Abstract
Background
The composition and content of fatty acids in the breast muscle are important factors influencing meat quality. In this study, we investigated the fatty acid composition and content in the breast muscle of Gushi chickens at different developmental stages (14 weeks, 22 weeks, and 30 weeks). Additionally, we utilized transcriptomic data from the same tissue and employed WGCNA and module identification methods to identify key genes associated with the fatty acid composition in Gushi chicken breast muscle and elucidate their regulatory networks.
Results
Among them, six modules (blue, brown, green, light yellow, purple, and red modules) showed significant correlations with fatty acid content and metabolic characteristics. Enrichment analysis revealed that these modules were involved in multiple signaling pathways related to fatty acid metabolism, including fatty acid metabolism, PPAR signaling pathway, and fatty acid biosynthesis. Through analysis of key genes, we identified 136 genes significantly associated with fatty acid phenotypic traits. Protein–protein interaction network analysis revealed that nine of these genes were closely related to fatty acid metabolism. Additionally, through correlation analysis of transcriptome data, we identified 51 key ceRNA regulatory networks, including six central genes, 7 miRNAs, and 28 lncRNAs.
Conclusion
This study successfully identified key genes closely associated with the fatty acid composition in Gushi chicken breast muscle, as well as their post-transcriptional regulatory networks. These findings provide new insights into the molecular regulatory mechanisms underlying the flavor characteristics of chicken meat and the composition of fatty acids in the breast muscle.
Similar content being viewed by others
Background
With the improvement of living standards, people are paying increasing attention to the taste and nutritional quality of meat. The breast muscle of chicken is highly favored due to its rich content of fatty acids, which directly influence the taste, flavor, tenderness, and nutritional value of chicken meat [1]. However, the composition and content of these fatty acids are regulated by various factors such as genetics and nutrition [2,3,4,5]. Therefore, it is of great significance to understand the molecular regulatory mechanisms behind the fatty acid composition and content in chicken breast muscle. This understanding can provide guidance for developing nutritional strategies or conducting genetic improvements, thereby optimizing the taste, flavor, and nutritional value of chicken breast muscle. However, the molecular regulatory mechanisms underlying these characteristics are currently not well understood.
As a renowned local chicken breed in China, Gushi chicken is highly acclaimed for its delicious taste, unique flavor, and nutritional richness. It is well known that the texture and tenderness of chicken breast are closely associated with the content and composition of its fatty acids. Abundant fat content gives the meat a richer taste and increased tenderness, resulting in juicier meat [6, 7]. Additionally, the presence of unsaturated fatty acids improves the flexibility of muscle fibers [7]. The nutritional value of different fatty acids is also a key factor influencing meat quality. According to nutritional standards, increasing the intake of polyunsaturated fatty acids (PUFAs) can reduce the risk of cardiovascular diseases, certain cancers, asthma, and diabetes [8,9,10]. Therefore, maintaining a balance between unsaturated and saturated fatty acids and reducing the intake of saturated fats is an effective strategy for lowering the risk of these diseases [11]. In comparison to other meat products, chicken meat is rich in higher levels of unsaturated fatty acids and lower levels of saturated fats, conversely, pork and ruminant meats have higher levels of saturated fats and lower levels of polyunsaturated fatty acids [12,13,14]. Therefore, further study on the composition and content of chicken breast muscle fat is of great significance to meat quality and healthy diet.
Previous studies on the relationship between fatty acids and meat quality have primarily focused on the field of nutrition, often describing the composition and content of fatty acids in specific meat types without considering their connection to gene regulation. However, the application of transcriptome sequencing technology and Weighted Gene Co-expression Network Analysis (WGCNA) offers a new approach to explore the relationship between phenotype and gene expression profiles [15]. For example, WGCNA analysis of beef fatty acids has identified insulin and MAPK signaling pathways as central pathways associated with fatty acids, and ACTA1 and ALDOA as potential regulators of fatty acid synthesis [16]. In the poultry field, Gai et al. performed a combined analysis of transcriptome and metabolome using WGCNA in Beijing-You chickens and found that CBS, GATM, GAD6, PNPLA1, ItaE, and AMPD1 were associated with the accumulation of flavor components [17]. Li et al. conducted WGCNA analysis on fat content and six fatty acids in Gushi chickens, identifying genes related to fatty acid metabolism, phospholipid metabolism, and cholesterol metabolism [18]. Furthermore, the study found that non-coding RNAs are also involved in the regulation of biological processes related to intramuscular fat deposition [19]. For instance, gga-miR-140-5p promotes chicken intramuscular adipocyte differentiation by targeting RXRG, while gga-miR-18b-3p acts as an inhibitor of intramuscular adipocyte differentiation by targeting ACOT13 [20, 21]. Currently, research on lncRNAs and their competing endogenous RNA (ceRNA) regulatory networks related to fatty acids is relatively limited. One example of ceRNA regulation is the lncRNA MFNCR, which stimulates the differentiation of intramuscular adipocytes in chickens by sequestering miR-128-3p and miR-27b-3p [22]. Therefore, due to the limitations of previous fatty acid determination methods and transcriptome technologies, the composition, content, and molecular regulatory mechanisms of fatty acids in Gushi chicken breast muscle remain unclear.
In the Gushi chicken production process, the chickens are at different developmental stages: 14 weeks for muscle development, 22 weeks for adipocyte development, and 30 weeks for the peak of egg production. Therefore, in this study, we utilized three different developmental stages of breast muscle tissue from the Gushi chicken to conduct the following investigations: (1) Accurately constructing the fatty acid profile in the breast muscle of the Gushi chicken to understand the variations in fatty acid composition and content across different stages. (2) Utilizing WGCNA to identify potential genes and signaling pathways that influence the composition and content of fatty acids in the pectoralis muscle, revealing key genes involved in the formation of fatty acid meat quality traits. (3) Establishing a post-transcriptional ceRNA regulatory network for fatty acids in the pectoralis muscle of the Gushi chicken to investigate the molecular regulatory network governing the meat quality traits of fatty acids at the transcriptional level. These research findings contribute to a deeper understanding of the nutritional characteristics and meat quality formation mechanisms in chicken, providing scientific evidence and guidance for chicken production and meat quality improvement.
Results
Phenotypic data associated with FA composition
Through GC–MS analysis, a total of 49 fatty acids were detected in the breast muscle tissue of Guishi chickens at three different developmental stages. Additionally, 16 metabolic characteristics of fatty acids were calculated (Table S1). Among them, there were 14 saturated fatty acids (SFA), 21 monounsaturated fatty acids (MUFA), and 14 polyunsaturated fatty acids (PUFA). SFA, MUFA, and PUFA accounted for approximately 51.95% to 64.63%, 23.70% to 34.80%, and 11.66% to 13.26% of the total fatty acid content, respectively. Furthermore, eight dominant fatty acids were identified in the breast muscle of Gushi chickens. These included two saturated fatty acids, namely palmitic acid (C16:0) and stearic acid (C18:0), as well as six unsaturated fatty acids, including vaccenic acid (C18:1N12), oleic acid (C18:1N9C), arachidonic acid (C20:4N6), palmitoleic acid (C14:1T), and linoleic acid (C18:2N6). These data will be utilized for the correlation analysis between gene co-expression modules and phenotypic traits, contributing to the further investigation of the relationship between genes and fatty acid phenotypic characteristics.
Weighted gene co-expression network construction and module identification
The WGCNA network of 12,062 genes was performed using the WGCNA R software package. Through calculation, we find that when the soft threshold β = 28, the scale-free network was guaranteed, because the scale independence reached 0.9 (Fig. 1A) and had a relatively high-average connectivity (Fig. 1B). A total of 20 gene co-expression modules were identified, based on hierarchical clustering of the calculated dissimilarity (Fig. 1C). The largest module contains 2899 genes, the smallest module contains 33 genes, and the number of genes in the specific module was shown in Table S2. In addition, 928 uncorrelated genes were assigned into a gray module that was ignored in further analysis, and the information of genes in all module was shown in Table S3.
Weighted gene co-expression network construction and module identification. A Scale-free index analysis of various soft threshold powers (β). B Average connectivity analysis of various soft threshold powers. C Clustering dendrogram of genes, with dissimilarity based on topological overlap, together with assigned module colors
FA composition trait-related modules in the breast muscle
In order to obtain specific modules that might be involved in FA composition in the breast muscle, this study evaluated the modular trait association between the 19 modules and 65 phenotypic traits. The results showed that six modules were highly correlated with fatty acid content and fatty acid metabolism traits (Fig. 2). Among the six modules, the purple and lightyellow modules were significant negative correlation to the C16:1 T, C17:1 T, Fatty.AI and Fatty,TI; the purple, lightyellow and blue modules were significant positive correlation to the C16:1, C18:1N7T, C18:1N12, C18:1N9C, C18:1N7, MUFA content, PUFA content, UFA content, UFA/SFA ratio, MUFA/SFA ratio, PUFA/SFA ratio, DBI, and UI in the breast muscle. In addition, the brown module was significantly positively correlated with C15:0, C16:0, C18:2N6 and C17:0; the green module was significantly positively correlated with C20:3N3, C24:0 and C22:6N3; the red module was significantly positively correlated with C20:2, C20:3N6 and C20:5N3. Through the cluster analysis of characteristic genes, the results show that the 19 modules could be divided into three clusters, and the modules had a high degree of interaction connectivity (Fig. 3). Among them, the purple and lightyellow modules belong to the same subclusters. In addition, the blue, brown and green modules belong to the same clusters, this suggests that these three modules had similar expression profiles. The red modules separately belong to another cluster. This suggests that genes in these six modules may play key roles during FA composition in the breast muscle of chickens.
Relationships between modules and FA composition traits in the breast muscle of Gushi chicken. Each row in the table corresponds to a module and each column to a trait. Boxes contains Pearson correlation coefficients and their associated p values. Red color indicates a positive correlation between modules and traits, while green color indicates a negative correlation between modules and traits, only modules with a p-value < 0.05 are displayed
Relationships between modules. Branches of the dendrogram (the meta‐modules) group together eigengenes that are positively correlated. Each row and column in the heatmap corresponds to one module eigengene (labeled by color). Red represents high adjacency (positive correlation), while blue represents low adjacency (negative correlation). Squares of red color along the diagonal are the meta‐modules
Enrichment analyses of module genes identified by weighted gene co-expression network analysis
In order to explore the biological functions related to genes in six modules highly related to fatty acid composition, we conducted functional enrichment analysis. The results of GO enrichment analysis showed that the blue module was significantly enriched in the process of macromolecular catabolism, including protein catabolic process, cellular macromolecule catabolic process, etc. In the purple module, significant enrichment of ubiquitin-protein transferase activity related GO terms was found. In addition, the GO terms that were significantly enriched (corrected P < 0.05) were not found in the green, brown, lightyellow and red modules. The results of KEGG enrichment analysis showed that the blue module was significantly enriched in lysine degradation, citrate cycle (TCA cycle), cysteine and methionine metabolism, inositol phosphate metabolism and oxidative phosphorylation, etc. No significantly enriched signaling pathways were found in the other modules. Based on the above results, among the six modules, we identified 31 pathways associated with lipid metabolism or fat deposition (Fig. 4), including fatty acid metabolism, PPAR signaling pathway, fatty acid biosynthesis, biosynthesis of unsaturated fatty acids, etc. These results indicate that the genes in these modules may affect the fatty acid composition of chicken breast muscle through the above pathways or biological processes.
The identification of hub genes and regulatory network construction
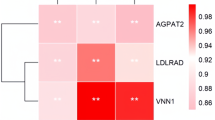
Based on the criteria of |module membership (MM)|≥ 0.8 and |gene significance (GS)|≥ 0.2, we have identified a set of pivotal genes from different colored modules. Specifically, the blue, brown, green, light yellow, purple, and red modules yielded 72, 37, 6, 3, 7, and 10 pivotal genes, respectively. Through protein–protein interaction (PPI) network analysis, we have successfully identified a significantly enriched lipid metabolism pathway consisting of 38 genes (Fig. 5A). It is noteworthy that these nine genes are closely related to fatty acid metabolism, including ACOT7, HSD17B4, ALDH3A2, CPT1A, ELOVL1, EPHX2, CPT2, GPX1, and HSD17B12. Additionally, through an association analysis of transcriptomic data encompassing miRNA and lncRNA from breast muscle samples across four developmental stages, we have discovered a total of 51 crucial ceRNA regulatory networks, comprising 6 hub genes, 7 miRNA, and 28 lncRNA (Fig. 5B). Notably, these 6 hub genes (RAPGEF1, AGPAT2, PISD, PCYT1A, PHOSPHO1, ELOVL1) were closely associated with the synthesis and metabolism of fatty acids.
The interaction network and ceRNA regulatory network of hub genes related to lipid metabolism pathway. A The network was constructed using Cytoscape 3.7 software. The node size and edge number are proportional to degree and connection strength, respectively. B The lncRNA-miRNA-mRNA ceRNA network related to lipid metabolism pathway. Green represents mRNA, red represents miRNA, and blue represents lncRNA
Discussion
It is widely acknowledged that the types and levels of fatty acids are widely recognized for their impact on the flavor of meat [7]. In order to gain a comprehensive understanding of the hub genes and their post-transcriptional regulatory networks involved in fatty acid regulation in the breast muscles of Gushi chickens, we quantified the contents of 49 different fatty acids in Gushi chicken breast muscle at different developmental stages, the related data of 16 kinds of fatty acid metabolism were calculated. By combining WGCNA with previous transcriptomic data, we have identified six modules that are highly correlated with fatty acid phenotypic traits. Subsequently, we conducted enrichment analysis and identified 136 crucial genes that have a significant impact on fatty acid production by filtering hub genes associated with GS and MM. Lastly, we constructed 51 ceRNA regulatory networks centered around the hub genes by integrating miRNA and lncRNA data.
The determination of fatty acids in the breast muscle of Gushi chickens revealed that C16:0, C18:0, C18:1N12, C18:1N9C, C20:4N6, C18:1N7, C18:2N6, and C14:1T are the predominant fatty acids. Most of these predominant fatty acids align with findings from studies on other chicken breeds [23, 24]. Saturated fatty acids play a crucial role in muscle fatty acid composition, with C16:0 being the most abundant long-chain saturated fatty acid in Gushi chicken breast muscle. Long-chain fatty acids serve various important functions in the human body, such as providing energy, forming cell membranes, and participating in hormone synthesis, making them essential components of the diet [25,26,27]. Compared to other livestock and poultry, chicken meat exhibits higher levels of unsaturated fatty acids, which is consistent with the findings of this study [12]. Among the detected predominant fatty acids are C18:1N9C, C18:2N6, and C20:4N6. C18:2N6 is an essential fatty acid that cannot be synthesized by the human body and must be obtained through dietary intake [28]. It plays a critical role in maintaining the stability and function of cell membranes and it is involved in the synthesis of various bioactive substances, such as anti-inflammatory agents and cell signaling molecules [29,30,31]. These findings further highlight the primary reasons for the rich nutritional composition of Gushi chicken meat.
In order to further investigate the relationship between genes and fatty acid phenotypes, we conducted WGCNA analysis and defined a total of 19 modules. Among these modules, the blue, brown, green, light yellow, purple, and red modules showed the highest correlation with fatty acid phenotypes. The enrichment results of these six modules encompassed various signaling pathways related to lipid synthesis and metabolism, including fatty acid metabolism, glycerolipid metabolism, fatty acid biosynthesis, PPAR signaling pathway, cellular lipid degradation, and insulin signaling pathway. Through analysis of hub genes, we identified 136 genes that are associated with fatty acid phenotypes. PPI analysis revealed the involvement of multiple genes in fatty acid metabolism. For example, ELOVL1 is involved in fatty acid chain elongation in the process of fatty acid synthesis [32]. It catalyzes the conversion of C18:00 fatty acyl-CoA to C20 and C22 fatty acids in the pathway of fatty acid synthesis [33]. Previous studies have shown that ELOVL1 is upregulated during chicken preadipocyte differentiation, suggesting its important role in the accumulation of lipid droplets in chicken adipocytes [34]. Furthermore, CPT1A catalyzes the transfer reaction between long-chain fatty acids and carnitine, facilitating the transport of fatty acids into mitochondria for fatty acid oxidation and energy production [35, 36]. A study found that downregulation of CPT1A leads to reduced fatty acid oxidation and increased accumulation of intramuscular fat in chickens [37]. Additionally, genes such as HSD17B4, ALDH3A2, EPHX2, CPT2, HSD17B12, and GPX1 are also involved in the oxidation process of fatty acids, although their specific roles may vary [38,39,40,41,42]. During the development of the Gushi chicken breast muscle, these genes may influence intramuscular fat content and fatty acid composition to varying degrees through their involvement in fatty acid oxidation. In addition, within the turquoise module, we identified the DYNLL2 gene, which had previously been established in our earlier research as playing a pivotal role in the proliferation and differentiation of myoblasts in breast muscles [43]. In this study, the turquoise module housing this gene is not correlated with the traits related to breast muscle fatty acid composition. This observation serves to reinforce the accuracy of our study's findings, shedding light on an objective reality.
The main component of fat in muscles is phospholipids, which account for approximately 60% to 70% of muscle fat content [44]. Phospholipids primarily consist of components containing C16 and C18 fatty acids, which are major factors influencing the volatile flavor of meat [45, 46]. In this study, we identified nine key genes associated with the biosynthesis pathway of phospholipids. These genes are PEMT, DGAT2, CRLS1, PLA2G6, AGK, LPCAT3, AGPAT5, EPT1, and AGPAT. AGPAT2 and AGPAT5 are intermediate enzymes involved in the biosynthesis of phospholipids and triglycerides (TAGs) in the glycerol-3-phosphate pathway [47]. AGPAT2 has been shown to play an important role in lipid metabolism in chickens [46]. AGPAT5, on the other hand, is considered a potential genetic marker for excellent pork quality [48]. In a study conducted on cattle, AGPAT5 was found to be significantly correlated with pre-slaughter weight, carcass weight, and meat weight [49]. Additionally, DGAT2 is a key gene involved in regulating the synthesis of triglycerides (TGs), as it facilitates the storage of excess fatty acids in lipid droplets [47]. In other studies, DGAT2 has been suggested to potentially regulate the deposition of intramuscular fat (IMF) in chicken meat [50]. These genes may regulate lipid deposition in the pectoralis muscle of Gushi chickens through the biosynthesis of phospholipids.
In this study, through the combined analysis with previous transcriptome data, a transcriptional post-regulatory network of some hub genes was discovered. Specifically, miR-34a-5p is likely regulated by multiple lncRNAs, thereby affecting the expression of AGPAT2. It is known that miR-34a-5p increases intracellular triglyceride and total cholesterol levels in chicken liver by inhibiting the ACSL1 gene [51]. In intramuscular fat, miR-34a-5p may regulate fat deposition through different pathways. Additionally, there is a potential regulatory network between miR-130b-3p and PISD, PCYT1A, and PHOSPHO1, which is regulated by galLB_NC_4846. miR-130b-3p is known to negatively regulate the accumulation of lipid droplets in goat intramuscular preadipocytes [52]. PISD and PCYT1A are associated with fatty acid synthesis and metabolism [47]. Furthermore, there is a potential targeting relationship between ELOVL1 and miR-22-3p. miR-22-3p is known to target ELOVL6 to regulate lipid synthesis in chicken liver [53]. Therefore, miR-22-3p may affect fat deposition in muscle by regulating ELOVL1. These findings suggest that post-transcriptional regulatory mechanisms, especially miRNAs and lncRNAs, play an important regulatory role in intramuscular fat deposition.
Conclusions
In conclusion, based on the data from fatty acids and transcriptomics in the breast muscles of Gushi chickens, we have identified signaling pathways highly associated with fatty acid content and composition. Through an analysis focused on hub genes and post-transcriptional regulatory mechanisms, we have defined a number of key genes and their interconnected ceRNA regulatory network. The study of these key genes may provide us with new insights into the formation of the flavor characteristics in the meat of Gushi chickens.
Materials and methods
Sample collection
In this study, Gushi chicken, a Chinese local chicken breed was used as the experimental animal from the Animal Center of Henan Agricultural University. Female chickens were raised in ladder cage under the same environment and standard feeding conditions. The feeding method was the same as our previous study [54]. In this study, three healthy chickens were randomly selected at 14, 22, and 30 weeks of age. Therefore, nine chickens were used in this study. Chickens were anesthetized by intravenous injection of sodium pentobarbital (40 mg/kg) at a concentration of 0.2% in the wing vein. Under deep anesthesia, these individuals were euthanized by intravenous KCL (1 ~ 2 mg/kg). Then their breast muscles tissues were collected, immediately frozen in liquid nitrogen and stored at − 80℃.
Collection of phenotypic data with meat traits
To obtain meat quality trait phenotypic data, we analyzed the different fatty acid composition contents of Gushi chicken breast muscles tissues at three developmental stages: 14, 22, and 30 weeks of age. The content of fatty acids in breast muscle tissue was performed by Trace 1310-ISQ 7000 gas chromatography-mass spectrometry (GC–MS) (Thermo Fisher Scientific, Shanghai, China), in accordance with the PRC National Standard “Meat and meat products—determination of fatty acids (GB/T 9695.2–2008).” In plain terms, we assessed the 49 kinds of fatty acids content in Gushi chicken breast muscle tissue and 16 kinds of fatty acid metabolism traits. The fatty acids content was expressed as the percentage of the breast muscle by fresh weight (μg/g).
mRNA expression data
The processing and analysis of mRNA expression data from the previously constructed Gushi chicken breast muscle transcriptome expression profile [55], with the sample accession of mRNA expression data of PRJNA516810. A total of 16,682 expressed genes were identified in Gushi chicken breast muscle tissue. Using the R language for data filtering, removing all genes that have a count of less than say 10 in more than 90% of the samples, the code was "gene_filter <—readcount[apply(readcount, 1, function(x) sum(x > 10) > (0.9 * ncol(readcount))),]". The resulting set of genes, composed of 12,062 genes post-filtering, was normalized using the CPM function from the edgeR package and then used in the subsequent WGCNA analysis.
Weighted gene co-expression network analysis
Weighted gene co-expression network was constructed for 12,062 genes using the WGCNA R software package [15]. Firstly, the Pearson correlation coefficient between each gene pair is calculated by using CPM value of selected genes, which is expressed as the connection strength between genes. The appropriate β value was selected through he R function PickSoftThreshold. Subsequently, we converted the adjacency matrix into the topological overlap matrix (TOM) to minimize the effects of noise and false positives by considering the (indirect) calculation method of intermediate nodes. Next, hierarchical clustering was used to identify modules and merged similar modules when each module contained at least 30 genes (minModuleSize = 30) and the threshold of merging similar modules was 0.25 (mergeCutHeight = 0.25).
Construct module-trait relationships
In order to identify the modules significantly related to FA composition, we performed principal component analysis on the gene expression matrix in each module, and used the module eigengene (ME) to represent the first principal component (PC1) of each module. Then, the modular trait association between MEs and phenotypic traits was evaluated by Pearson correlation coefficient. The correlation between each module and each trait was also indicated by the heatmap.
Functional enrichment analysis of genes in modules of interest
In order to explore the biological function of module genes related to fatty acid composition in breast muscle of Gushi chicken, R software package clusterProfile 4.0 was used for enrichment analyses of Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) [56]. The P-value < 0.05 was used as the enrichment cut-off criterion, and the visualization was performed using ggplot2.
Hub genes analysis
Under the weighted co-expression network, highly connected genes are called hub genes, which may play an important role in the biological regulatory mechanisms of the living organism. In this network, hub gene was determined by the correlation between gene expression profile and module membership (MM) and the correlation between gene and external traits (GS). Thus, the intramodular hub genes were chosen by |GS|> 0.2 and |MM|> 0.8 with a threshold of P-value < 0.05.
The expression of genes was controlled by various factors, such as the regulation of non-coding RNA, including miRNA, lncRNA, etc. Therefore, we used the identified differential miRNA and differential lncRNA data at 14, 22, 30 weeks from the same RNA sample transcriptome and small RNA library collected from the breast muscle tissues of Gushi chicken [54, 57]. The data were used to construct the lncRNA-miRNA-mRNA regulatory network by association analysis of hub gene, which is highly correlated with the fatty acid composition of the breast muscles. The Cytoscape 3.7 software was used for the visualization of the network [58].
Availability of data and materials
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information files. All the raw sequences have been deposited in the NCBI database Sequence Read Archive with the BioProject number PRJNA516810 (mRNA and lncRNA) and PRJNA516961 (miRNA).
References
Dal Bosco A, Cartoni Mancinelli A, Vaudo G, Cavallo M, Castellini C, Mattioli S. Indexing of fatty acids in poultry meat for its characterization in healthy human nutrition: a comprehensive application of the scientific literature and new proposals. Nutrients. 2022;14(15):3110.
Vanden Heuvel JP. Nutrigenomics and nutrigenetics of ω3 polyunsaturated fatty acids. Prog Mol Biol Transl Sci. 2012;108:75–112.
El-Bahr S, Shousha S, Shehab A, Khattab W, Ahmed-Farid O, Sabike I, El-Garhy O, Albokhadaim I, Albosadah K. Effect of dietary microalgae on growth performance, profiles of amino and fatty acids, antioxidant status, and meat quality of broiler chick ens. Animals. n.d. 10(5):761.
Pewan SB, Otto JR, Huerlimann R, Budd AM, Mwangi FW, Edmunds RC, Holman BWB, Henry MLE, Kinobe RT, Adegboye OA et al. Next generation sequencing of single nucleotide polymorphic DNA-marker s in selecting for intramuscular fat, fat melting point, omega-3 long- chain polyunsaturated fatty acids and meat eating quality in Tattykeel Australian White MARGRA Lamb. Foods. n.d. 10(10):2288.
Lemos MVAd, Pereira ASC, Regatieri IC, Feitosa FLB, Baldi F. Genetic factors that determine the meat fatty acids composition: InTech; 2017.
Wood JD, Enser M, Fisher AV, Nute GR, Sheard PR, Richardson RI, Hughes SI, Whittington FM. Fat deposition, fatty acid composition and meat quality: a review. Meat Sci. n.d. 78(4):343–358.
Wood JD, Richardson RI, Nute GR, Fisher AV, Campo MM, Kasapidou E, Sheard PR, Enser M. Effects of fatty acids on meat quality: a review. Meat Sci. 2004;66(1):21–32.
Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21(6):495–505.
Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56(8):365–79.
Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008;233(6):674–88.
Efsa Panel on Dietetic Products N, Allergies. Scientific opinion on dietary reference values for fats, including sat urated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010;8:1461.
Wood JD, Enser M. Factors influencing fatty acids in meat and the role of antioxidants in improving meat quality. Br J Nutr. 1997;78(Suppl 1):S49-60.
Figueiredo IL, Claus T, Oliveira Santos Júnior O, Almeida VC, Magon T, Visentainer JV. Fast derivatization of fatty acids in different meat samples for gas chromatography analysis. J Chromatogr A. 2016;1456:235–41.
Rule DC, Broughton KS, Shellito SM, Maiorano G. Comparison of muscle fatty acid profiles and cholesterol concentrations of bison, beef cattle, elk, and chicken. J Anim Sci. 2002;80(5):1202–11.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559.
de Oliveira PSN, Coutinho LL, Cesar ASM, Diniz W, de Souza MM, Andrade BG, Koltes JE, Mourão GB, Zerlotini A, Reecy JM, et al. Co-expression networks reveal potential regulatory roles of miRNAs in fatty acid composition of nelore Cattle. Front Genet. 2019;10:651.
Gai K, Ge Y, Liu D, Zhang H, Cong B, Guo S, Liu Y, Xing K, Qi X, Wang X, et al. Identification of key genes affecting flavor formation in Beijing-You chicken meat by transcriptome and metabolome analyses. Foods. 2023;12(5):1025.
Li G, Zhao Y, Li Y, Chen Y, Jin W, Sun G, Han R, Tian Y, Li H, Kang X. Weighted gene coexpression network analysis identifies specific transcriptional modules and hub genes related to intramuscular fat traits in chicken breast muscle. J Cell Biochem. 2019;120(8):13625–39.
Gong Y, Lin Z, Wang Y, Liu Y. Research progress of non-coding RNAs regulation on intramuscular adipocytes in domestic animals. Gene. 2023;860:147226.
Zhang M, Li DH, Li F, Sun JW, Jiang RR, Li ZJ, Han RL, Li GX, Liu XJ, Kang XT, et al. Integrated analysis of MiRNA and genes associated with meat quality reveals that Gga-MiR-140-5p affects intramuscular fat deposition in chickens. Cell Physiol Biochem. 2018;46(6):2421–33.
Sun G, Li F, Ma X, Sun J, Jiang R, Tian Y, Han R, Li G, Wang Y, Li Z, et al. gga-miRNA-18b-3p inhibits intramuscular adipocytes differentiation in chicken by targeting the ACOT13 Gene. Cells. 2019;8(6):556.
Zhang M, Li F, Sun JW, Li DH, Li WT, Jiang RR, Li ZJ, Liu XJ, Han RL, Li GX, et al. LncRNA IMFNCR promotes intramuscular adipocyte differentiation by sponging miR-128-3p and miR-27b-3p. Front Genet. 2019;10:42.
Milićević D, Vranić D, Mašić Z, Parunović N, Trbović D, Nedeljković-Trailović J, Petrović Z. The role of total fats, saturated/unsaturated fatty acids and cholesterol content in chicken meat as cardiovascular risk factors. Lipids Health Dis. 2014;13:42.
Ponte PI, Alves SP, Bessa RJ, Ferreira LM, Gama LT, Brás JL, Fontes CM, Prates JA. Influence of pasture intake on the fatty acid composition, and cholesterol, tocopherols, and tocotrienols content in meat from free-range broilers. Poult Sci. 2008;87(1):80–8.
Lapillonne A, Clarke SD, Heird WC. Plausible mechanisms for effects of long-chain polyunsaturated fatty acids on growth. J Pediatr. 2003;143(4 Suppl):S9-16.
Kyselová L, Vítová M, Řezanka T. Very long chain fatty acids. Prog Lipid Res. 2022;87:101180.
Abshirini M, Ilesanmi-Oyelere BL, Kruger MC. Potential modulatory mechanisms of action by long-chain polyunsaturated fatty acids on bone cell and chondrocyte metabolism. Prog Lipid Res. 2021;83:101113.
Spector AA, Kim HY. Discovery of essential fatty acids. J Lipid Res. 2015;56(1):11–21.
Chilton FH, Murphy RC, Wilson BA, Sergeant S, Ainsworth H, Seeds MC, Mathias RA. Diet-gene interactions and PUFA metabolism: a potential contributor to health disparities and human diseases. Nutrients. 2014;6(5):1993–2022.
Sauer LA, Dauchy RT, Blask DE. Polyunsaturated fatty acids, melatonin, and cancer prevention. Biochem Pharmacol. 2001;61(12):1455–62.
Kris-Etherton PM, Hecker KD, Binkoski AE. Polyunsaturated fatty acids and cardiovascular health. Nutr Rev. 2004;62(11):414–26.
Schackmann MJ, Ofman R, Dijkstra IM, Wanders RJ, Kemp S. Enzymatic characterization of ELOVL1, a key enzyme in very long-chain fatty acid synthesis. Biochim Biophys Acta. 2015;1851(2):231–7.
Nie L, Pascoa TC, Pike ACW, Bushell SR, Quigley A, Ruda GF, Chu A, Cole V, Speedman D, Moreira T, et al. The structural basis of fatty acid elongation by the ELOVL elongases. Nat Struct Mol Biol. 2021;28(6):512–20.
Wang D, Li X, Zhang P, Cao Y, Zhang K, Qin P, Guo Y, Li Z, Tian Y, Kang X, et al. ELOVL gene family plays a virtual role in response to breeding selection and lipid deposition in different tissues in chicken (Gallus gallus). BMC Genomics. 2022;23(1):705.
Du W, Zhang L, Brett-Morris A, Aguila B, Kerner J, Hoppel CL, Puchowicz M, Serra D, Herrero L, Rini BI, et al. HIF drives lipid deposition and cancer in ccRCC via repression of fatty acid metabolism. Nat Commun. 2017;8(1):1769.
Schlaepfer IR, Joshi M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology. 2020;161(2):bqz046.
Qiu F, Xie L, Ma JE, Luo W, Zhang L, Chao Z, Chen S, Nie Q, Lin Z, Zhang X. Lower expression of SLC27A1 enhances intramuscular fat deposition in chicken via down-regulated fatty acid oxidation mediated by CPT1A. Front Physiol. 2017;8:449.
Violante S, Achetib N, van Roermund CWT, Hagen J, Dodatko T, Vaz FM, Waterham HR, Chen H, Baes M, Yu C, et al. Peroxisomes can oxidize medium- and long-chain fatty acids through a pathway involving ABCD3 and HSD17B4. Faseb j. 2019;33(3):4355–64.
Kitamura T, Seki N, Kihara A. Phytosphingosine degradation pathway includes fatty acid α-oxidation reactions in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2017;114(13):E2616-e2623.
Zhou Y, Li X, Guan A, Zhou H, Zhu Y, Wang R, Li R. EPHX2 inhibits colon cancer progression by promoting fatty acid degradation. Front Oncol. 2022;12:870721.
Jiang N, Xie B, Xiao W, Fan M, Xu S, Duan Y, Hamsafar Y, Evans AC, Huang J, Zhou W, et al. Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion. Nat Commun. 2022;13(1):1511.
Li D, Yao H, Du L, Zeng X, Xiao Q. Thallium(I and III) exposure leads to liver damage and disorders of fatty acid metabolism in mice. Chemosphere. 2022;307(Pt 1):135618.
Li Y, Yuan P, Fan S, Zhai B, Jin W, Li D, Li H, Sun G, Han R, Liu X, et al. Weighted gene co-expression network indicates that the DYNLL2 is an important regulator of chicken breast muscle development and is regulated by miR-148a-3p. BMC Genomics. 2022;23(1):258.
Mougios V, Petridou A. Analysis of lipid profiles in skeletal muscles. Methods Mol Biol. 2012;798:325–55.
Chen DW, Balagiannis DP, Parker JK. Use of egg yolk phospholipids to generate chicken meat odorants. Food Chem. 2019;286:71–7.
Wang DY, Zhu YZ, Xu WM. Composition of intramuscular phospholipids and free fatty acids in three kinds of traditional Chinese duck meat products. Poult Sci. 2009;88(1):221–6.
Takeuchi K, Reue K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab. 2009;296(6):E1195-1209.
Molinero E, Pena RN, Estany J, Ros-Freixedes R. Identification of a missense variant in the porcine AGPAT gene family associated with intramuscular fat content through whole-genome sequencing. Anim Genet. 2022;53(6):782–93.
Edea Z, Jung KS, Shin SS, Yoo SW, Choi JW, Kim KS. Signatures of positive selection underlying beef production traits in Korean cattle breeds. J Anim Sci Technol. 2020;62(3):293–305.
Liu L, Cui H, Fu R, Zheng M, Liu R, Zhao G, Wen J. The regulation of IMF deposition in pectoralis major of fast- and slow- growing chickens at hatching. J Anim Sci Biotechnol. 2017;8:77.
Tian WH, Wang Z, Yue YX, Li H, Li ZJ, Han RL, Tian YD, Kang XT, Liu XJ. miR-34a-5p increases hepatic triglycerides and total cholesterol levels by regulating ACSL1 protein expression in laying hens. Int J Mol Sci. 2019;20(18):4420.
Li Y, He C, Ran L, Wang Y, Xiong Y, Wang Y, Zhu J, Lin Y. miR-130b duplex (miR-130b-3p/miR-130b-5p) negatively regulates goat intramuscular preadipocyte lipid droplets accumulation by inhibiting Krüppel-like factor 3 expression. J Anim Sci. 2023;101:skad184.
Ma Z, Li H, Zheng H, Jiang K, Yan F, Tian Y, Kang X, Wang Y, Liu X. Hepatic ELOVL6 mRNA is regulated by the gga-miR-22-3p in egg-laying hen. Gene. 2017;623:72–9.
Li Y, Jin W, Zhai B, Chen Y, Li G, Zhang Y, Guo Y, Sun G, Han R, Li Z, et al. LncRNAs and their regulatory networks in breast muscle tissue of Chinese Gushi chickens during late postnatal development. BMC Genomics. 2021;22(1):44.
Li Y, Chen Y, Jin W, Fu S, Li D, Zhang Y, Sun G, Jiang R, Han R, Li Z, et al. Analyses of MicroRNA and mRNA expression profiles reveal the crucial interaction networks and pathways for regulation of chicken breast muscle development. Front Genet. 2019;10:197.
Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, Feng T, Zhou L, Tang W, Zhan L, et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation (Camb). 2021;2(3):100141.
Fu S, Zhao Y, Li Y, Li G, Chen Y, Li Z, Sun G, Li H, Kang X, Yan F. Characterization of miRNA transcriptome profiles related to breast muscle development and intramuscular fat deposition in chickens. J Cell Biochem. 2018;119(8):7063–79.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (32072692), Key Research Project of the Shennong Laboratory (SN01-2022–05), and the Scientific Studio of Zhongyuan Scholars (NO.30601985), Joint Fund of Science and Technology Research and Development Program Foundation of Henan Province (232103810013). The funding bodies had no role in study design or in any aspect of the collection, analysis and interpretation of data or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Y.W. and G.L. conceived and designed the experiments. B.Z. and Y.Z. performed the data analysis, wrote the manuscript and prepared the figures. H.L., S.L., J.G., and H.Z. prepared the samples, figures and tables. Y.Z., H.L. and Y.T. was responsible for study supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal experiments were performed according to the Regulations for the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China, 2004). The protocols and guidelines were approved by the Institutional Animal Care and Use Committee of Henan Agricultural University, China. The study was carried out in compliance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. The phenotypic value associated with FA composition in Gushi chicken breast muscle at different developmental stages.
Additional file 2:
Table S2. The number of known genes in the 20 modules.
Additional file 3:
Table S3. The information of known genes in the 19 modules.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhai, B., Zhao, Y., Li, H. et al. Weighted gene co-expression network analysis identified hub genes critical to fatty acid composition in Gushi chicken breast muscle. BMC Genomics 24, 594 (2023). https://doi.org/10.1186/s12864-023-09685-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09685-8