way by electric shock and without anesthesia. After slaughter, the tissue samples of heart, liver, spleen, lung, kidney, muscle and back adipose were collected. These tissues were washed with sterile physiological saline, cut into small pieces, put into 1.5 mL centrifuge tubes and stored in liquid nitrogen. At the same time, adipose tissue was stored in phosphate buffer saline (PBS, HyClone, Logan, USA) with 1% penicillin and streptomycin (HyClone, Logan, USA) and brought back to the laboratory. Primary adipocytes were isolated using the tissue-block method. After fascia and blood vessels in the adipose tissue were removed with scissors and tweezers, the adipose tissue was cut into small pieces about 1 mm3 and placed in new sterile 90 mm petri dishes. The petri dishes were placed upside down at 37℃, in a 5% CO2 incubator 5 h later.
Vector construction, siRNA chemical synthesis and cell transfection
Construction of pcDNA3.1-HOXA9 overexpression plasmid and siRNA synthesis
According to the sequence of bovine HOXA9 (GenBank: NM_001105617.2) in NCBI database, CDS region of HOXA9 was amplified and inserted into pcDNA3.1 vector to construct overexpressed HOXA9 plasmid. Meanwhile, three interfering fragments targeting HOXA9 were designed and synthesized. Sequences are shown in Table 1. When the cell fusion degree of 6-well plates reached 60-80%, 2.5 µg plasmid DNA or 10 µL siRNA were transfected into each well. All transfection experiments were carried out according to the Lipofectamine™ 3000 Reagent USER GUIDE (Thermo Scientific, California, USA), but the cell culture medium was not changed after transfection.
Construction of dual-luciferase reporter plasmids and dual luciferase assay
The partial promoter of PPARγ (GenBank: NC_037349.1), CDK2 (GenBank: NC_037332.1), FABP4 (GenBank: NC_037341.1), and PCNA (GenBank: NC_037340.1) genes were cloned into pGL3-basic plasmid vector to construct dual luciferase reporter plasmids. When the cell fusion degree of 24-well plates reached 60-80%, 1 µg pcDNA3.1/pcDNA3.1-HOXA9, 0.8 µg dual luciferase reporter plasmids DNA, and 20 ng pRL-TK plasmids DNA were transfected into 293T cells. All transfection experiments were carried out according to the Lipofectamine™ 3000 Reagent USER GUIDE, but the cell culture medium was not changed after transfection. All dual luciferase experiments were performed according to the instructions Dual-Luciferase® Reporter Assay System.
Induced differentiation of preadipocytes and oil red O staining
Then the cell fusion degree of preadipocytes reached 90–100%, the growth medium (GM, DMEM + 10% fetal bovine serum) was replaced with induce medium (IM, GM containing 10 µg/mL of insulin, 1 µmol/L of dexamethasone, 0.5 mmol/L IBMX, and 1 µmol/L of rosiglitazone) to continue culture, after 2 days, IM was replaced with differentiation-maintaining medium (MM, GM containing 10 µg/mL of insulin and 1 µmol/L of rosiglitazone). MM was changed every 2 days and cells were collected after 8 days. For Oil Red O staining, mature adipocytes were washed with PBS and fixed with 4% formaldehyde solution for 30 min. Subsequently, cells dyed with Oil Red O working solution for 30 min, and decolored with 60% isopropanol. Then, hematoxylin was added to each well to counterstain nucleus, and cells were observed by a fluorescence microscope. Finally, 1 mL of 100% isopropanol was added to each well for quantitative analysis of lipid droplets, and the absorbance value was detected at 490 nm.
EdU and CCK8 assay
CCK8 kits (Meulunbio, Shanghai, China) and EdU kits (Beeyotime, Shanghai, China) were used to detected to cell proliferation. In CCK8 assay, preadipocytes were placed to 96-well petri dishes. After 0, 24, 48 and 72 h of transfection, 10 µL CCK8 solution was added to each well, and the absorbance at 450 nm was measured with a microplate reader (SYNERGY|LX, BioRad, Hercules, CA, USA) after cells were incubated in the dark for 1 h. For EdU staining, after transfection of plasmids and interfering fragments 48 h, cells were labeled with EdU solution and continued to culture for 6 h. Subsequently, cells were fixed with 4% formaldehyde and infiltrated with 0.3% Triton X-100 for 15 min. Then the click reaction mixture was added to each well and cells were incubated in the dark for 30 min. DAPI was added to dye the nucleus for 10 min, and finally cells were observed and imaged by a fluorescence microscope.
Flow Cytometry
The flow cell cycle
Cell cycle was detected by cell cycle kits (Beyptime, Shanghai, China). Preadipocytes were placed in a 6-well petri dish, after transfection for 48 h, cells were digested with trypsin. Cells were collected in 1.5 mL centrifuge tubes and added 1 mL precooled 70% ethanol. Kept cells stored refrigerator at 4 °C for 24 h. Then, the ethanol was removed and 1 mL PBS was added to wash cells. Subsequently, 0.5 mL propidium iodide staining solution was added to each tube of cells. Cells were resuspended and incubated in the dark at 37 °C for 30 min. Finally, cells were detected by flow cytometry (BD-C6 Plus, 2000).
Flow apoptosis
Apoptosis was detected by Annexin V-FITC cell apoptosis detection kits (Beyptime, Shanghai, China). Growth medium were collected into centrifuge tubes. Cells were digested with trypsin, 3 min later, the previously collected growth medium was added to the 6-well plates to stop digesting and cells were collected to centrifuge tubes. After centrifugation at 1000 rpm for 5 min, cells were resuspended with PBS buffer and stimulated in 50℃ water for 2–3 min. Centrifuge again, discarded the supernatant and resuspend cells. Then, 195 µL Annexin V-FITC was added into a tube, cells were resuspended gently and added 5 µL Annexin V-FITC. Finally, 10 µL propidium iodide staining solution was added into a tube. The apoptotic cells were detected by a flow cytometry (each treatment has three parallel replicates) after incubated 20 min in the dark at room temperature.
RNA extraction, cDNA synthesis and RT-qPCR
Total RNA was extracted from tissues and cells by TRIzol method. The operation was carried out according to the instructions of TRIzol kits (Vazyme, Nanjing, China). The concentration (ng/µL) and the value of OD260/280 of RNA were detected by a multifunctional full-wavelength enzyme-labeled instrument (SYNERGY|LX), and the quality of RNA was detected by 1% agarose gel electrophoresis. 100 ng RNA was reversely transcribed into cDNA according to the instructions of reverse transcription kits (TaKaRa, Kyoto, Japan). cDNA was used as a template (three parallel replicates were set for each biological sample), GAPDH as a standardized reference gene. RT-qPCR was performed according to the instructions of ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) (three parallel replicates for each treatment). Primer information is shown in Table 2.
Western blot
Cells protein were extracted by whole protein extraction kits (Epyzime, Shanghai, China). Cells were washed with PBS buffer, and 150 µL protein lysis (1% PMSF) was added to each well, which was collected in a 1.5 mL centrifuge tube after 5 min. Subsequently, cells were shaken with vortex for 30 s, kept them on ice 5 min. This operation was repeated 5 times. Later, cells were centrifuged at 12,000 r for 5 min. Then supernatant was placed in new centrifuge tubes. The BCA Protein Assay Kits (Epyzime, Shanghai, China) were used to determine protein concentration. 5× protein loading buffer (Epyzime, Shanghai, China) was added to the protein samples, boiled 10 min and stored them at -80℃. After prepared concentrated gel and separated gel (PAGE Gel Fast Preparation Kits, Epyzime, Shanghai, China), the protein samples subjected to electrophoresis, transfered to polyvinylidene fluoride membrane, sealed, primary antibody incubation and secondary antibody incubated. Finally, they were visualized by enhanced chemiluminescence (ECL) detection systems (Epyzime, Shanghai, China). The brands and dilution ratio of primary antibody and secondary antibody are showed in Table 3.
Data analysis
One-way analysis of variance (one-way ANOVA) using GraphPad Prism 9 software was carried out, and 2−∆∆Ct method was used to analyze the results of RT-qPCR. The data were expressed as mean ± standard error (SEM). Among them, * means P < 0.05, which means significant difference. ** means P < 0.01, which means the difference is extremely significant.
Results
Analysis of the expression pattern of HOXA9
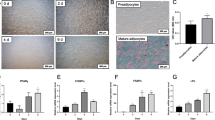
In order to clarify the role of HOXA9 in fat deposition, preadipocytes were isolated and induced to adipogenic differentiation. Oil Red O staining showed that the number and size of the lipid droplets increased significantly during adipocytes differentiation (Fig. 1-A), and the relative expression level of PPARγ and FABP4 significantly up-regulated (Fig. 1-B, C). Meanwhile, the expression level of HOXA9 was changed significantly during adipocytes differentiation (Fig. 1-D) and highly expressed in fat (Fig. 1-E). All the above date indicated that the adipocytes differentiation system was successfully established and HOXA9 may regulate the adipogenic differentiation of bovine preadipocytes.
Analysis of the expression pattern of HOXA9. (A) Oil Red O staining images of preadipocytes induced for 0 d and 8 d. (B-D) RT-qPCR analysis of the relative level of PPARγ, FABP4 and HOXA9 during preadipocytes differentiation. All date were compared with 0 d. (E) RT-qPCR analysis of the relative mRNA level of HOXA9 in different tissues of bovine. All data were compared with heart. GAPDH as a standardized reference gene. n = 3, * P < 0.05, ** P < 0.01
HOXA9 inhibits the differentiation of bovine adipocytes
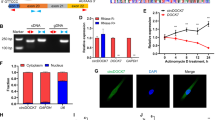
In order to explore the role of HOXA9 in adipocytes differentiation, pcDNA3.1-HOXA9 and pcDNA3.1 plasmids were transfected into preadipocytes to overexpress HOXA9. Results showed that the overexpression efficiency was more than 4000 times (Fig. 2-A, P < 0.01), achieving the high expression of HOXA9 in adipocytes. The high expression of HOXA9 in adipocytes not only decreased the mRNA expression level of PPARγ, CEBPα, CEBPβ, LPL and FABP4 (Fig. 2-B, P < 0.05), but also significantly decreased the protein expression level of FABP4, CEBPα and PPARγ (Fig. 2-C). Meanwhile, Oil Red O staining showed that the number and concentration of lipid droplets decreased significantly (Fig. 2-G, P < 0.01), of which were caused by overexpression of HOXA9. Furthermore, the interference experiments were carried out on the HOXA9. Three interference sequences (btaHOXA9-102, btaHOXA9-558 and btaHOXA9-727) targeting the HOXA9 were designed and transfected into preadipocytes to silence HOXA9 expression. RT-qPCR was used to measure the knockdown efficiency. It found that, compared to NC group, btaHOXA9-558 has the strongest knockdown efficiency more than 60% (Fig. 2-D, P < 0.01), so btaHOXA9-558 was selected for subsequent experiments. It showed that the mRNA expression level of adipogenic markers of PPARγ and CEBPβ (Fig. 2-E, P < 0.01) and the protein expression level of FABP4, CEBPα and PPARγ were significantly increased (Fig. 2-F) after transfection of btaHOXA9-558 and induced differentiation. Coincidently, Oil Red O staining also showed that the number and aggregation lipid droplets increased significantly (Fig. 2-G, P < 0.01).
HOXA9 inhibits the differentiation of bovine adipocytes.(A.D) Relative expression level of mRNA after overexpression/interference of HOXA9. (B.E) Relative expression level of mRNA of adipogenic markers after overexpression/interference of HOXA9. (C.F) Relative expression level of protein of adipogenic markers after overexpression/interference of HOXA9. (G) Oil Red O staining images (scale bar = 50 μm) of preadipocytes after overexpression/interference of HOXA9. GAPDH as a standardized reference gene. The data were expressed as mean ± SEM, n = 3, * P < 0.05, ** P < 0.01
HOXA9 inhibits the proliferation of bovine adipocytes
We have studied the role of HOXA9 in differentiation, so next to further validated the role of HOXA9 in regulating proliferation of bovine adipocytes. First of all, RT-qPCR showed that the relative mRNA expression level of the proliferation-related genes CDK1, CCNA2, PCNA and CCND1 were significantly decreased (Fig. 3-A, P < 0.05), and the protein expression level of CDK2 was also significantly decreased with transfection of pcDNA3.1-HOXA9 (Fig. 3-B, P < 0.05). Then, Cell proliferation was detected by CCK8 and EdU staining. The results showed that overexpression of HOXA9 significantly reduced the proliferation rate and EdU positive rate of preadipocytes (Fig. 3-C, G). Subsequently, we found that the cell cycle arrested in G0/G1 and S phases after overexpression of HOXA9, and the proportion of cells in G2 phase decreased significantly (Fig. 3-I, P < 0.05) through flow cytometry analysis. Meanwhile, the interference experiments showed opposite effect to the overexpression experiments. The relative expression level of mRNA and protein of proliferation markers were significantly increased (Fig. 3-D, E. P < 0.01), the cell viability was significantly higher than that of the control group at 24 and 48 h (Fig. 3-F. P < 0.01), and the positive rate of EdU was also significantly increased (Fig. 3-H) with transfection of btaHOXA9-558. Furthermore, results of flow cycle showed that the number of cells in G0/G1 phase decreased, and the proportion of cells in S phase and G2 phase increased after interference, which promoted cell proliferation (Fig. 3-J, P < 0.05).
HOXA9 inhibits the proliferation of bovine adipocytes. (A.D) Relative expression level of mRNA of proliferation markers after overexpression/interference of HOXA9. (B.E) Relative expression level of protein of proliferation markers after overexpression/interference of HOXA9. (C.F) The viability of cells was detected by CCK8 after overexpression/interference of HOXA9. (G.H) Cell proliferation rate was detected by EdU (scale bar = 500 μm) after overexpression/interference of HOXA9. (I.J) The cell cycle analysis after overexpression/interference of HOXA9. GAPDH as a standardized reference gene. The data were expressed as mean ± SEM, n = 3, * P < 0.05, ** P < 0.01
HOXA9 promotes the apoptosis of bovine preadipocytes
To further explore the role of HOXA9 on adipocytes apoptosis. RT-qPCR and WB results showed overexpression of HOXA9 up-regulate the relative mRNA expression level of apoptosis markers BAD and BAX and the expression level of BAX protein, and down-regulate the relative mRNA expression level of anti-apoptosis gene BCL2 (Fig. 4-A, B). But results were reversed with silence HOXA9 (Fig. 4-C, D). Meanwhile, Flow cytometry analysis showed that transfection of pcDNA3.1-HOXA9 plasmid significantly increased the apoptosis rate (Fig. 4-E), while the number of apoptosis cells decreased after interference (Fig. 4-F).
HOXA9 promotes the apoptosis of bovine preadipocytes. (A.C) Relative expression level of mRNA of apoptosis-related genes after overexpression/interference of HOXA9. (B.D) Relative expression level of protein of apoptosis-related genes after overexpression/interference of HOXA9. (E.F) The apoptosis was detected by flow cytometry after overexpression/interference of HOXA9. GAPDH as a standardized reference gene. The data were expressed as mean ± SEM, n = 3, * P < 0.05, ** P < 0.01
HOXA9 may target the PCNA promoter
HOXA9 inhibited the proliferation and differentiation of preadipocytes and promoted apoptosis by up-regulating and down-regulating multiple marker genes, we hypothesized that those may be potential targets of HOXA9. To verify this conjecture, the HOXA9 transcriptional binding motif (Fig. 5-E) and sites was first analyzed by bioinformatics (Fig. 5-A, B, C, D). Following pGL3-basic-PPARγ, pGL3-basic-CDK2, pGL3-basic-FABP4 and pGL3-basic-PCNA promoter dual luciferase reporter plasmids were transfected into 293T Cells, PCNA promoter had the most relative luciferase activity (Fig. 5-F). Further cotransfection of pcDNA3.1, pcDNA3.1-HOXA9 and pGL3-basic-PCNA showed that overexpression of HOXA9 significantly reduced relative luciferase activity (Fig. 5-G). It was indicated that HOXA9 may target the PCNA promoter to regulate adipocytes proliferation.
Discussion
Fat deposition is regulated by a complex signal transduction network formed by transcription factors, enzymes, hormones and signal pathways. Transcription factors control the transcription process by combining specific DNA sequences and play an important regulatory role in the development and deposition of fat [28]. HOXA9 is an important transcription factor, which is involved in the regulation of organ and morphogenesis, differentiation and adhesion, migration and cell cycle [29,30,31]. Previous studies showed that HOXA9 expression is significantly up-regulated in subcutaneous adipose tissues after bariatric surgery [34], which played an important role in regulating the function of adipose tissue. Sadkowski and others have reported that HOXA9 was a candidate gene for regulating intramuscular fat deposition of bovine [25]. In this study, HOXA9 was found to be abundant in adipose tissue, and the expression level of HOXA9 changed significantly during preadipocytes differentiation. It suggested that HOXA9 may play a key regulatory role in adipogenesis of bovine. Therefore, this study aimed to investigate the function of HOXA9 gene in ruminantia fat deposition.
As we all know, the differentiation of adipocytes involves cascaded regulation of multiple transcription factors. PPARγ is the core of regulation of adipocytes differentiation [32]. Ectopic expression of CEBPβ and CEBPδ bound to the PPARγ promoter to induce expression of PPARγ/RXR [33, 34], which in turn activated CEBPα, both of them form a positive feedback that initiated/maintained the differentiation of adipocytes and activated downstream genes [35, 36]. LPL and FABP4, as downstream genes of PPARγ, directly or indirectly affected by PPARγ to maintain lipogenesis [37, 38]. These genes were considered as markers of adipocytes differentiation. In this study, we found that HOXA9 was a negative transcription factor for fat deposition, which down-regulated not only the mRNA of adipogenic markers of PPARγ, CEBPα, CEBPβ, FABP4 and LPL, but also the protein expression of PPARγ, CEBPα and FABP4 at the molecular level. At the same time, overexpression of HOXA9 inhibited the number and aggregation of lipid droplets that inhibited adipocytes differentiation at the morphological level. Until now, there is no research about function and specific mechanism of HOXA9 for adipocytes differentiation in the existing literature, but some studies found that silence of HOXA9 promoted the differentiation of leukemia cells [18] and inhibited the differentiation of normal myeloid progenitor by inhibiting the activity of CEBPα gene + 8 kb enhancer [39]. Whether it has function in adipocytes remain to be explored.
The basis of lipogenesis is the increase of cell number and the accumulation of lipid droplets, so we further explored the effect of HOXA9 on proliferation and differentiation of preadipocytes. We revealed that HOXA9 negatively regulated the proliferation of adipocytes. PCNA, an auxiliary factor for the replication polymerases δ and ε (Pol δ and Pol ε), was a key factor in DNA replication and cell cycle regulation [40], existing mainly as a homotrimer whose expression increased in the late G1 to S phases of the cell cycle immediately before DNA synthesis [41]. HOXA9 bound to p21, cyclin D, and Gadd45 to regulate cell cycle progression [42, 43]. And it also bound to human DNA-(cytosine-5) methylchainase (MCMT) [44]. In this study, dual luciferase reporter assay system showed that overexpression of HOXA9 affected promoter activity of PCNA. We speculated that HOXA9 may target the PCNA promoter to regulate its expression and inhibit adipocytes proliferation. Meanwhile, HOXA9 was also an inhibitor of vascular smooth muscle cells (VSMC) proliferation and migration. After silencing, it reduced the expression of synthetic proteins (osteocalcin and PCNA) and enhanced the expression of contractile proteins (α-SMA and SM22α). Thus, it inhibited the proliferation of muscle cells mediated by ox-LDL [45]. However, HOXA9 regarded as the “switch” of cell proliferation in the process of myeloid leukemia, which can promote the expression of CDK6, CyclinD1 gene and telomerase RNA by triggering pleiotropic oncogenes Myc and Myb, and provide necessary cofactors to maintain the rapid proliferation of cells [46]. Moreover, the protein complex formed by HOXA9 and C/EBPα targeted Cdkn2a/b to overcome G1 phase blockage, promoted the proliferation of bone marrow cells and advanced the process of myeloid leukemia [47]. HOXA9 acted as a cancer promoter in head and neck squamous cell carcinoma (HNSCC) and laryngeal squamous cell carcinoma to promote the proliferation and migration of cancer cells [48, 49]. Therefore, HOXA9 played different regulation functions for proliferation between normal somatic cells and cancer cells. At the same time, this study also revealed that HOXA9 gene was a positive regulator of adipocytes apoptosis. Overexpression of HOXA9 significantly promoted the mRNA expression of BAD and BAX genes, reduced the expression of BCL2 gene, and increased the apoptosis rate. Moreover, previous studies showed that HOXA9 accelerated the apoptosis process of primary muscle satellite cells by affecting atrophic Signaling pathways [50] and negatively regulated downstream anti-apoptosis and autophagy-promoting genes (including BCL-XL, ULK1, ATG3 and ATG12) of NF-κB to promote the apoptosis of skin squamous cell carcinoma (cSCC) cells and inhibit autophagy [51]. This meant HOXA9 promoted cells apoptosis. Our results were consistent with the findings of these studies. In this study, we found that HOXA9 not only inhibited the accumulation of lipid droplets in phenotype, but also inhibited adipocytes proliferation at the molecular level, possibly by targeting PCNA. Meanwhile it promoted the process of adipocytes apoptosis, which was a negative regulator of fat deposition. However, the specific mechanisms remain to be further explored, especially the signaling pathway.
Conclusion
In a word, in this study, we confirmed that the HOXA9 has the ability to inhibit the proliferation and differentiation of adipocytes and promote apoptosis. It may play a regulatory role by targeting PCNA and it is a negative regulator of fat deposition for the first time. Therefore, HOXA9 gene may become a new key factor to regulate bovine fat deposition (the mechanism is shown in Fig. 6). This study expanded the key genes bank to explore the regulatory network of bovine fat deposition.
Data availability
The data presented in this study are available in the article.
References
Kruk ZA, Bottema MJ, Reyes-Veliz L, et al. Vitamin A and marbling attributes: intramuscular fat hyperplasia effects in cattle[J]. Meat Sci. 2018;137:139–46.
Haczeyni F, Bell-Anderson KS, Farrell GC. Causes and mechanisms of adipocyte enlargement and adipose expansion[J]. Obes Rev. 2018;19(3):406–20.
Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte[J]. Annu Rev Biochem. 2012;81:715–36.
Anghel SI, Wahli W. Fat poetry: a kingdom for PPAR gamma[J]. Cell Res. 2007;17(6):486–511.
Lee JE, Schmidt H, Lai B et al. Transcriptional and epigenomic regulation of Adipogenesis[J]. Mol Cell Biol. 2019, 39(11).
Rosen E, Eguchi J, Xu Z. Transcriptional targets in adipocyte biology[J]. Expert Opin Ther Targets. 2009;13(8):975–86.
Diez DCR, Storey KG. Opposing FGF and retinoid pathways: a signalling switch that controls differentiation and patterning onset in the extending vertebrate body axis[J]. BioEssays. 2004;26(8):857–69.
Ng Y, Tan SX, Chia SY, et al. HOXC10 suppresses browning of white adipose tissues[J]. Exp Mol Med. 2017;49(2):e292.
Tan H, Sim M, Tan SX, et al. HOXC10 suppresses Browning to maintain White Adipocyte Identity[J]. Diabetes. 2021;70(8):1654–63.
Yoneyama S, Guo Y, Lanktree MB, et al. Gene-centric meta-analyses for central adiposity traits in up to 57 412 individuals of European descent confirm known loci and reveal several novel associations[J]. Hum Mol Genet. 2014;23(9):2498–510.
Brune JE, Kern M, Kunath A, et al. Fat depot-specific expression of HOXC9 and HOXC10 may contribute to adverse fat distribution and related metabolic traits[J]. Obes (Silver Spring). 2016;24(1):51–9.
Cantile M, Procino A, D’Armiento M, et al. HOX gene network is involved in the transcriptional regulation of in vivo human adipogenesis[J]. J Cell Physiol. 2003;194(2):225–36.
Kumar V, Sekar M, Sarkar P, et al. Dynamics of HOX gene expression and regulation in adipocyte development[J]. Gene. 2021;768:145308.
Gesta S, Blüher M, Yamamoto Y, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution[J]. Proc Natl Acad Sci U S A. 2006;103(17):6676–81.
Popovic R, Erfurth F, Zeleznik-Le N. Transcriptional complexity of the HOXA9 locus[J]. Blood Cells Mol Dis. 2008;40(2):156–9.
Laslo P, Pongubala JM, Lancki DW, et al. Gene regulatory networks directing myeloid and lymphoid cell fates within the immune system[J]. Semin Immunol. 2008;20(4):228–35.
Luo B, Feng S, Li T et al. Transcription factor HOXB2 upregulates NUSAP1 to promote the proliferation, invasion and migration of nephroblastoma cells via the PI3K/Akt signaling pathway[J]. Mol Med Rep. 2022, 25(6).
Chen S, Yu J, Lv X, et al. HOXA9 is critical in the proliferation, differentiation, and malignancy of leukaemia cells both in vitro and in vivo[J]. Cell Biochem Funct. 2017;35(7):433–40.
Hu YL, Passegué E, Fong S, et al. Evidence that the Pim1 kinase gene is a direct target of HOXA9[J]. Blood. 2007;109(11):4732–8.
Aryal S, Zhang Y, Wren S, et al. Molecular regulators of HOXA9 in acute myeloid leukemia[J]. FEBS J. 2023;290(2):321–39.
Tang L, Peng L, Tan C, et al. Role of HOXA9 in solid tumors: mechanistic insights and therapeutic potential[J]. Cancer Cell Int. 2022;22(1):349.
Rusan M, Andersen RF, Jakobsen A, et al. Circulating HOXA9-methylated tumour DNA: a novel biomarker of response to poly (ADP-ribose) polymerase inhibition in BRCA-mutated epithelial ovarian cancer[J]. Eur J Cancer. 2020;125:121–9.
Karastergiou K, Fried SK, Xie H, et al. Distinct developmental signatures of human abdominal and gluteal subcutaneous adipose tissue depots[J]. J Clin Endocrinol Metab. 2013;98(1):362–71.
Xu Z, Wu J, Zhou J, et al. Integration of ATAC-seq and RNA-seq analysis identifies key genes affecting intramuscular fat content in pigs[J]. Front Nutr. 2022;9:1016956.
Sadkowski T, Ciecierska A, Majewska A, et al. Transcriptional background of beef marbling - novel genes implicated in intramuscular fat deposition[J]. Meat Sci. 2014;97(1):32–41.
Tai W, Gao X. Functional peptides for siRNA delivery[J]. Adv Drug Deliv Rev. 2017;110–111:157–68.
Prelich G. Gene overexpression: uses, mechanisms, and interpretation[J]. Genetics. 2012;190(3):841–54.
Lambert SA, Jolma A, Campitelli LF, et al. Hum Transcription Factors[J] Cell. 2018;172(4):650–65.
Gardiner DM, Blumberg B, Komine Y, et al. Regulation of HoxA expression in developing and regenerating axolotl limbs[J]. Development. 1995;121(6):1731–41.
Ko SY, Naora H. HOXA9 promotes homotypic and heterotypic cell interactions that facilitate ovarian cancer dissemination via its induction of P-cadherin[J]. Mol Cancer. 2014;13:170.
Sun M, Song CX, Huang H, et al. HMGA2/TET1/HOXA9 signaling pathway regulates breast cancer growth and metastasis[J]. Proc Natl Acad Sci U S A. 2013;110(24):9920–5.
Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation[J]. Physiol Rev. 1998;78(3):783–809.
Wu Z, Xie Y, Bucher NL, et al. Conditional ectopic expression of C/EBP beta in NIH-3T3 cells induces PPAR gamma and stimulates adipogenesis[J]. Genes Dev. 1995;9(19):2350–63.
Fajas L, Auboeuf D, Raspé E, et al. The organization, promoter analysis, and expression of the human PPARgamma gene[J]. J Biol Chem. 1997;272(30):18779–89.
Rosen ED, Sarraf P, Troy AE, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro[J]. Mol Cell. 1999;4(4):611–7.
Wu Z, Rosen ED, Brun R, et al. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity[J]. Mol Cell. 1999;3(2):151–8.
Gu H, Zhou Y, Yang J, et al. Targeted overexpression of PPARγ in skeletal muscle by random insertion and CRISPR/Cas9 transgenic pig cloning enhances oxidative fiber formation and intramuscular fat deposition[J]. FASEB J. 2021;35(2):e21308.
Prentice KJ, Saksi J, Hotamisligil GS. Adipokine FABP4 integrates energy stores and counterregulatory metabolic responses[J]. J Lipid Res. 2019;60(4):734–40.
Peng L, Guo H, Ma P, et al. HoxA9 binds and represses the cebpa + 8 kb enhancer[J]. PLoS ONE. 2019;14(5):e217604.
Slade D. Maneuvers on PCNA rings during DNA replication and Repair[J]. Genes (Basel). 2018, 9(8).
Takasaki Y, Deng JS, Tan EM. A nuclear antigen associated with cell proliferation and blast transformation[J]. J Exp Med. 1981;154(6):1899–909.
Xiong Y, Zhang H, Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA[J]. Cell. 1992;71(3):505–14.
Matsuoka S, Yamaguchi M, Matsukage A. D-type cyclin-binding regions of proliferating cell nuclear antigen[J]. J Biol Chem. 1994;269(15):11030–6.
Chuang LS, Ian HI, Koh TW, et al. Human DNA-(cytosine-5) methyltransferase-PCNA complex as a target for p21WAF1[J]. Science. 1997;277(5334):1996–2000.
Fu X, Fu P, Yang T, et al. Homeobox A9 is a novel mediator of vascular smooth muscle cell phenotypic switching and proliferation by regulating methyl-CpG binding protein 2[J]. Cell Signal. 2023;108:110695.
Zhong X, Prinz A, Steger J, et al. HoxA9 transforms murine myeloid cells by a feedback loop driving expression of key oncogenes and cell cycle control genes[J]. Blood Adv. 2018;2(22):3137–48.
Collins C, Wang J, Miao H, et al. C/EBPα is an essential collaborator in Hoxa9/Meis1-mediated leukemogenesis[J]. Proc Natl Acad Sci U S A. 2014;111(27):9899–904.
Sun Q, Zhang SY, Zhao JF, et al. HIF-1α or HOTTIP/CTCF promotes Head and Neck squamous cell Carcinoma Progression and Drug Resistance by Targeting HOXA9[J]. Mol Ther Nucleic Acids. 2020;20:164–75.
Sun X, Liu B, Ji W, et al. The role of HOXA9 in human laryngeal squamous cell carcinoma[J]. Oncol Res. 2013;20(10):467–72.
Lu X, Liang B, Li S, et al. Modulation of HOXA9 after skeletal muscle denervation and reinnervation[J]. Am J Physiol Cell Physiol. 2020;318(6):C1154–65.
Han S, Li X, Liang X et al. HOXA9 transcriptionally promotes apoptosis and represses autophagy by targeting NF-κB in cutaneous squamous cell Carcinoma[J]. Cells. 2019, 8(11).
Funding
This research was supported by the National Natural Science Foundation of China (U22A20506, 32072720),National Key R&D Program of China (2023ZD0404803-02), Key R&D projects in Ningxia Hui Autonomous Region (2021BEF01002, 2021NXZD1, 2023BCF01006), and the Autonomous Region Science and Technology Innovation Leading Talents Training Project (2020GKLRLX02).
Author information
Authors and Affiliations
Contributions
HL and YM: conceived and designed the research. HL: wrote the original manuscript. FX, HC, LS, SH, CB and YM: modified the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Animal experiments were conducted following the Regulations for the Administration of Affairs Concerning Experimental Animals, as well as the standards set by the Ningxia University of experimental animal management practices. This study and all experiments were approved by the Ethics Committee of Ningxia University (approval no: NXU-2022-063), and permission was obtained from Fumin Agricultural Technology Development. We sure that manuscript reporting adheres to the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, L., Feng, X., Hu, C. et al. HOXA9 gene inhibits proliferation and differentiation and promotes apoptosis of bovine preadipocytes. BMC Genomics 25, 358 (2024). https://doi.org/10.1186/s12864-024-10231-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-024-10231-3