Abstract
Glycoside hydrolase family 1 (GH1) β-glucosidases (BGLUs), are encoded by a large number of genes, which participate in the development and stress response of plants, particularly under biotic and abiotic stresses through the activation of phytohormones. However, there are few studies systematically analyzing stress or hormone-responsive BGLU genes in alfalfa. In this study, a total of 179 BGLU genes of the glycoside hydrolase family 1 were identified in the genome of alfalfa, and then were classified into five distinct clusters. Sequence alignments revealed several conserved and unique motifs among these MsBGLU proteins. Many cis-acting elements related to abiotic stresses and phytohormones were identified in the promoter of some MsBGLUs. Moreover, RNA-seq and RT-qPCR analyses showed that these MsBGLU genes exhibited distinct expression patterns in response to different abiotic stress and hormonal treatments. In summary, this study suggests that MsBGLU genes play crucial roles in response to various abiotic stresses and hormonal responses, and provides candidate genes for stress tolerance breeding in alfalfa.
Similar content being viewed by others
Introduction
Plants are exposed to various environmental stresses during their growth and development processes, including biotic and abiotic stresses, such as pathogens, salt stress, drought stress, and cold stress, etc. [1, 2], which seriously affect the yield and quality of crops [3]. To adapt to various stresses, plants produce a variety of defensive chemicals by coordinating a complex network of genes [4]. The glucosylated forms of several defensive chemicals include cyanogenic, isoflavone, and hydroxamic acid glucosides. In addition to protecting plants against harmful effects on their defense system, glucosylation may increase the solubility and stability of these chemicals, making them appropriate for storage in the vacuole or other organelles [5, 6]. Glycoside hydrolases (EC 3.2.1) belong to glycosylases (EC 3.2), which are a major class of hydrolases (EC 3). They are classified into a group of enzymes that hydrolyze the glycosidic bonds of carbohydrates [7]. So far, 161 families have been identified and classified in the CAZy (Carbohydrate-Active enZYmes) database [8, 9]. Among these families, the glycoside hydrolase family 1 is recognized for its β-glycosidase activity, which largely contributes to various developmental processes and stress responses in plants [10, 11]. BGLUs are groups of glycoside hydrolase 1 (GH1) family members, which can be found in all domains of living organisms, and have the essential function of removing non-reducing terminal glucosyl residues from glycosyl esters, oligosaccharides, and glycosides [9]. A large number of glycosidases have been found in higher plants, which play a variety of abundant functions, including defense, phytohormone activation, cell wall remodeling, scent release, microbe/insect interactions, and secondary metabolism [11, 12].
Several BGLU enzymes have been elucidated to be involved in multiple biological processes of plant growth and development, such as cell wall remodeling, lignification, and plant secondary metabolites [12, 13]. For example, AtBGLU20 plays a crucial part in pollen formation, as evidenced by the fact that knockdown mutants of AtBGLU20 have defective pollen grains but normal morphological growth [14]. In Cymbidium sinense, BGLU genes are upregulated during ovule development, when β-glucosidase may mediate hydrolysis of cellulose [15]. More importantly, substantial evidence also indicates that BGLU genes play important roles in the activation of defense compounds response to stresses and in the release of active plant phytohormones [16]. For instance, two stem-specific β-glucosidases, AtBGLU45 and AtBGLU46, are involved in the hydrolysis of monolignol glucoside coniferin, which is the storage form of monolignols. When lignin needs to be synthesized de novo under various stresses, glucoside coniferin in Arabidopsis thaliana could be used [17]. Besides, it has been demonstrated that AtBGLU26 plays an important role in plant protection against broad spectrum fungi in A. thaliana [18]. When A. thaliana was treated with salt and drought stress, AtBGLU48 protected plants against freezing. AtBGLU48 could increase cold, salt, and drought resistance in A. thaliana by protecting chloroplast membranes and preventing dehydration [19]. During dehydration, AtBGLU18, has the function of increasing ABA, and increased molecular weight under drought. Compared with wild-type plants, the mutants of AtBGLU18 exhibit earlier germination, defects in stomatal closure, and increased sensitivity to drought stress [20]. In Oryza sativa, OsBGLU10, OsBGLU24, and OsBGLU33 are associated with IAA and ABA signaling and play a particular role in seed germination, root elongation, and drought tolerance [21]. Taken together, all these studies suggest that BGLUs play an important role in plant growth, development, and stress resistance.
The BGLU gene members of the GH1 family have been identified in several plants through the genome sequencing, and their functions have been preliminarily analyzed. Alfalfa (Medicago sativa L.), well-known as the “Queen of forages”, is a perennial legume forage crop, due to its advantages of rich content in protein, vitamins, and other nutrients [22]. However, the growth and development of M. sativa were constrained by multiple environmental stress factors, such as drought, salt, and extreme temperature stresses [23, 24]. Therefore, it is essential to investigate the abiotic stress-responsive mechanism and screen new genes for genetic breeding of M. sativa. With the rapid development of sequencing technology, a large number of genomes and publicly available transcriptomic data have been collected. Genome-wide identification and analysis of BGLU genes have been conducted in many plants. However, the reference genome of alfalfa (cv. XinJiangDaYe) was not published until 2020 [25]; therefore, the basic information and functions of BGLU genes in alfalfa have not yet been clarified.
In this study, a total of 179 MsBGLU genes from the GH1 family were identified and characterized. We compared the protein sequences of A. thaliana and Medicago truncatula to the reference genome of alfalfa using BLAST method. At the same time, HMM method was used to find glyco_hydro_1 domain in The reference genome of alfalfa. Both of the results were intersected, and CD-search was used to examine, resulting in the identification of 179 MsBGLU genes. The comprehensive analyses on these MtBGLUs were performed, including sequence features, phylogenetic relationships, gene structures, motif composition, conserved domains, chromosomal localization, synteny analysis, and cis-acting elements in the promoter regions, and the evolutionary relationship between M. sativa, A. thaliana, M. truncatula, and Glycine max. In addition, MsBGLUs were found to participate in various biological processes by analyzing the expression patterns under cold, salt, drought, and ABA treatment, with the public transcriptomic data, as well as checked by quantitative real-time PCR (qRT-PCR) analysis. This study establishes the foundation for a deeper understanding of the functional characteristics of members in the MsBGLUs family and their utilization in molecular breeding to enhance resistance against various abiotic stresses in alfalfa.
Results
Identification of BGLU genes in the M. sativa genome
A total of 179 MsBGLU genes of the GH1 family were obtained by HMM and BLAST in the alfalfa (cv. XinJiangDaYe) genome after the removal of redundant sequences. MsBGLU1 to MsBGLU179 were named based on their locations on the alfalfa chromosomes. Physico-chemical properties of MsBGLU genes were listed in Supplementary Table S1, including instability index, aliphatic index, molecular weights, isoelectric points, average of hydropathicity (GRAVY), and possible subcellular localization. The lengths of the predicted precursor proteins ranged from 276 (MsBGLU23) to 1521 (MsBGLU16) amino acids (AAs), corresponding to protein molecular weights (MWs) ranging from 31.4 to 171.2 kDa, and the predicted pI ranged from 5.05 (MsBGLU125) to 9.34 (MsBGLU23). The GRAVY values were all negative except MsBGLU19, indicating that most of the MsBGLU proteins were hydrophilic. Based on the subcellular localization predictions, 56 (75.61%) proteins were found in the cytoplasm, 46 (25.70%) in the chloroplast, and 26 (14.53%) in the vacuole. The remaining proteins were located in the extracellular, endoplasmic reticulum, mitochondrion and nucleus. The results suggest that the MsBGLU proteins may have functions in diverse microcellular environments.
Multiple sequence alignment, phylogenetic analysis, and classification of MsBGLU genes
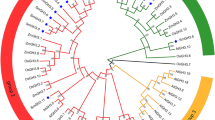
To assess the evolutionary relationships among MsBGLU genes, BGLU proteins from A. thaliana, M. truncatula, and M. sativa were used to construct a phylogenetic tree by MEGA-X using the NJ method. Based on the homology and classification of BGLU genes in A. thaliana and M. truncatula, the 179 MsBGLU proteins were divided into 5 major phylogenetic clusters: I, II, III, IV, and V groups with 108, 8, 23, 23, and 17 members, respectively (Fig. 1). Cluster I was the largest MsBGLU subfamily in this study. Cluster II contained 6 MtBGLUs and 8 MsBGLUs. The At_I Cluster with 16 members was all from A. thaliana, which was consistent with the previous study on M. truncatula [26].
Phylogenetic analysis of BGLUs from M. sativa, M. truncatula, and A. thaliana. Full-length protein sequences of BGLUs were used to construct the unrooted tree, by using MEGA-X based on the Neighbor-Joining (NJ) method with a bootstrap value of 1000 replicates. Subfamilies are highlighted with different colors. The red circles, green stars, and blue squares shapes represent BGLU proteins from M. sativa (Ms), M. truncatula (Mt), and A. thaliana (At), respectively
Conserved motif and gene structure analyses of MsBGLU
To evaluate the structural similarity and diversification of MsBGLU proteins, a total of 10 different conserved motifs were identified using the MEME tool. The sequences and SeqLogo values of the 10 conserved motifs were presented in Fig. 2. The results showed that the sequence length of the 10 motifs ranged from a minimum of 21 amino acids (Motif8, 9, 10) to a maximum of 57 amino acids (Motif1). Most MsBGLU contained all 10 motifs, indicating their conservation across the gene family. Each member of the MsBGLU gene family contained a minimum of 4 to a maximum of 10 of these motifs (Supplementary Figure S1, Supplementary Table S3); 63%, 23% and 10% of the MsBGLU members contained ten, nine and eight different motifs, respectively. MsBGLU23 contained only four motifs (Motif4, 6, 7, 9); two of MsBGLUs contained only six motifs and four of MsBGLUs contained seven motifs. Conserved motifs of BGLU proteins were located in the glyco_hydro_1 domain, and the distribution of these variable motifs may lead to a diversity of MsBGLU functions.
To understand the gene-structural variations of the BGLU family in alfalfa, the compositions of exon-introns were further analyzed using TBtools. The results showed that the number of exons in MsBGLU genes ranged from 7 (MsBGLU115) to 43 (MsBGLU16) (Supplementary Figure S1). Among the 179 MsBGLUs, 36%, 16%, 12% of MsBGLUs possessed 13, 12, and 14 exons, respectively. These results indicate that the intron/exon distribution of MsBGLUs is highly variable, with the structure of 13 exons being the main form of MsBGLUs (Supplementary Figure S1).
Chromosomal distribution and synteny analysis of MsBGLU genes
The 179 members of the MsBGLU genes were unevenly distributed across 28 chromosomes of alfalfa reference genome (cv. XinJiangDaYe) [25], except for chr1.1, chr1.3, chr8.3 and chr8.4 (Fig. 3). The chromosome with the highest number of BGLU genes (18) was chr4.4, whereas the fewest, only 1, were found on chr1.2 and chr1.4. The BGLU gene distributions also presented clustering on some chromosomes, such as MsBGLU139-MsBGLU146 on chr6.2 and MsBGLU89-MsBGLU100 on chr4.3 (Fig. 3).
As shown in Fig. 4 and Supplementary Table S3, a total of 150 pairs of homologous genes were found, involving 90 MsBGLU members in alfalfa genome. Among these duplication events, 15 were involved in duplications of the four allelic chromosomes. For example, the paralogy of MsBGLU5 on chr2.1 and MsBGLU10 on chr2.2, as well as MsBGLU19 on chr2.3 and MsBGLU30 on chr2.4, arose from genome duplication events. Among the 179 MsBGLU members, 80% MsBGLUs belonged to segmental duplications, while 7%, 9%, and 2% of MsBGLUs belonged to tandem, proximal and dispersed duplications, respectively (Supplementary Table S3). In addition, no singletons were observed. These results indicate that the segmental duplication is the key driving force in the evolution of MsBGLUs.
To further understand the possible evolutionary events of the BGLU genes in different groups, three comparative syntenic maps of M. sativa with A. thaliana, M. truncatula, and G. max were constructed. The results showed 12 collinear BGLU gene pairs between M. sativa and A. thaliana, 63 collinear BGLU gene pairs between M. sativa and G. max, and 76 orthologs between M. sativa and M. truncatula (Fig. 5, Supplementary Table S4). The number of orthologous gene pairs between MsBGLUs and AtBGLUs was significantly lower than the number between MsBGLUs and GmBGLUs or MsBGLUs and MtBGLUs. This is likely because M. sativa, M. truncatula, and G. max are all legumes.
To explore the evolution type among these duplication events on different chromosomes, the Ka/Ks values were calculated using TBtools software. As shown in Supplementary Table S5, the MsBGLU10/MsBGLU30 gene pairs had a Ka/Ks ratio of 4.96, indicating a high degree of positive selection. However, the Ka/Ks values of 99% of the paralogous MsBGLU gene pairs were below 1, implying that the MsBGLUs might have undergone a strong purification selection pressure during evolution.
Analysis of cis-acting elements in the promoter regions of MsBGLU genes
The cis-acting elements in the 2000 bp sequence of the promoter region were analyzed using PlantCARE software. Twenty-eight types of cis-acting elements were found associated with responses to stresses and phytohormones in the promoters (Fig. 6, Supplementary Figure S2, Supplementary Table S6). Among plant growth and development clusters, we detected 67 circadian responsive elements (Circadian), 80 O2-site, 72 CAT-box, and 80 GCN4_motif elements. In terms of stress-responsive clusters, we found 104 low-temperature responsive elements (LTR), 98 TC-rich repeats, 119 drought responsive elements (MBS), 133 W-boxes, 254 wound responsive elements (WUN-motif and WRE3), and 391 antioxidant response element (ARE). Among the phytohormone responsive elements, we detected 59 auxin responsive elements (TGA-element), 134 gibberellin responsive elements (P-box, TATC-box, and GARE-motif), 132 salicylic acid responsive elements (TCA-element), 418 MeJA responsive elements (TGACG-motif and CGTCA-motif), 273 ERE (ethylene responses), and 304 ABA responsive elements (Abscisic acid responses, ABRE). Additionally, many light-responsive regulatory elements were identified in the promoter regions of most MsBGLU genes (Supplementary Table S6). Taken together, these results suggest that MsBGLUs are likely involved in plant growth, development, and abiotic/biotic stress responses.
Expression analysis of MsBGLU genes in alfalfa under abiotic stresses
Based on the previous research, we collected transcriptome data under cold, ABA, drought, and salt treatments to clarify the role of the MsBGLU genes. The expression levels of these genes were shown in a heatmap (Supplementary Figure S3). The results showed that multiple MsBGLU genes were associated with various abiotic stresses. The expression levels of two MsBGLU genes (MsBGLU78 and MsBGLU119) were significantly increased under cold stress (Fig. 7A). Under the ABA treatment, the expression levels of five MsBGLUs (MsBGLU99, MsBGLU63, MsBGLU62, MsBGLU78, MsBGLU49) were significantly upregulated, while three (MsBGLU91, MsBGLU70, MsBGLU73) were downregulated (Fig. 7B).
Combining these different treatment results, we also found that multiple MsBGLU genes were involved in two or more adversity responses (Fig. 7). MsBGLU78 and MsBGLU119 were upregulated in these four treatments. Under ABA, drought, and salt treatment, MsBGLU99, MsBGLU63, and MsBGLU62 were upregulated, while MsBGLU91, MsBGLU70, and MsBGLU73 were downregulated. Notably, MsBGLU35 and MsBGLU42 were only highly expressed under cold stress.
Validation of the expression profile of MsBGLU genes by qPCR
Among all 179 MsBGLU genes, six of them (MsBGLU99, MsBGLU69, MsBGLU35, MsBGLU19, MsBGLU78, and MsBGLU49) were highly induced by various stresses. To confirm the result, RT-qPCR was further performed. As shown in Fig. 8, the expression profiles of the six candidate genes were basically consistent with RNA-Seq results. Under cold stress (Fig. 8C), the expression levels of MsBGLU19 and MsBGLU78 were significantly up-regulated at 48 h and then decreased; the expression levels of MsBGLU49 and MsBGLU99 were significantly up-regulated at 2 h and then decreased gradually. The expression level of MsBGLU69 reached the highest at 6 h, and MsBGLU35 was comparatively higher at 24 and 48 h. These results were consistent with the above RNA-seq data (Fig. 7). Under mannitol stress (Fig. 8A), MsBGLU35 was significantly up-regulated to a certain level. MsBGLU78 was obviously down-regulated during the late treatment stages. MsBGLU49, 69, and 99 reached the highest at 24 h, 12 h, and 48 h, respectively. However, the expression level of MsBGLU19 was not changed significantly. Under NaCl stress (Fig. 8B), the expression level of MsBGLU19 increased to 72 h gradually, whereas MsBGLU35, 69 were significantly up-regulated at 6 h and then decreased gradually. The expression level of MsBGLU99 reached the highest at 2 h. Besides, NaCl had no significant effect on the expression levels of MsBGLU49 and MsBGLU78. Under ABA treatment (Fig. 8D), the expression levels of MsBGLU19 and MsBGLU35 were greatly elevated. Additionally, MsBGLU78 was remarkably diminished after 12 h of ABA treatment, whereas ABA showed no evident effect on the expression levels of MsBGLU49, 69, and 99 genes. For IAA treatment (Fig. 8E), the expression levels of MsBGLU35 and MsBGLU69 were only up-regulated after 6 h. MsBGLU19, 49, 78, and 99 showed no significant increment in response to the IAA supplement. Collectively, the results confirm that these MsBGLU genes was most likely key BGLU genes involved in response to stress and hormone stimuli in M. sativa.
qRT-PCR analysis for 6 representative MsBGLU genes in response to (A) Mannitol, (B) NaCl, (C) Cold, (D) ABA, and (E) IAA treatment. Data were normalized with MsActin gene. The vertical bars indicate standard error. Error bars were obtained from three measurements. Lowercase letter(s) above the bars indicate significant differences (α = 0.05, Duncan’s t-test among the treatments)
Discussion
β-glucosidase genes play diverse and important roles in response to abiotic stress and plant development [27], including roles in cell-wall oligosaccharides, lignification, activation of phytohormones, defense, secondary metabolism [12, 16]. It is noteworthy that BGLUs participate in most of these above processes through an effective mechanism, namely hydrolyzing (activating) inactive and stable glucoside compounds, and increasing cellular ABA levels to protect plants from various stresses. Because of their important roles, BGLU genes have been identified and functionally analyzed genome-wide in many plant species. However, a systematic analysis and in-depth study of the BGLU genes at the whole-genome level is still lacking in alfalfa, because of its autotetraploid and difficulty in genome assembly [25]. In this study, a total of 179 MsBGLU gene members were identified in the alfalfa reference genome (Supplementary Table S1). The number of BGLU genes in alfalfa was higher than those in A. thaliana (n = 47) [28], O. sativa (n = 40) [29], Zea mays (n = 26) [30], M. truncatula (n = 51) [26], and Brassica rapa (n = 64) [14]. That is mainly due to the fact the genome of ‘XinJiangDaYe’ is an allele-aware chromosome-level genome consisting of 32 allelic chromosomes. Hence, MsBGLU genes identified include a portion of allelic genes. Another reason for the increase in MsBGLU numbers could be gene duplication events.
It is well known that gene duplication plays a vital role in the rapid expansion and evolution of gene families [31]. In this study, 143 pairs of segmental duplication genes and 13 pairs of tandem duplication were detected in the alfalfa BGLU gene family, supporting the inference that MsBGLU gene family expands through segmental and tandem duplication events, and the former played a predominant role. This observation is in accordance with PtBGLU genes [27]; conversely, the evolution of MtBGLU genes was mainly driven by tandem duplication [26]. It is not difficult to speculate that the number of gene duplications is related to the number of chromosomes and family/subfamily members. Synteny maps among the three representative species (A. thaliana, M. truncatula, and G. max) and alfalfa were constructed to better understand the phylogenetic relationships. There were only 12 gene pairs between MsBGLU and AtBGLU, but 63, and 76 gene pairs between MsBGLU and GmBGLU, and MtBGLU, respectively (Fig. 5, Supplementary Table S4). It is likely that M. sativa, G. max, and M. truncatula belong to the leguminous family, while A. thaliana belongs to the cruciferous family. There were relatively more collinear gene pairs between M. sativa, G. max, and M. truncatula, indicating a closer genetic relationship. Especially, the genetic relationship between M. sativa and M. truncatula is closer than that of G. max, because both of them belong to the same genus of Medicago.
On the basis of functional characterization of homologs in different plant species, gene functions were analyzed and predicted by phylogenetic tree analysis. MsBGLU19/25/26 were clustered in cluster IV with AtBGLU45/46 (Fig. 1), which hydrolyzed lignin precursors in A. thaliana [17]. This suggests that MsBGLU19/25/26 may be involved in lignin metabolism in M. sativa. As glucosyltransferase, AtBGLU6 and AtBGLU10 in cluster V play an important role in the biosynthesis of flavonoid glycosides in A. thaliana [28, 32]. It is speculated that the BGLUs of cluster V in M. sativa may have the similar function of synthesise flavonoid glycosides to resist abotic stress. Notably, cluster I contained more than half (108/179) of MsBGLU family members and twelve members of A. thaliana. The genes of this cluster were mainly expressed in roots that accumulate natural isoflavonoids [33], which participate in plant-microbe interactions, such as symbiotic or defensive mechanisms against pathogens infection. In M. truncatula, four BGLUs participate in methyl jasmonate (MJ) signal-induced hydrolysis of stored isoflavone glucosides for phytoalexin medicarpin accumulation [26]. Therefore, it is inferred that the BGLUs of cluster I in M. sativa may have the similar function of hydrolyzing isoflavonoid glucosides to defensive aglycones under adverse stresses. Besides, 16 out of 47 AtBGLUs were grouped into the cluster At_I. Actually, orthologous genes of this cluster are also present in other species of Brassicales order, but not in closely related M. sativa and M. truncatula counterparts (At I) in Fig. 1, indicating they might have diverged before M. sativa and A. truncatula. Similarly, eight MsBGLUs in cluster II have no closely related A. thaliana homologs. Interestingly, in each group (except for At_1), MsBGLUs have homologous genes of MtBGLUs, likely due to the relatively close evolutionary distance between M. sativa and M. truncatula.
Many studies provide evidence that BGLU genes are involved in response to various plant abiotic stresses [17, 29, 34]. For instance, AtBGLU18 with an ER retention signal is induced by dehydration and contributes significantly to drought resistance in A. thaliana [20]; chloroplast-localized Os3BGLU6 is responsive to drought and ABA treatments, and disruption of Os3BGLU6 in rice results in lower ABA content and higher stomatal density [35]; MtBGLU21, MtBGLU22, MtBGLU28, and MtBGLU30 are strongly induced by abiotic stresses and hormone treatments, indicating their important roles in development and stress tolerance [26]. Alfalfa has high tolerance to abiotic stress, but the role of MsBGLUs in abiotic stress has not been clarified. In the present study, we found that MsBGLU78 was homologous gene of MtBGLU19, while MsBGLU69 and MsBGLU99 were homologous genes of MtBGLU28 and MsBGLU21, respectively (Fig. 1). The expression levels of MsBGLU19 and MsBGLU35 were found to be induced by cold stress (Fig. 8). Therefore, based on phylogenetic analysis and expression analysis, we inferred that MsBGLU19, 35, 69, 78 and 99 may perform the function of regulating resistance to abiotic stress in alfalfa. Furthermore, there were plentiful stress and hormone responsive elements detected in the promoter regions of MsBGLUs, and the expression patterns of most MsBGLU genes exhibited differential responses to various abiotic stresses at the transcriptional level, indicating the functional diversity of them. The qRT-PCR quantitative data showed that the expression of MsBGLU69 was induced by IAA, and MsBGLU99 was induced by mannitol and NaCl to some extent; the previous studies showed that their homolog MtBGLU28 and MtBGLU21 in M. truncatula respectively were induced by IAA, ABA, SA and GA3 treatments [26], indicating their potential roles in various signaling network. Additionally, the expression of MsBGLU35 was induced not only by abiotic stresses but also by hormone treatments, and MsBGLU19 was induced by cold stress and ABA treatment, indicating the important roles against stresses (Fig. 8). Besides, root-specific AtBGLU42 has been known to enhance the ability of beneficial rhizosphere bacteria to stimulate the immune system, while regulating the iron deficiency response in A. thaliana roots [36], and its homolog MsBGLU49 in alfalfa was induced by mannitol stress (Fig. 8), indicating the diversified functions between AtBGLUs and MsBGLUs. Previous study also showed that MtBGLU19 was induced by NaCl stress [26], but the expression level of its homolog MsBGLU78 was decreased by NaCl stress to 12 h, then increased gradually. The different expression patterns exhibited among MsBGLUs under stress may be due to their different defense mechanisms, and further in-depth research is needed.
Alfalfa, as one of the most important forage crops in the world [37], often suffers from various adverse effects like drought and salt stress, resulting in yield and quality loss [3]. The BGLU gene family is involved in plant growth, development, and abiotic stress response. Therefore, it is important to mine MsBGLU genes related to stress tolerance and develop molecular breeding strategies for the genetic improvement of alfalfa. Our genome-wide identification and expression analysis of MsBGLU genes provide knowledge of candidate genes of abiotic stress resistance in alfalfa, which may be helpful for better understanding the biological roles of MsBGLU genes and for the further functional analysis of them.
Conclusion
In this study, a comprehensive and systematical investigation of GH1 β-glucosidases in M. sativa was carried out. A total of 179 MsBGLUs were identified from autotetraploid cultivated alfalfa genome. They were classified into five main clusters, with highly identical amino acid sequences and motif compositions. The conserved domain, gene structure, genomic location, cis-element, and expression pattern were conducted. The cis-acting element responsive to plant hormones (ABA, auxin, MeJA, salicylic acid, and gibberellin) and stresses were found in the promoter of some MsBGLUs. Moreover, expression levels of MsBGLUs were analyzed in response to various treatments (drought, salt, ABA, and cold) based on available RNA-seq data and qPCR validation, indicating that MsBGLUs played important roles in M. sativa stress tolerance, especially MsBGLU19, MsBGLU35, MsBGLU49, MsBGLU69, MsBGLU78, and MsBGLU99. Overall, the study provides valuable resources for understanding the potential molecular function of MsBGLU genes, and lays a foundation for further improvement of stress resistance and hormone signaling transduction in alfalfa.
Materials and methods
Plant materials, growth conditions, and stress treatments
The alfalfa cultivar ‘XinJiangDaYe’ used in this experiment was obtained from College of Grassland and Environment Sciences of Xinjiang Agricultural University. First, the seeds that were full and uniform in shape were selected and sterilized in 1% NaClO solution for 10 min. Then they were placed onto two layers of filter paper moistened with distilled water in circular Petri dishes in the dark at 22 °C for 3 days. Then, seedlings were transplanted to square plastic pots with vermiculite in a greenhouse for growth (16/8 h light/dark photoperiod, 25 °C/23°C day/night temperature, 52% relative humidity). The 6-week-old alfalfa seedlings were subjected to different treatments. For cold stress, alfalfa seedlings were transferred to an artificial climate chamber at 10 °C. For drought stress, plants were irrigated with 400 mM mannitol solution to simulate drought stress. For salt stress, 300 mM NaCl was used to simulate salt stress. Seedling leaves were collected at seven time points (0, 2, 6, 12, 24, 48, and 72 h), including three biological replicates, and the samples treated for 0 h were used as controls. For phytohormone treatments, plants were treated with 100 µM abscisic acids (ABA) and 100 µM auxin (IAA), respectively. Seedling leaves were collected at six time points (0, 1, 6, 12, 24, and 48 h). All samples were immediately frozen in liquid nitrogen and stored at − 80 °C before RNA extraction.
Identification of MsBGLU genes of GH1 family in alfalfa reference genome
The reference genome sequence (Xinjiangdaye) of alfalfa was downloaded from Figshare (https://figshare.com, accessed on 12 November 2022). To more comprehensively investigate the BGLU genes, the BGLU protein sequences of A. thaliana were obtained from genome databases of A. thaliana (TAIR, http://www.arabidopsis.org/, accessed on 1 March 2023); the BGLU protein sequences of M. truncatula, a model plant for legumes, were acquired as described by Yang et al. [26]; the reference genome of G. max (Wm82.a4.v1) was downloaded from the Pytozome (https://phytozome-next.jgi.doe.gov/). The BGLU candidate members of GH1 family (BGLU was referred as GH1 family hereinafter) of alfalfa were carried out by two steps. First, a hidden Markov model (HMM) profile of Glyco_hydro_1 (PF00232) was downloaded from the Pfam database (http://pfam.xfam.org/) and used as the query (P < 1e−10) to search the M. sativa protein sequence data with HMMER3 software using default parameters [38]. Second, BGLU protein sequences of A. thaliana and M. truncatula were utilized as the query sequences for BLAST analysis against the cultivar XinJiangDaYe genome database with an E-value cutoff 1e−10. After removing all of the redundant sequences, the candidate MsBGLU protein sequences were screened via NCBI Batch CD-search (https://www.ncbi.nlm.nih.gov) to confirm the conserved BGLU domain. Finally, the physicochemical properties of MsBGLUs including molecular weight (MW) and theoretical isoelectric points (pI) were analyzed using ProtParam (http://web.expasy.org/protparam/, accessed on 6 May 2023). The subcellular localization of MsBGLUs was predicted using the WoLF PSORT (https://wolfpsort.hgc.jp/, accessed on 6 May 2023).
Multiple sequence alignment, phylogenetic analysis, and classification of the MsBGLU genes
In order to analyze the evolutionary relationships of the obtained BGLUs in alfalfa, the BGLU protein sequences of M. sativa, A. thaliana, and M. truncatula were used for multiple sequence alignment with Muscle [39] and trimmed automatically with TrimAL [40]. Subsequently, an unrooted phylogenetic tree was constructed based on the neighbor-joining (NJ) method with 1000 replicated bootstrap values using MEGA 7.0 software. According to the classification of MtBGLU, all the identified MsBGLU genes were divided into 5 groups [26]. The online program iTOL (https://itol.embl.de/, accessed on 7 May 2023) was used to optimize the evolutionary tree [41].
Gene structure and motif analysis of MsBGLU genes
Understanding the gene structure can help reveal gene functions, regulation, and evolution. To visualize the exon-intron structure of MsBGLU genes, the gene structure was displayed using Tbtools [42], according to the alfalfa genome annotation file [25]. Conserved motifs of MsBGLU proteins were identified using the MEME program (http://meme-suite.org/tools/meme) with parameters of maximum of 10 motifs and a range of motif width 6 to 200 and displayed using TBtools software [42].
Chromosomal locations, gene duplication, and syntenic analysis with several plant species
To better recognize the genomic distribution of MsBGLU genes, a chromosomal location map of the MsBGLU genes was drafted based on the alfalfa genome annotation files using TBtools [42]. A multiple Collinearity Scan toolkit (MCScanX) was adopted to analyze the duplication events of alfalfa BGLU genes with default parameters [43].
Gene duplication is a major source to produce new genes, which leads to the proliferation and diversification of TFs genes in the plant kingdom. To exhibit the interspecific synteny relationship between alfalfa and the other three representative model plant species (A. thaliana, M. truncatula, and G. max), the syntenic maps were constructed using Dual Synteny Plotter of TBtools software [42].
Cis-regulatory element analysis in the promoter region of MsBGLU genes
A thorough analysis of the cis-regulatory elements in MsBGLU genes may provide further insight into their function. The cis-regulatory elements in the upstream 2000 bp promoter sequences were submitted to the online program PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 6 May 2023) [44].
Expression analysis of MsBGLU genes under abiotic stresses
In previous studies, to acquire additional genetic information of alfalfa under abiotic stresses, RNA-Seq projects of alfalfa under cold (SRR7091780-SRR7091794), drought, salt, and ABA treatments (SRR7160313-SRR7160357) were performed [22]. In this study, to realize the roles of MsBGLU genes in response to abiotic stresses, the clean reads were mapped to the “Xinjiangdaye” genome by using Hisat2 software [31]. The gene expression level was estimated by using FPKM value (fragments per kilobase of transcript per million reads mapped). Subsequently, TBtools software was used for data visualization [43].
Expression analysis by qRT-PCR
Six MsBGLU genes were selected for qRT-PCR experiments to validate the RNA-Seq data. Total RNAs were extracted using Eastep® Super total RNA Extraction kit (Promega, Shanghai, China) according to the manufacturer’s instructions. Subsequently, a NanoDrop ND1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA) was applied to determine the concentration of each sample. The first-strand cDNA synthesis was performed using HiScript®III RT SuperMix for qPCR (+ gDNA wiper) (Vazyme Biotech, Beijing, China) according to the manufacturer’s instructions. The expression patterns of MsBGLU genes were explored by using the SYBR Green RT-PCR Kit (Takara). The reaction was conducted as follows: 94 °C for 30 s, followed by 45 cycles of 94 °C, for 5 s, and 60 °C for 34 s. Each reaction was carried out in three biological repetitions, and the relative expression was calculated using the 2−∆∆Ct method. The results were analyzed by means ± SE. The Medicago actin gene was amplified as the reference gene to normalize the amount of the template. The primer sequences used in this study were shown in detail in Supplementary Table S1. The specific primers for qRT-PCR were designed using the Primer Premier 5 software and listed in Supplementary Table S7.
Availability of data and materials
RNA-seq raw data used in this study was downloaded from the SRA database in NCBI. It could be accessed with accession numbers PRJNA450305 and PRJNA454564. PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 6 May 2023).
References
Mammadov J, Buyyarapu R, Guttikonda SK, Parliament K, Abdurakhmonov IY, Kumpatla SP. Wild relatives of Maize, Rice, Cotton, and soybean: treasure troves for tolerance to biotic and abiotic stresses. Front Plant Sci. 2018;9: 886.
Türkan I, Demiral T. Recent developments in understanding salinity tolerance. Environ Exp Bot. 2009;67:2–9.
He F, Wei C, Zhang Y, Long R, Li M, Wang Z, et al. Genome-wide association analysis coupled with transcriptome analysis reveals candidate genes related to salt stress in alfalfa (Medicago sativa L). Front Plant Sci. 2021;12:826584.
VanEtten HD, Mansfield JW, Bailey JA, Farmer EE. Two classes of plant antibiotics: Phytoalexins versus Phytoanticipins. Plant Cell. 1994;6:1191–2.
Jones P, Vogt T. Glycosyltransferases in secondary plant metabolism: tranquilizers and stimulant controllers. Planta. 2001;213:164–74.
Suzuki H, Takahashi S, Watanabe R, Fukushima Y, Fujita N, Noguchi A, et al. An isoflavone conjugate-hydrolyzing beta-glucosidase from the roots of soybean (Glycine max) seedlings: purification, gene cloning, phylogenetics, and cellular localization. J Biol Chem. 2006;281:30251–9.
Chandrasekar B, Colby TD, Emran Khan Emon A, Jiang JJ, Hong TN, Villamor JG, et al. Broad-range glycosidase activity profiling. Mol Cell Proteomics. 2014;13:2787–800.
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The carbohydrate-active EnZymes database (CAZy): an expert resource for glycogenomics. NAR. 2008;37:D233-238.
Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. NAR. 2014;42:D490-495.
Henrissat B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J. 1991;280:309–16.
Opassiri R, Pomthong B, Onkoksoong T, Akiyama T, Esen A, Ketudat Cairns JR. Analysis of rice glycosyl hydrolase family 1 and expression of Os4bglu12 β-glucosidase. BMC Plant Biol. 2006;6: 33.
Ketudat Cairns JR, Esen A. β-Glucosidases. Cell Mol Life Sci. 2010;67:3389–405.
Ketudat Cairns JR, Mahong B, Baiya S, Jeon J-S. β-Glucosidases: Multitasking, moonlighting or simply misunderstood? Plant Sci. 2015;241:246–59.
Dong X, Jiang Y, Hur Y. Genome-wide analysis of Glycoside hydrolase family 1 β-glucosidase genes in Brassica rapa and their potential role in pollen development. Int J Mol Sci. 2019;20:E1663.
Zeng D, Que C, Teixeira da Silva JA, Xu S, Li D. Comparative transcriptomic and metabolic analyses reveal the molecular mechanism of ovule development in the orchid, Cymbidium sinense. Front Plant Sci. 2021;12:814275.
Morant AV, Bjarnholt N, Kragh ME, Kjærgaard CH, Jørgensen K, Paquette SM, et al. The β-glucosidases responsible for bioactivation of hydroxynitrile glucosides in Lotus japonicus. Plant Physiol. 2008;147:1072–91.
Chapelle A, Morreel K, Vanholme R, Le-Bris P, Morin H, Lapierre C, et al. Impact of the absence of stem-specific β-glucosidases on lignin and monolignols. Plant Physiol. 2012;160:1204–17.
Bednarek P, Piślewska-Bednarek M, Svatoš A, Schneider B, Doubský J, Mansurova MA, et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Sci Total Environ. 2009;323:101–6.
Wang K, Hersh HL, Benning C. SENSITIVE TO FREEZING2 aides in resilience to salt and drought in freezing-sensitive tomato. Plant Physiol. 2016;172:1432–42.
Lee KH, Piao HL, Kim H-Y, Choi SM, Jiang F, Hartung W, et al. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell. 2006;126:1109–20.
Ren R, Li D, Zhen C, Chen D, Chen X. Specific roles of Os4BGlu10, Os6BGlu24, and Os9BGlu33 in seed germination, root elongation, and drought tolerance in rice. Planta. 2019;249:1851–61.
Dong X, Deng H, Ma W, Zhou Q, Liu Z. Genome-wide identification of the MADS-box transcription factor family in autotetraploid cultivated alfalfa (Medicago sativa L.) and expression analysis under abiotic stress. BMC Genom. 2021;22:603.
Kaiwen G, Zisong X, Yuze H, Qi S, Yue W, Yanhui C, et al. Effects of salt concentration, pH, and their interaction on plant growth, nutrient uptake, and photochemistry of alfalfa (Medicago sativa) leaves. Plant Signal Behav. 2020;15: 1832373.
Mouradi M, Farissi M, Bouizgaren A, Makoudi B, Kabbadj A, Véry A-A, et al. Effects of water deficit on growth, nodulation and physiological and biochemical processes in Medicago sativa-rhizobia symbiotic association. Arid Land Res Manag. 2016;30:193–208.
Chen H, Zeng Y, Yang Y, Huang L, Tang B, Zhang H, et al. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nat Commun. 2020;11:2494.
Yang J, Ma L, Jiang W, Yao Y, Tang Y, Pang Y. Comprehensive identification and characterization of abiotic stress and hormone responsive glycosyl hydrolase family 1 genes in Medicago truncatula. Plant Physiol Biochem. 2021;158:21–33.
Bian Z, Wang D, Liu Y, Xi Y, Wang X, Meng S. Analysis of Populus glycosyl hydrolase family I members and their potential role in the ABA treatment and drought stress response. Plant Physiol Biochem. 2021;163:178–88.
Miyahara T, Sakiyama R, Ozeki Y, Sasaki N. Acyl-glucose-dependent glucosyltransferase catalyzes the final step of anthocyanin formation in Arabidopsis. J Plant Physiol. 2013;170:619–24.
Cao Y, Yang J, Liu T, Su Z, Zhu F, Chen M, et al. A phylogenetically informed comparison of GH1 hydrolases between Arabidopsis and rice response to stressors. Front Plant Sci. 2017;8:350.
Gómez-Anduro GA, Ceniceros-Ojeda EA, Casados-Vázquez LE, Bencivenni C, Sierra-Beltrán AP, Murillo-Amador B, et al. Genome-wide analysis of the beta-glucosidase gene family in maize (Zea mays L. var B73). Plant Mol Biol. 2011;77:159–83.
Kim D, Langmead B, Salzberg SLJNM. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–60.
Roepke J, Bozzo GG. Arabidopsis thaliana β-glucosidase BGLU15 Attacks flavonol 3-O-β-glucoside-7-O-α-rhamnosides. Phytochemistry. 2015;109:14–24.
Veitch NC. Isoflavonoids of the leguminosae. Nat Prod Rep. 2013;30:988–1027.
Xu Z-Y, Lee KH, Dong T, Jeong JC, Jin JB, Kanno Y, et al. A vacuolar β-glucosidase homolog that possesses glucose-conjugated abscisic acid hydrolyzing activity plays an important role in osmotic stress responses in Arabidopsis. Plant Cell. 2012;24:2184–99.
Wang C, Chen S, Dong Y, Ren R, Chen D, Chen X. Chloroplastic Os3BGlu6 contributes significantly to cellular ABA pools and impacts drought tolerance and photosynthesis in rice. New Phytol. 2020;226:1042–54.
Zamioudis C, Hanson J, Pieterse CMJ. β-Glucosidase BGLU42 is a MYB72-dependent key regulator of rhizobacteria-induced systemic resistance and modulates iron Deficiency responses in Arabidopsis roots. New Phytol. 2014;204:368–79.
Chen L, He F, Long R, Zhang F, Li M, Wang Z, et al. A global alfalfa diversity panel reveals genomic selection signatures in Chinese varieties and genomic associations with root development. J Integr Plant Biol. 2021;63:1937–51.
Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, et al. The pfam protein families database: towards a more sustainable future. NAR. 2016;44:D279-285.
Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. NAR. 2004;32:1792–7.
Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–3.
Letunic I, Bork P. Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. NAR. 2021;49:W293-296.
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13:1194–202.
Wang Y, Tang H, DeBarry JD, Tan X, Li J, Wang X, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. NAR. 2012;40:e49.
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. NAR. 2002;30:325–7.
Acknowledgements
Not applicable.
Funding
This work was supported by the Project of Science and Technology Innovation 2030 (2022ZD04011), China Agriculture Research System of MOF and MARA (CARS-34), the Key Research and Development Program of Shaanxi Province (2022ZDLNY01-07), and Ph.D. Start-up Fund of Northwest A&F University (Grant No: 2452021055).
Author information
Authors and Affiliations
Contributions
Kong HM and Cao YM planned and designed the study; Kong HM prepared the figures and tables and wrote the manuscript; Song JX carried out the expression analysis and supervised the experiments; Ma SH, Yang J, Li Q, Li ZX, and Xie ZG curated the software and data; Kong HM and Song JX performed the experiments; Shao ZT, Yang PZ and Cao YM reviewed and edited the manuscript; Xie ZG, Cao YM and Yang PZ provide resources and fund support; All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Protein property of MsBGLU proteins. Table S2. Information for each MsBGLU motif. Table S3. Number of homologous gene pairs. Table S4. One-to-one orthologous relationships between M.sativa, A. thaliana, G.max and M.truncatula. Table S5. The BGLU protein sequence in Medicago sativa. Table S6. Name and position of cis-acting elements in MsBGLUs. Table S7. List of primers used in this research. Fig. S1. Phylogenetic relationships, conserved patterns, and gene structure of BGLU in M. sativa. The motifs, numbered 1-10, are displayed in different colored boxes. Fig. S2. Analysis of cis-acting elements in the putative promoter of MsBGLUs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kong, H., Song, J., Ma, S. et al. Genome-wide identification and expression analysis of the glycosyl hydrolase family 1 genes in Medicago sativa revealed their potential roles in response to multiple abiotic stresses. BMC Genomics 25, 20 (2024). https://doi.org/10.1186/s12864-023-09918-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09918-w