Abstract
Background
Vitamin A and retinoic acid (RA, a metabolite of vitamin A), are inextricably involved to the development of skeletal muscle in animals. However, the mechanisms regulating skeletal muscle development by vitamin A remain poorly reported. The current study designed to investigate the underlying mechanism of vitamin A affecting myogenic differentiation of lamb myoblasts through transcriptome sequencing (RNA-Seq) and gene function validation experiments. It provides a theoretical basis for elucidating the regulation of vitamin A on skeletal muscle development as well as for improving the economic benefits of the mutton sheep industry.
Results
Newborn lambs were injected with 7,500 IU vitamin A, and longissimus dorsi (LD) muscle tissue was surgically sampled for RNA-Seq analysis and primary myoblasts isolation at 3 weeks of age. The results showed that a total of 14 down-regulated and 3 up-regulated genes, were identified between control and vitamin A groups. Among them, BHLHE40 expression was upregulated in vitamin A group lambs. Furthermore, BHLHE40 expression is significantly increased after initiation of differentiation in myoblasts, and RA addition during differentiation greatly promoted BHLHE40 mRNA expression. In vitro, RA inhibited myoblasts proliferation and promoted myoblasts myogenic differentiation through BHLHE40. Moreover, BHLHE40 was proved to inhibit the expression of the DNA binding inhibitor 3 (ID3), and meanwhile, ID3 could effectively promote myoblasts proliferation and inhibit myoblasts myogenic differentiation.
Conclusions
Taken together, our results suggested that vitamin A inhibited myoblasts proliferation and promoted myoblasts myogenic differentiation by inhibiting ID3 expression through BHLHE40.
Similar content being viewed by others
Introduction
Skeletal muscle consists of multinucleated muscle fibers formed by the fusion of myoblasts, and accounts for approximately 40% of total body weight. Skeletal muscle performs numerous biological functions, including voluntary movement, controlling body temperature and regulating metabolism [1, 2]. Meanwhile, skeletal muscle, as the main source of animal protein, seriously affects the meat yield of farm animals at harvest [3, 4]. During myogenesis, myoblasts derived from the somatic mesoderm undergo a highly coordinated process of myogenic differentiation, culminating in the formation of mature skeletal muscle [5] and this event requires the concerted action of numerous transcription factors and signaling pathways [6, 7]. Hence, elucidating the underlying mechanisms of myoblast differentiation is critical for understanding of skeletal muscle development.
Class E basic helix-loop-helix protein 40 (BHLHE40, Aliases: BHLHB2/STRA13/DEC1/Sharp2) is a transcriptional repressor expressed in different cells and is mainly involved in the control of circadian rhythms and cell differentiation. It has been shown that the expression level of BHLHE40 is related to cell growth arrest [8]. For myogenesis, the absence of BHLHE40 leads to muscle necrosis in mice [9]. ID3 is a member of the HLH superfamily and it lacks the basic DNA-binding structural domain and represses transcription by forming non-functional dimers that are unable to bind to DNA [10]. Thus, ID3 is widely recognized as a negative regulator of the differentiation process. Previous studies suggest that ID3 is negatively involved in myogenesis by inhibiting the activation of myogenic regulatory factors during myoblast differentiation [10,11,12].
Vitamin A is essential for growth and development in humans and animals. RA, the most important metabolite of vitamin A, performs the vast majority of vitamin A metabolic functions. After entering the nucleus, RA regulates the expression of target genes by binding to RA receptors [13]. Studies have shown that RA promotes myogenesis in zebrafish [14] and Angus cattle [15], while inhibit differentiation of chick embryo limb myoblasts [16]. Our previous publication indicated that intramuscular injection of vitamin A increased final body weight and loin eye area of lambs, and RA promoted sheep primary myoblasts early differentiation in vitro [17]. In the current study, we continue to investigate the mechanism by which vitamin A affect sheep skeletal muscle growth.
Materials and methods
Animal treatment and sample collection
A total of 80 purebred healthy Hu sheep were randomly selected, and all ewes were on their third pregnancy. To avoid the influence of sire, we used semen from one Dorper ram for artificial insemination. Every 3 ewes were placed in a shed and the ewes were fed a diet that met the National Research Council nutritional requirements for ewes. Ewes were exposed to consistent environmental conditions and had free access to clean water and salt blocks. The number of fetuses carried by the ewes was observed at day 35 of gestation using an ultrasonic detector. Only ewes with 2 fetuses were used for further experimentation. After the birth of lambs, male twins weighing 3.5 ± 0.5 Kg were selected and randomly assigned to control and treatment groups of 8 rams each.
Based on the previous studies’ recommendation [15, 18], 7500 IU vitamin A palmitate (vitamin A group, product no. PHR1235, Sigma) or an equivalent volume of corn oil (Control group, product no. c8267, Sigma) were injected into the biceps femoris muscle of 2-day-old lambs. After that, lambs in control and vitamin A group were injected once a week at a fixed time point for 3 weeks. An appropriate amount of LD muscle samples of 3-week-old lambs were surgically obtained for subsequent experiments, then the wound was cleaned and the skin was sutured.
Transcriptome sequencing analysis (RNA-seq)
Total RNA in muscle tissue was extracted using a Trizol kit (Invitrogen, Carlsbad, CA, USA) and assessed for RNA quality. Eukaryotic mRNA was enriched using Oligo (dT) beads and the enriched mRNA was fragmented using fragmentation buffer and then reverse transcribed into cDNA. The obtained double-stranded cDNA was purified and end-repaired and ligated to the Illumina sequencing adapter for polymerase chain reaction (PCR) amplification. The obtained cDNA library was sequenced using Illumina Novaseq6000 (Gene Denovo Biotechnology Co., Guangzhou, China). The resulting reads were filtered by fastp [19] (version 0.18.0) to obtain high-quality, clean reads. The rRNA mapping reads were removed using Bowtie2 [20] (version 2.2.8). Then, mapping of paired-end clean reads to the reference genome was performed using HISAT2. 2.4 [21]. Using a reference-based method, the number of mapped reads for each sample was assembled using StringTie v1.3.1 [22, 23]. The FPKM (Fragments per kilobase of transcript per million mapped reads) values were calculated for each transcribed region using the RSEM [24] software to quantify its expression abundance and variation.
Principal component analysis (PCA)
Principal component analysis (PCA) was performed with R package gmodels (http://www.r-project.org/) in this experiment. PCA is the degree of interpretation, which is largely used to reveal the structure/relationship of the samples/datas, and usually results are considered reliable with PCA greater than 50%.
Differentially expressed gene (DEG) analysis
RNAs between two different groups were analyzed for differential expression using DESeq2 [25] software (and between two samples with edgeR) [26]. Genes/transcripts with a false discovery rate (FDR) parameter below 0.05 and an absolute fold change of ≥ 2 were considered as differentially expressed genes/transcripts. Differentially expressed genes are shown in additional file 1.
Target gene prediction for BHLHE40
The target gene prediction of BHLHE40 was performed using online databases. First, three online databases, (GTRD, Cistrome Data Browser and hTFtarget) were used to predict the target genes of BHLHE40, and then the intersection of the three databases was taken to be the predicted target genes of BHLHE40. Finally, the target genes with stable binding sites to BHLHE40 and involved in the proliferation and differentiation of skeletal muscle cells were screened by reviewing the references and using the JASPAR online database.
Isolation, culture and treatment of primary myoblasts
Surgically obtained skeletal muscle tissue was washed three times with PBS, and then the membrane, fat, and other surrounding non-muscle tissues were removed. The muscle was minced with scissors and placed in a 15 mL centrifuge tube, and digested with 0.1% collagenase type I for 45 min on a shaker at 37 °C, then centrifuged at 500 × g for 5 min, and the precipitate was retained. Using Dulbecco’s Modified Eagle’s medium (DMEM) resuspended the muscle pellet, and passed it sequentially through 100 μm and 40 μm cell strainers, then the filtrate was collected and centrifuged at 500 × g for 5 min. Finally, the cell pellet was resuspended with DMEM containing 20% fetal bovine serum (FBS), 0.1% penicillin, and 0.1% streptomycin and cultured in an incubator at 37 °C and 5% CO2. For studies of primary myoblasts differentiation, the medium containing 20% FBS was replaced with differentiation medium containing 2% horse serum. Based on previous study [27], the retinoic acid concentration in subsequent cell experiments was 100 nM.
Transfection
In order to verify the function of BHLHE40 on the proliferation and differentiation of myoblasts, we transfected the cells with si-BHLHE40 and BHLHE40 overexpression vectors (Genomeditech, Shanghai, China) by using lipofectamine 3000, to inhibit and overexpress BHLHE40, respectively. Negative control siRNA (si-NC) and si-BHLHE40 sequences were shown in Table 1. To further investigate the effect of ID3 (target gene of BHLHE40) on the proliferation and differentiation of myoblasts, we transfected the cells with si-ID3 and ID3 overexpression vectors (Genomeditech, Shanghai, China) using lipofectamine 3000. Negative control siRNA (si-NC) and si-ID3 sequences were shown in Table 2. Overexpression vector information for BHLHE40 and ID3 are shown in additional file 2.
Wound healing scratch assay
Sheep primary myoblasts were seeded into 12-well plates to form a fused monolayer in incubator at 37 °C and 5% CO2. Then, a sterile 200 µL pipette tip was used to leave a scratch, and the sheep primary myoblasts were washed with PBS. For the BHLHE40 silencing assay, myoblasts were treated with si-NC, si-NC + RA, si-BHLHE40 and si-BHLHE40 + RA, and for the BHLHE40 overexpression assay, myoblasts were treated with empty vector and BHLHE40 overexpression vector. Moreover, for the ID3 silencing assay, myoblasts were treated with si-NC and si-ID3, and for the ID3 overexpression assay, myoblasts were treated with empty vector and ID3 overexpression vector. Three replicate wells for each treatment in the above test, and using a DMi8 microscope (Leica, Germany) to monitor cell migration at 0, 12 and 24 h after scratch formation.
5-ethynyl-2’-deoxyuridine staining assay (EdU)
EdU staining assays were performed using the Cell-Light EdU DNA Cell Proliferation Kit (RiboBio, Guangzhou, China). Specifically, sheep primary myoblasts were seeded into 12-well plates in incubator at 37 °C and 5% CO2, and the cells were treated exactly as in the scratch assay. Sheep primary myoblasts in logarithmic growth phase were treated for 24 h, and then cultured with EdU medium for 2 h and washed twice with PBS. Paraformaldehyde (4%) was used to fix the sheep primary myoblasts for 30 min and washed twice with PBS. After that, sheep primary myoblasts were incubated with 0.5% TritonX-100 for 10 min, washed with PBS, and incubated with Apollo stain for 10 min. Finally, DNA was stained with Hoechst 33,342 for 30 min and images were captured with a DMi8 microscope (Leica, Germany).
Immunocytochemical staining
Sheep primary myoblasts were cultured to 100% fusion and induced to form myotubes, and paraformaldehyde (4%) was used to fix the cells on ice for 10 min. Using 0.25% Triton X-100 permeabilized myoblasts for 10 min and blocked with 1% bovine serum albumin for 1.5 h. Primary antibody (MHC) was used to incubate myoblasts overnight at 4 °C, and then corresponding secondary antibody was used to incubate myoblasts for 1 h at room temperature. Finally, DNA was stained with DAPI for 3 min and images were acquired under a DMi8 microscope (Leica, Germany). Finally, DNA was stained with DAPI for 3 min and images were acquired under a DMi8 microscope (Leica, Germany).
Dual-luciferase reporter assay
Detailed information on plasmid construction of sheep ID3 promoter reporter gene is provided in additional file 3. When the cell coverage reached 70%, the medium was replaced with fresh medium. The sea cucumber endogenous plasmid and promoter reporter gene plasmid were transfected into the target cells 2 h later. After 48 h, the cell samples were collected, and the assay was performed according to the instructions of the reporter gene assay kit (Genomeditech, Shanghai, China). Briefly, the collected cell samples were sufficiently lysed, centrifuged at 1000 × g for 5 min, and the supernatant was retained. The Renilla Luciferase Assay working solution was prepared at room temperature. Finally, the parameters were set and analyzed using a full-featured microplate detector.
Real-time PCR (qRT-PCR)
TRIzol reagent (Takara, Dalian, China) was used to extract total RNA on muscle and cells. Then, using a one-step reverse transcription kit (Takara, Dalian, China) to reverse transcribe the RNA to cDNA, and further analyzed for quantitative analysis of the target genes using a SYBR Green RT-PCR kit (TAKARA Co, Ltd) and a CFX RT-PCR detection system (Bio-Rad, Hercules, CA). The primer sequences for the target genes are shown in Table 3, and at least three replicates were performed for each sample. The data obtained in the experiments were analyzed for the relative expression of the target genes using the 2−ΔΔCt method with β-actin as an internal reference gene.
Western blotting
Protein lysis buffer (1% NaF, 1% Na3VO4, 1% PMSF, 2% β-mercaptoethanol, 0.1% protease inhibitor, 1 × loading buffer) was added to the cell samples, and the mixture was placed on ice for 10 min. Lysed cells were collected in 1.5 mL EP tubes, and the extracted proteins were denatured in boiling water for 10 min at 100 °C. Then, centrifugation was performed at 12,000 × g for 5 min, and the supernatant was collected and placed on ice. The soluble proteins were separated by SDS-PAGE at room temperature (80 V, 0.5 h, 120 V, 1.5 h) and then transferred to nitrocellulose membrane (100 V, 1.5 h). Skim milk (5%) was used to block the nitrocellulose membrane for 1 h at room temperature, and then incubated with primary antibody to the target protein overnight at 4 °C, and corresponding secondary antibody was incubated for 1 h at room temperature. Finally, the target proteins were visualized with an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, Nevada, USA) and the band densities were normalized to the β-actin content. (The blots were cut prior to hybridisation with antibodies, and images of blots for all replicates were provided in the additional file 4)
Antibodies against CDK4 (bs-0633R), Cyclin D1 (bs-0623R), MyoD (bs-23809R), MyoG (bs-3550), MHC (bs-18070R) and β-actin (bs-0061R) were purchased from Biosynthesis Biotechnology Co., Ltd. (Beijing, China). PCNA (#2586 s) was purchased from Cell Signaling (Danvers, MA, USA). Goat anti-rabbit secondary antibody (926 − 32,211) was from LI-COR Biosciences (Lincoln, NE, US), and anti-mouse fluoresce secondary antibodies (#4408) was from Cell Signaling (Danvers, MA, USA). ID3 (#DF12520) was from Affinity Biosciences (Jiangsu, China).
Statistical analysis
All data in this experiment were processed using GraphPad Prism 9 software (Monrovia, CA, USA). The normal distribution and homogeneity of variance analysis were conducted. The comparison of two groups of data was performed using student’s t-test, and the comparison of multiple groups of data was performed using one-way analysis of variance (ANOVA) followed by Tukey’s test. Data were shown as the mean ± SEM. P < 0.05 was considered to be significantly different.
Results
Vitamin a promotes the BHLHE40 expression in muscle and differentiated myoblasts
Transcriptome sequencing analysis was performed to identify the target genes by which vitamin A affects skeletal muscle development. Principal component analysis (PCA) showed that a significant difference was observed between vitamin A and control groups (Fig. 1A). As shown in Fig. 1B, vitamin A was found to significantly up-regulate the expression of BHLHE40 through RNA-seq. Further analysis revealed a fully conserved RARE site in the promoter region of the BHLHE40 gene (Fig. 1C), which suggested that vitamin A regulated the expression of BHLHE40. Moreover, BHLHE40 was found to up-regulate during myoblasts myogenic differentiation through q-PCR (Fig. 1D, P < 0.05), and RA increased the mRNA content of BHLHE40 in vitro as confirmed by q-PCR (Fig. 1E, P < 0.01). The q-PCR further validated the mRNA expression of PIX3, ABRA, ANKRD1, SLC16A9 and BHLHE40. The results showed that the mRNA expression of PIX3, ABRA, ANKRD1 and SLC16A9 were decreased significantly (Fig. 1F, P < 0.05), while that of BHLHE40 was increased significantly with VA treatment (Fig. 1F, P < 0.05), which were identical to the results of RNA-seq (additional file 1).
Vitamin A promotes the BHLHE40 expression in muscle and differentiated myoblasts. (A) Principal component analysis (PCA) for all samples. (B) Differently expressed genes volcano plots for all samples. (C) Pattern map of RARE sites in the promoter region of the BHLHE40 gene. (D) BHLHE40 relative mRNA content at 0 d, 3 d and 6 d of sheep primary myoblasts myogenic differentiation. (E) BHLHE40 relative mRNA content in control and RA groups of sheep primary myoblasts myogenic differentiation. (F) Relative mRNA contents of differentially expressed genes. (Mean ± SEM; n = 3 in each group, *P < 0.05, **P < 0.01)
RA inhibits the proliferation of sheep primary myoblasts through BHLHE40
As shown in Fig. 2A and B, RA was shown to significantly inhibit migration ability and EdU labeling of sheep primary myoblasts through cell scratch and EdU assays, respectively. The protein abundance of CDK4, CyclinD1 and PCNA were found to significantly decrease through western blotting (Fig. 2C, P < 0.05). After silencing BHLHE40, the migration ability and EdU labeling of sheep primary myoblasts were found to significantly enhance through cell scratch and EdU assays, respectively (Fig. 2A and B), and the protein abundance of CDK4, CyclinD1 and PCNA were found to be greatly increased through western blotting (Fig. 2C, P < 0.05). Silencing of BHLHE40 with concomitant addition of RA did not restore the migration capacity, EdU labeling, and protein abundance of CDK4, CyclinD1 and PCNA in sheep primary myoblasts as revealed by cell scratch, EdU, and western blotting assays (Fig. 2A–C, P > 0.05). Overexpression of BHLHE40 was found to significantly inhibit the proliferation of sheep primary myoblasts through cell scratch, EdU, and western blotting assays, including decreased cell migration ability (Fig. 2D), reduced EdU labeling (Fig. 2E), and decreased abundance of CDK4, CyclinD1 and PCNA proteins (Fig. 2F, P < 0.05).
RA inhibits the proliferation of sheep primary myoblasts through BHLHE40. (A) Representative images of wound healing scratch assay after inhibition of BHLHE40. (B) Representative images of EdU staining assay after inhibition of BHLHE40. (C) Protein abundance of CDK4, Cyclin D1 and PCNA after inhibition of BHLHE40. (D) Representative images of wound healing scratch assay after overexpression of BHLHE40. (E) Representative images of EdU staining assay after overexpression of BHLHE40. (F) Protein abundance of CDK4, Cyclin D1 and PCNA after overexpression of BHLHE40. (Mean ± SEM; n = 3 in each group, *P < 0.05. Values with different letters indicated significant differences. The blots were cut prior to hybridisation with antibodies, and images of blots for all replicates were provided in the additional file 4)
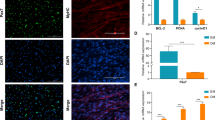
RA promotes the differentiation of sheep primary myoblasts through BHLHE40
As shown in Fig. 3A and B, RA was found to significantly promote the differentiation of sheep primary myoblasts and increased the myotube fusion index through cell immunofluorescence staining assay (Fig. 3A and B, P < 0.05). The mRNA contents of MYOD and MYOG, and the protein abundance of MyoD, MyoG and MHC were found to increase significantly through q-PCR and western blotting (Fig. 3C and D, P < 0.05). After silencing BHLHE40, the differentiation ability of sheep primary myoblasts was found to significantly inhibit through cell immunofluorescence staining assay, and the myotube fusion index was significantly reduced (Fig. 3A and B, P < 0.05). The mRNA contents of MYOD and MYOG, and the protein abundance of MyoD, MyoG and MHC were found to decrease significantly through q-PCR and western blotting (Fig. 3C and D, P < 0.05). Silencing of BHLHE40 with concomitant addition of RA did not restore the differentiation ability of sheep primary myoblasts, myotube fusion index, MYOD and MYOG mRNA contents and MyoD, MyoG and MHC protein abundance as revealed by cell immunofluorescence staining, q-PCR and western blotting (Fig. 3A–D, P > 0.05). After transfecting the cells with the BHLHE40 overexpression plasmid, the myotube fusion index was found to significantly increase through cell immunofluorescence staining assay (Fig. 3E and F, P < 0.01), the relative mRNA contents of MYOD and MYOG were found to significantly increase through q-PCR (Fig. 3G, P < 0.05), and the protein abundance of MyoD, MyoG, and MHC were also found to significantly increase through western blotting (Fig. 3H, P < 0.05).
RA promotes the differentiation of sheep primary myoblasts through BHLHE40. (A) Representative images of MHC immunofluorescence staining after inhibition of BHLHE40. (B) Fusion index. (C) Relative mRNA contents of MYF5, MYOD and MYOG after inhibition of BHLHE40. (D) Protein abundance of MyoD, MyoG and MHC after inhibition of BHLHE40. (E) Representative images of MHC immunofluorescence staining after overexpression of BHLHE40. (F) Fusion index. (G) Relative mRNA contents of MYF5, MYOD and MYOG after overexpression of BHLHE40. (H) Protein abundance of MyoD, MyoG and MHC after overexpression of BHLHE40. (Mean ± SEM; n = 3 in each group, *P < 0.05, **P < 0.01. Values with different letters indicated significant differences. The blots were cut prior to hybridisation with antibodies, and images of blots for all replicates were provided in the additional file 4)
BHLHE40 targets and inhibits ID3 gene expression
The intersecting genes predicted by online databases (GTRD, Cistrome Data Browser and hTFtarget) were taken as target genes of BHLHE40. Review of references revealed that ID3 negatively regulates the differentiation process of skeletal muscle cells. In addition, the prediction by JASPAR database revealed the presence of a high-scoring binding site of BHLHE40 in the promoter region of ID3 gene. Therefore, ID3 was chosen as the target gene of BHLHE40 for further studies. As shown in Fig. 4A, the mRNA content of ID3 in lambs of vitamin A group was significantly reduced as demonstrated by qPCR (Fig. 4A, P < 0.05). The level of ID3 mRNA was found to be decreased with myoblast differentiation through qPCR (Fig. 4B, P < 0.01). Moreover, RA was shown to significantly suppress the mRNA and protein expression levels of ID3 through q-PCR and western blotting (Fig. 4C and D, P < 0.01). To demonstrate the targeting relationship between BHLHE40 and ID3, we analyzed changes of ID3 after silencing and overexpressing BHLHE40. The results revealed that ID3 mRNA and protein abundance were negatively correlated with BHLHE40 levels through q-PCR and western blotting (Fig. 4E–G, P < 0.05). Furthermore, dual luciferase reporter assay showed that BHLHE40 repressed the expression of ID3 gene (Fig. 4H, P < 0.01).
BHLHE40 targets and inhibits ID3 gene expression. (A) Relative mRNA content of ID3 in control and Vitamin A groups lamb. (B) Relative mRNA content of ID3 during differentiation of myoblasts. (C) Relative mRNA content of ID3 in control and RA groups of differentiated myoblasts. (D) Protein abundance of ID3 in control and RA groups of differentiated myoblasts. (E) Relative mRNA content of ID3 after inhibition or overexpression of BHLHE40. (F) Protein abundance of ID3 after inhibition of BHLHE40. (G) Protein abundance of ID3 after overexpression of BHLHE40. (H) Dual luciferase reporter assay. (Mean ± SEM; n = 3 in each group, *P < 0.05, **P < 0.01. The blots were cut prior to hybridisation with antibodies, and images of blots for all replicates were provided in the additional file 4)
ID3 promotes the proliferation of sheep primary myoblasts
As shown in Fig. 5A and B, it was found that silencing ID3 significantly inhibited migration ability and EdU labeling, and decreased the abundance of CDK4, CyclinD1 and PCNA of sheep primary myoblasts through cell scratch, EdU, and western blotting assays (Fig. 5C, P < 0.01). After transfecting ID3 overexpression vector into the cells, the proliferation ability of myoblasts was found to be significantly elevated through cell scratch, EdU and western blotting assays, including enhanced cell migration ability (Fig. 5D), increased EdU labeling (Fig. 5E) and increased the abundance of CDK4, CyclinD1 and PCNA of sheep primary myoblasts (Fig. 5F, P < 0.01).
ID3 promotes the proliferation of sheep primary myoblasts. (A) Representative images of wound healing scratch assay after inhibition of ID3. (B) Representative images of EdU staining assay after inhibition of ID3. (C) Protein abundance of CDK4, Cyclin D1 and PCNA after inhibition of ID3. (D) Representative images of wound healing scratch assay after overexpression of ID3. (E) Representative images of EdU staining assay after overexpression of ID3. (F) Protein abundance of CDK4, Cyclin D1 and PCNA after overexpression of ID3. (Mean ± SEM; n = 3 in each group, **P < 0.01. The blots were cut prior to hybridisation with antibodies, and images of blots for all replicates were provided in the additional file 4)
ID3 inhibits the differentiation of sheep primary myoblasts
As shown in Fig. 6A and B, the sheep primary myoblasts differentiation ability and myotube fusion index was found to significantly increase after silencing ID3 through cell immunofluorescence staining assay (Fig. 6A and B, P < 0.01). It was that the mRNA contents of MYOD and MYOG were significantly elevated through q-PCR (Fig. 6C, P < 0.01), as was the protein abundance of MyoD, MyoG and MHC through western blotting (Fig. 6D, P < 0.05). In addition, after transfecting ID3 overexpression vector into the cells, the differentiation ability and myotube fusion index of sheep primary myoblasts were found to be significantly reduced through cell immunofluorescence staining assay (Fig. 6E and F, P < 0.01), the mRNA contents of MYOD and MYOG were found to be significantly decreased through q-PCR (Fig. 6G, P < 0.05), and the protein abundance of MyoD, MyoG, and MHC were also found to be significantly decreased through western blotting (Fig. 6H, P < 0.05).
ID3 inhibits the differentiation of sheep primary myoblasts. (A) Representative images of MHC immunofluorescence staining after inhibition of ID3. (B) Fusion index. (C) Relative mRNA contents of MYF5, MYOD and MYOG after inhibition of ID3. (D) Protein abundance of MyoD, MyoG and MHC after inhibition of ID3. (E) Representative images of MHC immunofluorescence staining after overexpression of ID3. (F) Fusion index. (G) Relative mRNA contents of MYF5, MYOD and MYOG after overexpression of ID3. (H) Protein abundance of MyoD, MyoG and MHC after overexpression of ID3. (Mean ± SEM; n = 3 in each group, *P < 0.05, **P < 0.01. The blots were cut prior to hybridisation with antibodies, and images of blots for all replicates were provided in the additional file 4)
Discussion
Sheep as a meat animal is widely favored by the public for its rich nutritional value [28,29,30,31]. In recent years, lamb production has increased in many countries and regions to meet consumer demand [32, 33]. Therefore, understanding the mechanisms of skeletal muscle development is urgently needed to improve meat production in sheep. In previous studies, we found that intramuscular injection of vitamin A promotes muscle development in lambs [17]. However, molecular regulation during skeletal muscle development is highly sophisticated and complex. For example, the sonic hedgehog (Shh), notch and wnt signaling are able to determine the direction of myogenic progenitor cell differentiation [34,35,36]. Myogenic regular factors (MRFs) are essential in myogenesis, and when myoblasts exit the cell cycle, they fuse to form multinucleated myotubes under the regulation of MRFs, and further fuse to form mature myofibers. Moreover, interactions among numerous transcription factors are involved in regulating skeletal muscle formation and development [37, 38].
In the current study, to investigate the mechanisms that vitamin A affects skeletal muscle development, transcriptome sequencing technology was used to sequence skeletal muscle tissues from 3-week-old lambs. Among the differentially expressed genes, vitamin A was found to upregulate BHLHE40 expression through analysis and screening, and this gene is believed to be involved in the control of cell differentiation. It has been shown that stra13 (BHLHE40) promotes myoblast differentiation by antagonizing Notch signaling [39]. Moreover, there are at least 2 RA-responsive regions in the nucleotide sequence of the SHARP-2 (BHLHE40) gene, which means that RA is able to stimulate the transcription of the SHARP-2 gene [40]. Consistently, the mRNA level of BHLHE40 was significantly increased after sheep primary myoblasts differentiation. Further analysis revealed the presence of a binding site for RARE in the promoter region of the BHLHE40 gene. In addition, RA greatly increased the mRNA content of BHLHE40 during sheep primary myoblasts myogenic differentiation in this study, implying that vitamin A promoted muscle development by up-regulating BHLHE40 expression.
RA, a metabolite of vitamin A, is an important factor involved in cell growth and differentiation [41]. Currently, there is increasing evidence that RA inhibits cell proliferation and promotes cell differentiation in different tissues or cells [27, 42,43,44]. More importantly, BHLHE40, a transcriptional repressor, has been shown to be a target gene of RA in P19 cells [45], which is consistent with the sequencing results of this study. In addition, Stra13 was shown to inhibit myoblast proliferation and promote myoblast differentiation in mice [39]. In the present experiments, silencing of BHLHE40 promoted myoblast proliferation, and silencing of BHLHE40 with concomitant addition of RA did not attenuate the proliferative capacity of myoblasts. In contrast, overexpression of BHLHE40 significantly inhibited myoblast proliferation. Thus, the above results suggested that the inhibition of RA on myoblast proliferation was realized through BHLHE40. Normally, during cell differentiation, the proliferative capacity is progressively limited and eventually exits the cell cycle [46]. For skeletal myogenesis, where proliferation and differentiation of myoblasts are opposite processes, overexpression of cyclin D1 inhibits MyoD activity [47]. Consistently, silencing of BHLHE40 inhibited myoblast differentiation, and silencing of BHLHE40 with concomitant addition of RA did not attenuate the inhibitory effect on myoblast differentiation. However, overexpression of BHLHE40 promoted myoblast differentiation in this research. Hence, the above findings suggested that RA promoted myoblast differentiation by upregulating BHLHE40.
Transcription factors are a class of proteins that regulate gene expression [48]. BHLHE40 belongs to the class B helix-loop-helix proteins, which contain an orange (O) domain, and is a typical transcriptional repressor [49]. BHLHE40 not only binds to the E-box sequence of genes to inhibit the transcription of target genes, but also inhibits the transcription of target genes by interacting with other transcription factors [50]. In this study, the target genes of BHLHE40 were predicted by online databases (GTRD, Cistrome Data Browser and hTFtarget databases). Subsequently, prediction and analysis using the JASPAR database revealed the presence of a high-score binding site for BHLHE40 in the promoter region of ID3 gene. Numerous researches have shown that ID3 gene is involved in a variety of cellular processes, including cell proliferation, differentiation, apoptosis and tumor transformation [51,52,53]. The present experiment revealed that the mRNA level of ID3 was significantly decreased in the skeletal muscle of vitamin A group lambs, and the mRNA level of ID3 was also significantly decreased with the differentiation of sheep primary myoblasts. Moreover, the mRNA and protein abundance of ID3 was inversely correlated with BHLHE40 by silencing and overexpressing BHLHE40, suggesting that BHLHE40 may negatively regulate ID3 expression. Actually, many researchers have shown that BHLHE40 functions as a nuclear transcriptional repressor. For example, St-Pierre et al., showed that Stra13 directly represses the transcription of the class B E-box and related sites [54]. It has also been reported that DEC1 represses the expression of DEC2 through binding to the proximal promoter E-box [55]. Since the alterations in myoblast proliferation and differentiation were caused by changes in the ID3 gene, we analyzed the effect of RA on ID3 gene levels, and the results corresponded to the in vivo experiments. This is consistent with previous findings where López-Carballo et al., found a significant reduction in ID3 mRNA levels during RA-induced differentiation of SH-SY5Y neuroblastoma cells [56]. Subsequently, a dual luciferase reporter assay confirmed that BHLHE40 inhibits the activity of the ID3 gene promoter and represses the transcription of ID3 gene. During myogenesis, myogenic regulators are highly coordinated interplay with the class I E proteins and class V Id proteins in the differentiation of myoblasts [57]. For ID family members (ID1, ID2, ID3 and ID4), ID3 has the highest expression in myoblasts [58], implying that ID3 is very important for myoblast proliferation and differentiation. In C2C12 cells, ID3 expression delays cell exit from the cell cycle, causing inhibition of cell differentiation [11, 59]. Consistently, our experiments showed that silencing ID3 inhibited myoblast proliferation and promoted myoblast differentiation. Accordingly, overexpression of ID3 showed the opposite result. However, there were many target genes of BHLHE40 predicted from online databases. Further experiments are needed to demonstrate whether BHLHE40 may be involved in regulating more genes in the process of vitamin A promoted sheep primary myoblasts myogenic differentiation.
The current results demonstrated that RA inhibited myoblast proliferation and promoted myoblast differentiation through BHLHE40-targeted inhibition of ID3 expression, which provided a scientific basis for understanding skeletal muscle development in sheep. Moreover, this research could provide a reference for the fields of muscle tissue regeneration and repair, as well as the treatment of muscle diseases and injuries.
Conclusion
Intramuscular injection of vitamin A in newborn lambs inhibited myoblasts proliferation and promoted myoblasts myogenic differentiation by inhibiting ID3 gene transcription through up-regulation of BHLHE40 expression, ultimately affected skeletal muscle development of lambs.
Data availability
The datasets presented in this study can be found in online repositories (https://ngdc.cncb.ac.cn/gsa/). The name of the repositories and accession number can be found below: Genome Sequence Archive (GSA), accession number PRJCA021156 (n=6).
Abbreviations
- RA:
-
Retinoic acid
- RNA-Seq:
-
Transcriptome sequencing
- LD:
-
Longissimus dorsi
- ID3:
-
DNA binding inhibitor 3
- PCR:
-
Polymerase chain reaction
- PCA:
-
Principal component analysis
- DEG:
-
Differentially expressed gene
- FDR:
-
False discovery rate
- DMEM:
-
Dulbecco’s Modified Eagle’s medium
- FBS:
-
Fetal bovine serum
- EdU:
-
5-ethynyl-2’-deoxyuridine
References
McCuller C, Jessu R, Callahan AL. Physiology, skeletal muscle. StatPearls [Internet]. StatPearls Publishing; 2022.
Ahmad K, Shaikh S, Chun HJ, Ali S, Lim JH, Ahmad SS, Lee EJ, Choi I. Extracellular matrix: the critical contributor to skeletal muscle regeneration—a comprehensive review. Inflamm Regeneration. 2023;43(1):58.
Sheet S, Jang SS, Kim JH, Park W, Kim D. A transcriptomic analysis of skeletal muscle tissues reveals promising candidate genes and pathways accountable for different daily weight gain in Hanwoo cattle. Sci Rep. 2024;14(1):315.
Li X, Liu X, Song P, Zhao J, Zhang J, Zhao J. Skeletal muscle mass, meat quality and antioxidant status in growing lambs supplemented with guanidinoacetic acid. Meat Sci. 2022;192:108906.
Relaix F, Bencze M, Borok M, Der Vartanian A, Gattazzo F, Mademtzoglou D, Perez-Diaz S, Prola A, Reyes-Fernandez P, Rotini A. Perspectives on skeletal muscle stem cells. Nat Commun. 2021;12(1):692.
Zhang D, Wu S, Zhang X, Ren S, Tang Z, Gao F. Coordinated transcriptional and post-transcriptional epigenetic regulation during skeletal muscle development and growth in pigs. J Anim Sci Biotechnol. 2022;13(1):146.
Schiaffino S, Reggiani C, Akimoto T, Blaauw B. Molecular mechanisms of skeletal muscle hypertrophy. J Neuromuscul Dis. 2021;8(2):169–83.
Sun H, Taneja R. Stra13 expression is associated with growth arrest and represses transcription through histone deacetylase (HDAC)-dependent and HDAC-independent mechanisms. PNAS. 2000;97(8):4058–63.
Vercherat C, Chung TK, Yalcin S, Gulbagci N, Gopinadhan S, Ghaffari S, Taneja R. Stra13 regulates oxidative stress mediated skeletal muscle degeneration. Hum Mol Genet. 2009;18(22):4304–16.
AlSudais H, Lala-Tabbert N, Wiper-Bergeron N. CCAAT/Enhancer binding protein β inhibits myogenic differentiation via ID3. Sci Rep. 2018;8(1):16613.
Hayashi S, Sakata S, Kawamura S, Tokutake Y, Yonekura S. XBP1u is involved in C2C12 myoblast differentiation via Accelerated Proteasomal degradation of Id3. Front Physiol. 2022;13:27.
Dey P, Soyer MA, Dey BK. MicroRNA-24-3p promotes skeletal muscle differentiation and regeneration by regulating HMGA1. Cell Mol Life Sci. 2022;79(3):170.
Lara-Ramírez R, Zieger E, Schubert M. Retinoic acid signaling in spinal cord development. Int J Biochem Cell Biol. 2013;45(7):1302–13.
Hamade A, Deries M, Begemann G, Bally-Cuif L, Genêt C, Sabatier F, Bonnieu A, Cousin X. Retinoic acid activates myogenesis in vivo through Fgf8 signalling. Dev Biol. 2006;289(1):127–40.
Wang B, Nie W, Fu X, de Avila JM, Ma Y, Zhu MJ, Maquivar M, Parish SM, Busboom JR, Nelson ML. Neonatal vitamin a injection promotes cattle muscle growth and increases oxidative muscle fibers. J Anim Sci Biotechnol. 2018;9(1):1–10.
Mok GF, Cardenas R, Anderton H, Campbell KH, Sweetman D. Interactions between FGF18 and retinoic acid regulate differentiation of chick embryo limb myoblasts. Dev Biol. 2014;396(2):214–23.
Song P, Chen X, Zhao J, Li Q, Li X, Wang Y, Wang B, Zhao J. Vitamin a injection at birth improves muscle growth in lambs. Anim Nutr. 2023;14:204–12.
Harris CL, Wang B, Deavila JM, Busboom JR, Maquivar M, Parish SM, McCann B, Nelson ML, Du M. Vitamin a administration at birth promotes calf growth and intramuscular fat development in Angus beef cattle. J Anim Sci Biotechnol. 2018;9:1–9.
Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884–90.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–9.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–60.
Pertea M, Pertea GM, Antonescu CM, Chang T-C, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33(3):290–5.
Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc. 2016;11(9):1650–67.
Li B, Dewey CN. RSEM. Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:1–16.
Love MI, Huber W, Anders S. Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):1–21.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–40.
Li Q, Zhang T, Zhang R, Qin X, Zhao J. All-trans retinoic acid regulates sheep primary myoblast proliferation and differentiation in vitro. Domest Anim Endocrin. 2020;71:106394.
Pannier L, Pethick DW, Boyce M, Ball A, Jacob RH, Gardner GE. Associations of genetic and non-genetic factors with concentrations of iron and zinc in the longissimus muscle of lamb. Meat Sci 2014;96(2):1111–9.
Ponnampalam EN, Burnett VF, Norng S, Hopkins DL, Plozza T, Jacobs JL. Muscle antioxidant (vitamin E) and major fatty acid groups, lipid oxidation and retail colour of meat from lambs fed a roughage based diet with flaxseed or algae. Meat Sci. 2016;111:154–60.
Talebi R, Ghaffari MR, Zeinalabedini M, Abdoli R, Mardi M. Genetic basis of muscle-related traits in sheep: a review. Anim Genet. 2022;53(6):723–39.
Battacone G, Lunesu MF, Manso T, Vieira C, Pulina G, Nudda A. The quality of meat in milk fed lambs is affected by the ewe diet: a review. Meat Sci. 2023;109374.
Mazinani M, Rude B. Population, world production and quality of sheep and goat products. Am J Anim Vet Sci. 2020;15(4):291–9.
Caihong H. Methods to improve the economic benefits of raising Mutton Sheep. Agricultural Forestry Econ Manage. 2023;6(2):68–73.
Girardi F, Le Grand F. Wnt signaling in skeletal muscle development and regeneration. Prog Mol Biol Transl Sci. 2018;153:157–79.
Abdullah S. SUFU in SHH signalling mediated myogenesis. 2023.
Mierzejewski B, Grabowska I, Michalska Z, Zdunczyk K, Zareba F, Irhashava A, Chrzaszcz M, Patrycy M, Streminska W, Janczyk-Ilach K. SDF-1 and NOTCH signaling in myogenic cell differentiation: the role of miRNA10a, 425, and 5100. Stem Cell Res Ther. 2023;14(1):204.
Mohammadabadi M, Bordbar F, Jensen J, Du M, Guo W. Key genes regulating skeletal muscle development and growth in farm animals. Animals. 2021;11(3):835.
Shirakawa T, Toyono T, Inoue A, Matsubara T, Kawamoto T, Kokabu S. Factors regulating or regulated by myogenic regulatory factors in skeletal muscle stem cells. Cells. 2022;11(9):1493.
Sun H, Li L, Vercherat C, Gulbagci NT, Acharjee S, Li J, Chung TK, Thin TH, Taneja R. Stra13 regulates satellite cell activation by antagonizing notch signaling. J Cell Biol. 2007;177(4):647–57.
Hayashi M, Misaki T, Yoshida S, Tokutake H, Aruga T, Yoda K, Kenmochi E, Saito K, Togashi M, Nakano M. Retinoic acid stimulates transcription of the rat SHARP-2 gene via multiple pathways. J Biochem. 2021;170(3):427–34.
Chen Q, Ross AC. Retinoic acid regulates cell cycle progression and cell differentiation in human monocytic THP-1 cells. Exp Cell Res. 2004;297(1):68–81.
Donato LJ, Suh JH, Noy N. Suppression of mammary carcinoma cell growth by retinoic acid: the cell cycle control gene Btg2 is a direct target for retinoic acid receptor signaling. Cancer Res. 2007;67(2):609–15.
Egly CR, Chambon P. F9 embryocarcinoma cells: a cell autonomous model to study the functional selectivity of RARs and RXRs in retinoid signaling. Histol Histopathol. 2001;16(3):909–22.
Park DJ, Chumakov AM, Vuong PT, Chih DY, Gombart AF, Miller WH, Koeffler HP. CCAAT/enhancer binding protein ε is a potential retinoid target gene in acute promyelocytic leukemia treatment. J Clin Investig. 1999;103(10):1399–408.
Boudjelal M, Taneja R, Matsubara S, Bouillet P, Dollé P, Chambon P. Overexpression of Stra13, a novel retinoic acid-inducible gene of the basic helix–loop–helix family, inhibits mesodermal and promotes neuronal differentiation of P19 cells. Genes Dev. 1997;11(16):2052–65.
Zhu L, Skoultchi AI. Coordinating cell proliferation and differentiation. Curr Opin Genet Dev. 2001;11(1):91–7.
Skapek SX, Rhee J, Spicer DB, Lassar AB. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science. 1995;267(5200):1022–4.
Weidemüller P, Kholmatov M, Petsalaki E, Zaugg JB. Transcription factors: bridge between cell signaling and gene regulation. Proteomics. 2021;21(23–24):2000034.
Davis RL, Turner DL. Vertebrate hairy and enhancer of split related proteins: transcriptional repressors regulating cellular differentiation and embryonic patterning. Oncogene. 2001;20(58):8342–57.
Yamada K, Miyamoto K. Basic helix-loop-helix transcription factors, BHLHB2 and BHLHB3; their gene expressions are regulated by multiple extracellular stimuli. Front Biosci. 2005;10(SUPPL 3):3151–71.
Lee S-H, Hao E, Kiselyuk A, Shapiro J, Shields DJ, Lowy A, Levine F, Itkin-Ansari P. The Id3/E47 axis mediates cell-cycle control in human pancreatic ducts and adenocarcinoma. Mol Cancer Res. 2011;9(6):782–90.
Chaudhary J, Johnson J, Kim G, Skinner MK. Hormonal regulation and differential actions of the helix-loop-helix transcriptional inhibitors of differentiation (Id1, Id2, Id3, and Id4) in sertoli cells. Endocrinology. 2001;142(5):1727–36.
Bakr A, Hey J, Sigismondo G, Liu C-S, Sadik A, Goyal A, Cross A, Iyer RL, Müller P, Trauernicht M. ID3 promotes homologous recombination via non-transcriptional and transcriptional mechanisms and its loss confers sensitivity to PARP inhibition. Nucleic Acids Res. 2021;49(20):11666–89.
St-Pierre B, Flock G, Zacksenhaus E, Egan SE. Stra13 homodimers repress transcription through class B E-box elements. J Biol Chem. 2002;277(48):46544–51.
Li Y, Xie M, Song X, Gragen S, Sachdeva K, Wan Y, Yan B. DEC1 negatively regulates the expression of DEC2 through binding to the E-box in the proximal promoter. J Biol Chem. 2003;278(19):16899–907.
López-Carballo G, Moreno L, Masiá S, Pérez P, Barettino D. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neural differentiation of SH-SY5Y human neuroblastoma cells. J Biol Chem. 2002;277(28):25297–304.
Roschger C, Cabrele C. The Id-protein family in developmental and cancer-associated pathways. Cell Commun Signal. 2017;15(1):1–26.
Atherton GT, Travers H, Deed R, Norton JD. Regulation of cell differentiation in C2C12 myoblasts by the Id3 Helix-Loop-Helix Protein. Cell Growth Differ. 1996;7(8):1059–66.
Melnikova IN, Christy BA. Muscle cell differentiation is inhibited by the helix-loop-helix protein Id3. Cell Growth Differ. 1996;7(8):1067–80.
Acknowledgements
The authors appreciate the support and assistance provided by the staff at Taigu Paulson Pastoral.
Funding
This work was supported by the National Key R&D Program of China (2023YFD1301302); and the Distinguished and Excellent Young Scholar Cultivation Project of Shanxi Agricultural University (2022JQPYGC01).
Author information
Authors and Affiliations
Contributions
PS designed and carried out the experiments. PS, JZ, WZ and XL collected animal samples. BJ performed data curation and analysis. PS wrote the original draft. JZ and XL reviewed and edited this manuscript. JZ secured the funding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Animal protocol was approved by the Institutional Animal Conservation and Utilization Committee of Shanxi Agricultural University (Sxnd202028).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Song, P., Zhao, J., Zhang, W. et al. Vitamin a potentiates sheep myoblasts myogenic differentiation through BHLHE40-modulated ID3 expression. BMC Genomics 25, 244 (2024). https://doi.org/10.1186/s12864-024-10161-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-024-10161-0