Abstract
Background
Haemorrhagic septicaemia (HS) is a highly fatal and predominant disease in livestock, particularly cattle and buffalo in the tropical regions of the world. Pasteurella multocida (P. multocida), serotypes B:2 and E:2, are reported to be the main causes of HS wherein serotype B:2 is more common in Asian countries including Pakistan and costs heavy financial losses every year. As yet, very little molecular and genomic information related to the HS-associated serotypes of P. multocida isolated from Pakistan is available. Therefore, this study aimed to explore the characteristics of novel bovine isolates of P. multocida serotype B:2 at the genomic level and perform comparative genomic analysis of various P. multocida strains from Pakistan to better understand the genetic basis of pathogenesis and virulence.
Results
To understand the genomic variability and pathogenomics, we characterized three HS-associated P. multocida serotype B:2 strains isolated from the Faisalabad (PM1), Peshawar (PM2) and Okara (PM3) districts of Punjab, Pakistan. Together with the other nine publicly available Pakistani-origin P. multocida strains and a reference strain Pm70, a comparative genomic analysis was performed. The sequenced strains were characterized as serotype B and belong to ST-122. The strains contain no plasmids; however, each strain contains at least two complete prophages. The pan-genome analysis revealed a higher number of core genes indicating a close resemblance to the studied genomes and very few genes (1%) of the core genome serve as a part of virulence, disease, and defense mechanisms. We further identified that studied P. multocida B:2 strains harbor common antibiotic resistance genes, specifically PBP3 and EF-Tu. Remarkably, the distribution of virulence factors revealed that OmpH and plpE were not present in any P. multocida B:2 strains while the presence of these antigens was reported uniformly in all serotypes of P. multocida.
Conclusion
This study's findings indicate the absence of OmpH and PlpE in the analyzed P. multocida B:2 strains, which are known surface antigens and provide protective immunity against P. multocida infection. The availability of additional genomic data on P. multocida B:2 strains from Pakistan will facilitate the development of localized therapeutic agents and rapid diagnostic tools specifically targeting HS-associated P. multocida B:2 strains.
Similar content being viewed by others
Background
Pasteurella multocida (P. multocida) is a Gram-negative, facultative anaerobe and an economically important veterinary pathogen. It exists both as a commensal and an opportunist pathogen, found in nasopharyngeal microflora as well as in the proximal gastrointestinal tract of animals [1]. It is responsible for a variety of acute to chronic respiratory infections in diverse wild and domestic animal species including poultry, livestock and pet animal throughout the world [2,3,4]. It was the first species of the genus Pasteurella isolated by Louis Pasteur in 1880 and was found to cause fowl cholera in birds [5]. Over a period of more than a century, it is well established that P. multocida plays a principal role in inducing serious infections like haemorrhagic septicaemia (HS) in cattle and buffaloes [6, 7], bovine respiratory disease (BRD) in calves [8], fowl cholera (FC) in poultry and porcine atrophic rhinitis (AR) in pigs and rabbits [9].
HS is an acute and highly fatal disease in cattle and water buffaloes [2]. It is a specific form of septicemic pasteurellosis mainly caused by P. multocida serotypes B:2 (predominant in Asia) and E:2 (predominant in Africa). During the period between 2005–2019, varying rates of HS infection were documented in cattle across different parts of the world: 100% in America and Europe, 85.4% in Africa, and 67.2% in Asia [10]. The highest disease incidence has been reported in South and Southeast Asian countries including Bhutan, China, India, Indonesia, Mongolia, Myanmar, Philippines, Sri Lanka and Malaysia [11,12,13]. Outbreaks of HS are common in Cambodia with high mortality rates in buffaloes (98%) and cattle (97%) [14]. Similarly, sporadic epidemics in South Asia countries mostly occur throughout in the year affecting large herd populations [15,16,17]. It has been considered an economically significant disease because of the higher mortality rate (80–100%) in infected animals as compared to other infectious diseases [7]. The seroprevalence of HS has been extensively reported in Pakistan [16, 18, 19]. In this context, buffaloes exhibit greater average morbidity (12.56%) and mortality (22.44%) compared to cattle (with respective rates of 2.42% and 6.46%) [20], indicating that buffaloes are more susceptible to HS [21]. The annual economic losses due to HS have been estimated at around $350 million in Pakistan in 2002 [13].
Antibiotic treatments have been found effective only if the treatment started soon after the diagnosis of HS infection at early stages [22]. Antimicrobials including oxytetracycline, sulfamethoxazole, a combination of streptomycin and penicillin, and sulphaquinoxaline are being used to treat HS [12]. However, these treatments are associated with a significant cost. Moreover, HS progresses rapidly, and therefore, antibiotic therapy is often unsuccessful at a later stage [23]. On the other hand, unnecessary overuse of antimicrobials has induced multi-drug resistance in the pathogens. Recent studies reported that strains of P. multocida are resistant to multiple antimicrobials commonly used to treat HS. These include amoxicillin, tetracycline, lincomycin, penicillin, oxytetracycline, chloramphenicol, gentamicin, and enrofloxacin [24, 25]. In a recent comparative genomic analysis of HS-associated strains from different geographical regions, including 12 from Pakistan, 2 from Thailand, and HS-associated North American strain M1404, alongside non-HS strains (Pm70, 37,950, HN06, and 3480) identified 35 unique genes in Pakistani isolates TX1 and BUKK [26]. These unique genes encode elements having significant similarity with an integrative conjugative element (ICEPmu1), previously reported in strain 36,950 [27]. This element encodes several acquired antimicrobial resistance genes including aminoglycoside resistance genes aph(3’)-lc, strA, and strB, sulphonamide resistance gene sul2, beta-lactamase resistance gene blaTEM-1B, tetracycline resistance gene tet(H), and catA2 conferring resistance to chloramphenicol [26]. Though several antimicrobials are available, most of them are becoming ineffective against emerging multi-drug resistant P. multocida and posing challenges for treating HS infections.
Several genes encoding putative virulence factors (VFs) have been identified to date [4], with multiple studies indicating a correlation between the prevalence of VFs and the host-specific disease manifestation [28]. Molecular level studies reported the presence of hsf1, hgbB, pfhA, and tbpA while absence of pmHAS, plpE, and tadD in all bovine isolates [29]. In contrarily, nanB, sodC, hgbA, exbB, ompH, ptfA, nanB and hbgA were found present in B:2 strain of P. multocida causing fowl cholera in layer chicken in Bangladesh [30].
Unfortunately, there is currently very little molecular and genomic information available regarding the HS-associated serotypes of P. multocida strains isolated from Pakistan. Therefore, this study was aimed to explore the genomic characteristics of bovine isolates of P. multocida serotype B:2 from Pakistan. This involve conducting a comparative genomic analysis of various P. multocida strains from Pakistan and a reference strain Pm70. This analysis aimed to enhance our understanding of the genetic basis of pathogenesis, genetic diversity, virulence, and identification of cross-protective vaccine candidates for the prevailing strains.
Results
Characteristics of P. multocida isolates
After 24 h of incubation at 37 ℃ very regular, transparent, small (approximately 1–1.5 mm in diameter) and greyish colonies were observed on brain heart infusion (BHI) agar plates while no growth was observed on MacConkey agar (Fig. 1).
The kmt1 and 6B genes are unique DNA fragments that are used to distinguish P. multocida species and the serotype B:2. The kmt1 gene identifies P. multocida species (species-specific) whereas the 6B gene distinguishes HS causing B:2 serotype of P. multocida (type-specific). The PCR amplified kmt1 gene fragment (KMT1SP6-KMT1T7 primers) of size 460 bp revealed that all three isolates belong to Pasteurella species, while the amplification of 6B gene (KTSP61-KTT72 primers) specifically produced a fragment of 620 bp, confirmed that they belong to serotype B:2 (Fig. 2).
Molecular characterization of P. multocida isolates (PM1, PM2 and PM3). A The amplified product of kmt1 gene using Pasteurella species-specific primers and (B) amplified product of 6B gene using HS causing P. multocida B:2–type-specific primers. Full-length gels are presented in the Supplementary Figure (S1)
Genomic features
The genome sequencing of PM1 produced 806,011 reads, with a total read size of 490,054,688 bp, providing a 203-fold coverage of the entire genome. Likewise, for PM2, a total of 806,478 reads were generated, with a total read size of 409,690,824 bp, achieving high 178-fold coverage. Similarly, for PM3, a total of 6,986,762 reads were generated, with a total read size of 705,035,298 bp, attaining 299-fold coverage. The de novo assembly of PM1, PM2, and PM3 generated 128, 33, and 23 contigs with N50 values of 289,611 bp, 166,244 bp, and 289,471 bp, respectively and L50 values 3, 4, and 3, respectively. (Table 1). The cumulative genome size of PM1 is 2,413,477 bp, PM2 is 2,300,632 bp, and PM3 is 2,350,552 bp having 41%, 40.4%, and 40.3% G + C contents, respectively. Genome annotation predicted 2,377 genes in PM1, 2,192 in PM2, and 2,249 in PM3. These predicted genes were further characterized as 2,281 protein coding sequences (CDS) in PM1, 2,098 in PM2, and 2,158 in PM3. PM1 and PM2 harbor 8 rRNAs while PM3 contains 4 rRNAs. Four ncRNAs are present in each genome (Table 1).
The multi-host multi-locus sequence typing (MLST) scheme analyses assigned ST-44 to all sequenced strains as 100% nucleotide identity over alleles was observed for the genes adk, aroA, deoD, gdhA, g6pd, mdh, and pgi. On the other hand, the RIRDC MLST scheme showed that all the strains belong to ST-122 due to 100% nucleotide identity over alleles for the genes adk, est, pmi, zwf, mdh, gdh, and pgi.
Identification of prophage regions and plasmids in the sequenced strains
The P. multocida strain PM1 contains 3 complete phage regions with lengths of 39.1Kb (Mannhe_vB_MhS_587AP2), 37.7Kb (Escher_D108), and 15.6Kb (Salmon_118970_sal3) (Table 2). Two complete prophages, Entero_Mu (37.7 Kb) and Salmon_118970_sal3 (30.4 Kb), along with one incomplete prophage, Pseudo_phiR18 (14.3 Kb), were found in strain PM2 (Table 2). Similarly, strain PM3 harbors two complete prophages, Escher_D108 (37.7 Kb) and Mannhe_vB_MhS_535AP2 (39.1 Kb), along with one incomplete prophage, Pseudo_phiR18 (14.3 Kb) (Table 2). No plasmid was identified in any sequenced genomes by the PlasmidFinder.
Pan-genome estimation and phylogenetic analysis
The pan-genome consists of 2,488 genes, of which 2187 are core, 93 accessory, and 208 unique genes (Fig. 3A). In pan-genome plot, the number of pan-genes increases with the increase of new genomes suggesting that the pan-genome of Pakistani P. multocida strains is relatively stable (Fig. 3B). In contrast, the core-genome curve becomes steady after adding certain genomes, indicating a stable core-genome (Fig. 3B).
Phylogenetic analysis of PM1, PM2, and PM3 with other publicly available Pakistani P. multocida strains was performed based on whole-genome SNPs as well as core-genome SNPs. The whole-genome SNPs-based phylogenetic analysis revealed that the three newly sequenced strains, PM1, PM2, and PM3, form cluster together nearest to the previously sequenced strain Faisal (Fig. 4). Additionally, a core-genome SNP-based phylogenetic analysis was also performed (Supplementary Figure S2). Following an extensive comparison of the phylogenetic trees, it was observed that the whole-genome SNP-based phylogeny demonstrated consistency with the core-genome SNP-based phylogeny and provided comparable results.
Whole genome SNPs-based phylogenetic relationship (A) and distribution of core and accessory genes (B) of the sequenced strains (PM1, PM2, and PM3) with 9 other Pakistani P. multocida strains (PVAcc, V1, TX1, Islm, Karachi, BUKK, Faisal, ATTK, and Pesh). The heatmap shows the presence and absence of genes
Functional annotation of core and unique genes
The functional annotation of the core genome in the studied strains reveals that the largest category of P. multocida strains comprises 184 genes (18%), which are involved in the biosynthesis of amino acids and their derivatives. Following closely, the second largest category of the core genome consists of 149 genes (15%) associated with protein metabolism, while the category containing genes found in phages, prophages, transposable elements, and plasmids, known as the mobilome, comprises no genes (0%) and stands as the least represented category (Fig. 5). Further analysis revealed that among the core genome of P. multocida, a total of 14 genes (1%) were identified to play crucial roles in virulence, disease, and defense mechanisms. Specifically, two genes (parC; GO:0003677 and gyrA; GO:0003918) were found to confer resistance to fluoroquinolones, while seven genes (CopZ; GO:0030001, CIA; GO:0004008, CRD; GO:0005507, CcmE; GO:0017004, CcmF; GO:0006461, ScsB; GO:0016020, and ScsC; GO:0015035) were associated with copper homeostasis and tolerance. Additionally, five genes (Rv0667; GO:0003899, Rv0668; GO:0003899, Rv1641; GO:0003743, Rv1642; GO:0003735, and Rv1643; GO:0003723) were found to be involved in invasion and intracellular resistance.
The pan-genome analysis identified 23 strain-specific genes (uniquely present) in PM1; however, no unique genes were found in strains PM2 and PM3. Among these genes, one gene (MO; GO:0016491) was associated with resistance to a toxic compound, another (XO; GO:0004855) with purine utilization, a third (Val-mt; GO:0004832) with protein biosynthesis, and finally, one gene (Ppx; GO:4309) with phosphorus metabolism. The remaining genes unique to strain PM1 were found to encode hypothetical proteins.
Presence of genes associated with antimicrobial resistance and virulence
The binary heatmap of the presence and absence of antibiotic resistance determinants shows that a PBP3 gene conferring resistance to beta-lactam antibiotics and an elfamycin resistance gene EF-Tu are present in all the studied genomes (Fig. 6). Whereas, BUKK and TX1 strains harbor additional antibiotic resistance determinants including aminoglycoside resistance genes aph(3')-la, aph(3’’)-Ib, and aph(6)-ld, a TEM-1 gene conferring resistance to penicillins and the first generation cephalosporins, a plasmid associated gene sul2 conferring resistance to sulfonamide, a tetracycline resistance gene tetR, a chloramphenicol resistance gene catII, and a tet(B) gene conferring resistance to tetracycline and doxycycline (Fig. 6).
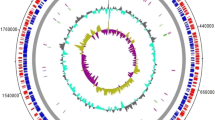
The virulence genes comparison analysis, however, predicted many common genes in Pakistani P. multocida and reference strain Pm70 (Fig. 7). These include HgbA, HgbB, hsf-1, Omp16, Omp87, OmpH2, TbpA, PtfA, ComE, PlpB, PlpP, PlpD, PfhB1, PfhB2, sodA, kmt1, tonB, nanB, nanH, exbD, fur, sodC, and exbB (Table 4). The reference strain Pm70 has additional virulence genes OmpA, OmpH, OmpH1, OmpH3, PlpE, tadD, and PmHAS, which were found absent in all the Pakistani strains (Fig. 7),
Comparison of virulence genes in Pakistani P. multocida strains and the reference strain Pm70. The genomes are presented with different colors in the ring, and the image was generated with BRIG (http://brig.sourceforge.net)
Discussion
HS is an acute, highly lethal, and widespread disease in tropical areas of the world that affects livestock, particularly cattle and buffalo [31]. P. multocida serotypes B:2 and E:2 are believed to be the main causes of HS, which develops in the later stages of pasteurellosis. Serotype B:2 is more prevalent in Asian countries including Pakistan than serotype E:2, which is more common in African countries [10]. HS is considered an endemic in Pakistan, where it causes livestock farmers to suffer significant financial losses every year [13, 20]. Hence in this study, we attempted to characterize HS-associated P. multocida strains (PM1, PM2, and PM3) from Pakistan at the genomic level. Together with nine other P. multocida strains of Pakistani origin and a reference strain Pm70, comparative genomic analysis was performed to better understand the genomic dynamics that may help in genomic epidemiology and reverse vaccinological applications.
The genome size of the sequenced P. multocida strains ranges from 2.3 to 2.4 Mbp with G + C contents ranging from 40.3% to 41% which is consistent with previous studies [32]. The MLST genotyping of P. multocida is used to determine disease conditions in different hosts such as HS in cattle/buffalo, fowl cholera in poultry, septicaemia in sheep and goats, atrophic rhinitis in pigs, snuffles in rabbits [33]. The MLST scheme assigned ST-44 to all sequenced strains under study, while the in silico RIRDC MLST revealed that all the sequenced strains (PM1, PM2, and PM3) belong to ST-122 and molecular analysis showed that they are serotype B:2. The P. multocida isolates with RIRDC ST-122 and multi host MLST ST-44 are strongly associated with bovine HS in Southeast Asia. Notably, P. multocida ST-122 serotype B:2 is predominant in Pakistan and has been extensively reported in bovine HS, predominant pasteurellosis in bovine [26, 33].
Bacterial populations frequently exchange genetic determinants such as adhesins, toxins, and antibiotic resistance genes through horizontal gene transfer (HGT). These genetic determinants are found on mobile genetic elements such as plasmids and prophages. Although not all P. multocida strains harbor plasmids, plasmids of different sizes (1 up to 100 kb) have been reported in P. multocida isolates from various sources [34, 35]. The genome of P. multocida PM1, PM2 and PM3 strains sequenced in this study contains no plasmids; however, each strain contains at least two complete prophages. The prophages are hotspots for HGT and the integration of genes associated with stress tolerance, virulence, and antimicrobial resistance [36].
The pan-genome of 13 strains accounted for a total of 2,488 genes, of which 2,187 are core, 93 accessory, and 208 unique genes. The number of pan genes increases with the increase of new genomes, which suggests that the pan-genome of isolates used in the study, is still open. In contrast, the core genes become steady after the addition of certain genomes, which indicates that the core genome is stable. The higher number of core genes and the low number of unique genes in Pakistani strains is indicative of high-level similarity among the strains. This is also supported by phylogenetic analysis of the studied strains i.e., the whole-genome SNPs-based phylogenetic analysis revealed that strains PM1, PM2 and PM3 cluster together nearest to the previously sequenced strain Faisal (Fig. 4). Interestingly, the genomes of all Pakistan-origin P. multocida strains neatly formed two clades, and the placement of newly sequenced strains in a clade with Faisal strain showed that the Faisal strain might be the ancestor of the newly sequenced strains (PM1, PM2, and PM3) (Fig. 4). The analysis was performed based on whole-genome SNP phylogeny as it gives a better resolution of very closely related organisms entailing more data as compared to core genome phylogeny (Fig. 4 and Supplementary Figure S2). It is interesting to mention that PM1 and PM3 have been isolated from buffaloes of the neighbouring cities, similar to that of the Faisal strain (Fig. 4; Table 3). This has been previously reported as well that HS strains formed clades based on their region of isolation (26). However, this might not always be the case as our data has shown HS strains from different regions with different climates might cluster together as well. For example, strains V1, Pesh, Karachi, Islm and ATTK cluster together but they have been isolated from distant cities of different regions (Fig. 4). However, further studies on a large set of Pakistani-origin P. multocida genomes would be required to confirm this observation. It was further identified that the major content of the core genome is involved in the biosynthesis of amino acids and protein metabolism and few genes of the core genome serve as a part of virulence and defense. Functional annotation of strain-specific genes showed that genes unique to strain PM1 are involved in resistance to copper (MO; GO:0016491), purine utilization (XO; GO:0004855), protein biosynthesis (Val-mt; GO:0004832), and phosphorus metabolism (Val-mt; GO:0004832).
The presence of resistance genes in bacteria suggests their high adaptability in a harsh environment. The Comprehensive Antibiotic Resistance Database (CARD) detected a PBP3 gene (conferring resistance to beta-lactam antibiotics) and EF-Tu (conferring resistance to elfamycin) in all the studied strains including reference strain Pm70. In addition to these two common resistance genes, BUKK and TX1 strains contain several other resistance determinants including aminoglycoside resistance genes aph(3')-la, aph(3’’)-Ib, and aph(6)-ld, a TEM-1 gene conferring resistance to penicillin and the first-generation cephalosporins, a plasmid associated gene sul2 conferring resistance to sulfonamide, a tetracycline resistance gene tetR, a chloramphenicol resistance gene catII, and a tet(B) gene conferring resistance to tetracycline and doxycycline. This indicates that BUKK and TX1 strains harbor more resistance-associated genes and are possibly phenotypically resistant to more antibiotics compared to other Pakistani strains and the reference strain Pm70.
It is well known that the expression of various virulent factors of P. multocida plays a critical role in pathogenesis. They serve a variety of important roles, including but not limited to adhesion and colonization in the host, enzymatic activity, and iron acquisition, etc. that leads to the development of pasteurellosis and persistence in the host environment [38]. The most important VFs include capsules, lipopolysaccharides (LPS), outer-membrane proteins (OMPs), and fimbriae. Furthermore, some VFs are believed to be a major contributing element to HS pathogenesis and all serotype B:2 strains contain ptfA encoding type 4 fimbriae, OmpH and Oma87 encoding outer membrane proteins, pfhB encoding filamentous hemagglutinin, tbpA encoding a transferrin-binding protein, sodC encoding superoxide dismutase, hgbA encoding a haemoglobin binding protein, and nanH encoding neuraminidases [10, 39].
Bacterial adhesion and colonization to the epithelial surface are essential to establish infection [3]. In our study VFs such as ptfA (encoding type 4 fimbrial subunit), PfhB1, PfhB2 and hsf-1 were present in all the studied genomes. They are important in surface adhesion and play a major role in the colonization of the upper respiratory tract. Iron is essential for bacterial growth and survival in the host. P. multocida has evolved multiple mechanisms for iron uptake. We found many genes (tbpA, HgbA, HgbB, fur, exbD, exbB, and tonB) in our studied genomes encoding proteins with predicted roles in iron acquisition and iron transport [4]. VFs encoding extracellular enzymes such as neuraminidase (nanB and nanH) were also found in the studied strains. These VFs are reported to enhance metabolic activity, and are critical for the pathogenesis of P. multocida in the host. The superoxide dismutase genes (sodA and sodC), which play an important role in protection against oxidative stress are also found present in all the analyzed strains. Outer membrane proteins are the main component of the bacterial outer membrane and are critical for infection and pathogenesis. Notably, Omp16, Omp87, OmpH2, PlpB, PlpP, and PlpD were uniformly present across all the studied genomes. These results are consistent with earlier studies [40, 41].
Surprisingly, OmpH, OmpH1, OmpH3, and PlpE were not found in any Pakistani P. multocida strains while these surface antigens are often used in vaccine studies. For instance, OmpH [42] and PlpE [43, 44] conferred protective immunity against P. multocida infection. A few previous studies reported the presence of the plpE gene across different strains of P. multocida, irrespective of serotypes [45, 46]. For example, there was high plpE gene sequence homology (˃ 90%) among Indian isolates (A: 3. B: 2 and D: 1) and the reported sequences from other serotypes [X-73 (A: 1), P-470 (A: 3), P-1059 (A: 3)] of P. multocida indicating the universal presence of plpE gene across all serotypes [45]. However, recent comparative genomic studies, including our present investigation, contradict the previous findings and suggest that outer membrane protein PlpE was not identified in any genome from capsular serogroup B. Instead, it is significantly associated with capsular serogroup A and F [29]. Similarly, outer membrane proteins OmpH1 and OmpH3 are significantly associated with capsular serogroup A as compared to all other serogroups [5, 29].
Beyond the mere presence of particular VFs in the genomes, their expression levels play an important role in determining pathogenicity and disease manifestation in the host. When conducting a comparative genome analysis between the highly virulent strain PmCQ2 and naturally attenuated strain PmCQ6 of P. multocida (serotype A), it was reported that they exhibited high genome similarity (99%), sharing common virulent factors. The differences in pathogenicity between the two strains was attributed to the differential expression of virulence genes [47]. Similarly, molecular variations within virulence-associated genes may affect both host specificity and virulence. Structural characterization of an abundant outer membrane protein A (OmpA) in P. multocida revealed the presence of four hypervariable extracellular loops, predicted to be more antigenic in bovine isolates compared to porcine isolates [48]. These loop regions, which contain charged residues, are suggested to be important for adherence to the host cell and potentially play an important role in the development of HS in bovine. Hence, many factors, including molecular-level variation within VFs, their presence or absence, and most importantly their differential expression, collectively contribute to shaping the pathogenicity of bacterial strains.
Conclusions
This study comprised a comparative genomic analysis of three novel strains of HS-associated P. multocida serotype B:2 with nine publicly available Pakistani origin P. multocida strains and a reference strain Pm70. The core-genome SNPs-based phylogenetic analysis indicated a close resemblance among Pakistani strains and few genes (1%) of the core-genome serve as a part of virulence and defense. Surprisingly, VFs like OmpH and PlpE were not found in any Pakistani P. multocida strains while these surface proteins are used in vaccine studies and reported to provide protective immunity. Therefore, this study warrants further research to investigate the diversity and prevalence of PlpE and OmpH genes in HS-associated Pakistani P. multocida strains to develop domestic HS-associated P. multocida specific therapeutics.
Methods
Bacterial strain isolation and characterization
Pasteurella multocida isolate PM1 was isolated from the heart blood of an infected buffalo in Faisalabad, PM2 from the blood of an infected buffalo in Peshawar, and PM3 was isolated from a buffalo (PM3) died apparently with the symptoms of HS in Okara, Pakistan. The morphological characteristics of the isolates were observed by growing on BHI and MacConkey agar by incubating at 37 ℃ for 16–18 h. The genomic DNAs (gDNAs) were extracted from purified cultures using GeneJET Genomic DNA Purification Kit, cat # K0721 (Thermo Fisher). The extracted gDNAs were quantified using nanodrop (NanoDrop 2000c, Thermo Scientific), and the integrity and purity of the extracted gDNAs were tested through agarose gel electrophoresis. The serotypes were confirmed by molecular analysis of gDNAs through species-specific and type-specific PCR [49]. The kmt1 gene was amplified using KMT1SP6-KMT1T7 primers that identify P. multocida species (species-specific) whereas the 6B gene was amplified using KTSP61-KTT72 primers that distinguish HS-associated P. multocida serotype B:2 strains (type-specific).
Genome sequencing and annotation
The whole-genome shotgun sequencing of newly isolated P. multocida strains PM1, PM2 and PM3 was achieved using Illumina HiSeq 2500 platform. The sequence reads were trimmed using Trimmomatic 0.30 [50], and the trimmed reads were de novo assembled using SPAdes (version 3.12.0) [51]. The generated contigs were annotated by Prokka at default parameters [52].
MLST genotyping and identification of prophage regions and plasmids
The in silico MLST genotyping was performed at PubMLST using the P. multocida typing database (https://pubmlst.org/bigsdb?db=pubmlst_pmultocida_seqdef) [53]. The PubMLST hosts two separate MLST schemes (Multi-host MLST and RIRDC MLST) for P. multocida. The multi-host MLST scheme includes isolates from a range of hosts such as birds, pigs, sheep, and cattle, and is based on seven housekeeping genes (adk, aroA, deoD, gdhA, g6pd, mdh, and pgi). The RIRDC MLST scheme is based on seven housekeeping genes (adk, est, pmi, zwf, mdh, gdh, and pgi) developed to investigate avian isolates.
The prophages in the sequenced genomes were identified and annotated using PHASTER [54] and the plasmid replicons (rep) were identified by PlasmidFinder 2.1 at the default parameters [55].
Pan-genome estimation and phylogenetic relationships
The Pakistani origin P. multocida genomes (n = 9) available at NCBI were downloaded with a reference strain Pm70. In addition, three genomes of P. multocida (sequenced in the current study) were also included (Table 3). To proceed with pan-genome analysis all selected genomes were first annotated by Prokka at default parameters [52]. The pan-genome analysis and core-genome SNPs based phylogenetic relationship were inferred using the in-house pipeline PanRV [56]. For, whole-genome SNPs-based phylogenetic analysis, the genomes were uploaded to the online server CSI Phylogeny 1.4 hosted at https://cge.food.dtu.dk/services/CSIPhylogeny/ [57] with the following default setting: minimum depth at SNP positions 10, relative depth at SNP positions 10, the minimum distance between SNPs (prune) 10, minimum SNP quality 30, and minimum Z-score of 1.96. The SNP calling was performed against P. multocida strain PVAcc (serotype B:2) and a maximum likelihood tree was generated using FastTree 2 tool [58].
Functional annotation of core and unique genes
The functional annotation of core and unique genes was achieved using Rapid Annotation using Subsystem Technology (RAST), available at https://rast.nmpdr.org/rast.cgi [59]. RAST conducts BLAST search against the SEED database and provides high-quality functional annotations [60].
Comparison of genes associated with antimicrobial resistance and virulence
The acquired genes and chromosomal mutations conferring antibiotic resistance were identified using CARD [61]. For the identification of virulence-associated genes, 29 known P. multocida VFs were retrieved from the NCBI database (Table 4). The BLASTp search was performed to identify the VFs in the selected genomes based on sequence similarity (genes exhibiting < 80% sequence similarity were deemed present). Subsequently, a comparative analysis of VFs with the reference strain Pm70 was performed using BRIG (Blast Ring Image Generator) [62].
Availability of data and materials
The whole-genome sequence data of PM1, PM2, and PM3 have been deposited at DDBJ/ENA/GenBank under the accession number JAEMBU000000000, JAFFJB000000000, and SDAS00000000, respectively.
Abbreviations
- HS:
-
Haemorrhagic septicaemia
- BRD:
-
Bovine respiratory disease
- FC:
-
Fowl cholera
- AR:
-
Atrophic rhinitis
- CDS:
-
Coding sequences
- MLST:
-
Multi-locus sequence typing
- HGT:
-
Horizontal gene transfer
- CARD:
-
Comprehensive antibiotic resistance database
- COGs:
-
Cluster of orthologous genes
- FAM:
-
Functional annotation module
- SNP:
-
Single nucleotide polymorphism
- ICE:
-
Integrative conjugative element
- VFs:
-
Virulence factors
- LPS:
-
Lipopolysaccharides
- OMPs:
-
Outer-membrane proteins
- BHI:
-
Brain heart infusion
- gDNA:
-
Genomic DNA
References
Annas S, Zamri-Saad M, Jesse FFA, Zunita Z. New sites of localisation of Pasteurella multocida B:2 in buffalo surviving experimental haemorrhagic septicaemia. BMC Vet Res. 2014;10(1):88.
Rimler RB: Pasteurella multocida and fowl cholera. In: Pasteurella and pasteurellosis. Edited by C. Adlam, Rutter JM. London: Academic Press Limited; 1989: 37–73.
Wilkie IW, Harper M, Boyce JD, Adler B. Pasteurella multocida: Diseases and Pathogenesis. In: Pasteurella multocida: Molecular Biology, Toxins and Infection. Edited by Aktories K, Orth JHC, Adler B. Berlin, Heidelberg: Springer Berlin Heidelberg; 2012: 1–22.
Harper M, Boyce JD, Adler B. Pasteurella multocida pathogenesis: 125 years after Pasteur. FEMS Microbiol Lett. 2006;265(1):1–10.
Peng Z, Liang W, Liu W, Wu B, Tang B, Tan C, Zhou R, Chen H. Genomic characterization of Pasteurella multocida HB01, a serotype A bovine isolate from China. Gene. 2016;581(1):85–93.
Petersen KD, Christensen JP, Permin A, Bisgaard M: Virulence of Pasteurella multocida subsp. multocida isolated from outbreaks of fowl cholera in wild birds for domestic poultry and game birds. Avian Pathol. 2001; 30(1):27–31.
De Alwis MC. Haemorrhagic septicaemia–a general review. Br Vet J. 1992;148(2):99–112.
Dabo SM, Taylor JD, Confer AW. Pasteurella multocida and bovine respiratory disease. Anim Health Res Rev. 2007;8(2):129–50.
Carter GR. Studies on Pasteurella multocida. I. A hemagglutination test for the identification of serological types. Am J Vet Res. 1955; 16(60):481–484.
Almoheer R, Abd Wahid ME, Zakaria HA, Jonet MAB, Al-shaibani MM, Al-Gheethi A, Addis SNK. Spatial, temporal, and demographic patterns in the prevalence of Hemorrhagic Septicemia in 41 Countries in 2005–2019: A systematic analysis with special focus on the potential development of a new-generation vaccine. Vaccines. 2022;10(2):1–17.
OIE: Manual of diagnostic tests and vaccines for terrestrial animals. In: Haemorrhagic septicaemia (Pasteurella multocida serotypes 6:b and 6:e) World Organization of Animal Health. Office International Des Epizooties (OIE), Paris, France. ; 2021: 1–16.
Spickler AR. Hemorrhagic Septicemia. 2019. Retrieved from http://www.cfsph.iastate.edu/DiseaseInfo/factsheets.php.
Benkirane A, De Alwis M. Haemorrhagic septicaemia, its significance, prevention and control in Asia. Vet Med (Praha). 2002;47(8):234–40.
Savoeurn I, Suon S, Windsor P. The epidemiology, diagnosis and control of haemorrhagic septicaemia of cattle and buffalo in Cambodia. Cattle health, production and trade in Cambodia. ACIAR Proceedings No. 138. 2013:50–2.
Mondal SP, Yamage M. A retrospective study on the epidemiology of anthrax, foot and mouth disease, haemorrhagic septicaemia, peste des petits ruminants and rabies in Bangladesh, 2010–2012. PLoS One. 2014;9(8):1–11.
Khan A, Saddique U, Ahmad R, Khan H, Mohammad Y, Zubair M. Serosurveillance of hemorrhagic septicemia in cattle and buffaloes in district Malakand. NWFP J Agric Biol Sci. 2006;1:11–4.
Shome R, Deka RP, Sahay S, Grace D, Lindahl JF. Seroprevalence of hemorrhagic septicemia in dairy cows in Assam. India Infect Ecol Epidemiol. 2019;9(1):1–4.
Sheikh MA, Anzam M, Shakoori AR. Observations on haemorrhagic septicaemia in Pakistan livestock. Zentralbl Veterinarmed B. 1996;43(5):293–304.
Moustafa A, Ali S, Bennett M, Hyndman T, Robertson I, Edwards J. A case–control study of haemorrhagic septicaemia in buffaloes and cattle in Karachi, Pakistan, in 2012. Transbound Emerg Dis. 2017;64(2):520–7.
Farooq U, Hussain M, Irshad H, Badar N, Munir R, Ali Q. Status of haemorrhagic septicaemia based on epidemiology in Pakistan. Pak Vet J. 2007;27(2):67–72.
Shivachandra SB, Viswas KN, Kumar AA. A review of hemorrhagic septicemia in cattle and buffalo. Anim Health Res Rev. 2011;12(1):67–82.
Khan A, Saleemi MK, Khan MZ, Gul ST, Irfan M, Qamar MS. Hemorrhagic septicemia in buffalo (Bubalus bubalis) calves under sub-tropical conditions in Pakistan. Pak J Zool. 2011;43:295–302.
Ahmad TA, Rammah SS, Sheweita SA, Haroun M, El-Sayed LH. Development of immunization trials against Pasteurella multocida. Vaccine. 2014;32(8):909–17.
Vu-Khac H, Trinh TH, Nguyen TG, Nguyen XT, Nguyen TT. Prevalence of virulence factor, antibiotic resistance, and serotype genes of Pasteurella multocida strains isolated from pigs in Vietnam. Vet World. 2020;13(5):896–904.
Cuevas I, Carbonero A, Cano D, Garcia-Bocanegra I, Amaro MA, Borge C. Antimicrobial resistance of Pasteurella multocida type B isolates associated with acute septicemia in pigs and cattle in Spain. BMC Vet Res. 2020;16(1):1–9.
Moustafa AM, Seemann T, Gladman S, Adler B, Harper M, Boyce JD, Bennett MD. Comparative genomic analysis of Asian haemorrhagic septicaemia-associated strains of Pasteurella multocida identifies more than 90 haemorrhagic septicaemia-specific genes. PLoS One. 2015;10(7):1–16.
Michael GB, Kadlec K, Sweeney MT, Brzuszkiewicz E, Liesegang H, Daniel R, Murray RW, Watts JL, Schwarz S. ICEPmu1, an integrative conjugative element (ICE) of Pasteurella multocida: analysis of the regions that comprise 12 antimicrobial resistance genes. J Antimicrob Chemother. 2012;67(1):84–90.
Okay S, Kızıldoğan AK. Comparative genome analysis of five Pasteurella multocida strains to decipher the diversification in pathogenicity and host specialization. Gene. 2015;567(1):58–72.
Smith E, Miller E, Aguayo JM, Figueroa CF, Nezworski J, Studniski M, Wileman B, Johnson T. Genomic diversity and molecular epidemiology of Pasteurella multocida. PLoS One. 2021;16(4):1–22.
Saha O, Ranga RI, Rahman MS, Hoque MN, Hossain MA, Sultana M. Genome-wide diversity and differentiation of two novel multidrug-resistant populations of Pasteurella multocida type B: 2 from fowl cholera. bioRxiv 2020.
Prajapati A, Yogisharadhya R, Mohanty NN, Mendem SK, Nizamuddin A, Chanda MM, Shivachandra SB. Comparative genome analysis of Pasteurella multocida serogroup B:2 strains causing haemorrhagic septicaemia (HS) in bovines. Gene. 2022;826: 146452.
Jabeen S, Yap HY, Abdullah FFJ, Zakaria Z, Isa NM, Tan YC, Joo YS, Satharasinghe DA, Omar AR. Complete genome sequence analysis and characterization of selected iron regulation genes of Pasteurella multocida serotype A strain PMTB2. 1. Genes. 2019, 10(2):1–21.
Peng Z, Liang W, Wang F, Xu Z, Xie Z, Lian Z, Hua L, Zhou R, Chen H, Wu B. Genetic and phylogenetic characteristics of Pasteurella multocida isolates from different host species. Front Microbiol. 2018;9:1–13.
San Millan A, Giufré M, Escudero JA, Hidalgo L, Gutierrez B, Cerquetti M, Gonzalez-Zorn B. Contribution of ROB-1 and PBP3 mutations to the resistance phenotype of a β-lactamase-positive amoxicillin/clavulanic acid-resistant Haemophilus influenzae carrying plasmid pB1000 in Italy. J Antimicrob Chemother. 2011;66(1):96–9.
Wilson BA, Ho M. Pasteurella multocida: from zoonosis to cellular microbiology. Clin Microbiol Rev. 2013;26(3):631–55.
Ramisetty BCM, Sudhakari PA. Bacterial “Grounded” prophages: Hotspots for genetic renovation and innovation. Front Genet. 2019;10:1–17.
May BJ, Zhang Q, Li LL, Paustian ML, Whittam TS, Kapur V. Complete genomic sequence of Pasteurella multocida, Pm70. Proc Natl Acad Sci. 2001;98(6):3460–5.
Prajapati A, Chanda MM, Yogisharadhya R, Parveen A, Ummer J, Dhayalan A, Mohanty NN, Shivachandra SB. Comparative genetic diversity analysis based on virulence and repetitive genes profiling of circulating Pasteurella multocida isolates from animal hosts. Infect Genet Evol. 2020;85:1–11.
Verma S, Sharma M, Katoch S, Verma L, Kumar S, Dogra V, Chahota R, Dhar P, Singh G. Profiling of virulence associated genes of Pasteurella multocida isolated from cattle. Vet Res Commun. 2013;37(1):83–9.
Furian TQ, Borges KA, Laviniki V. Rocha SLdS, Almeida CNd, Nascimento VPd, Salle CTP, Moraes HLdS: Virulence genes and antimicrobial resistance of Pasteurella multocida isolated from poultry and swine. Braz J Microbiol. 2016;47(1):210–6.
Kim J, Kim JW, Oh S-I, So B, Kim W-I, Kim H-Y. Characterisation of Pasteurella multocida isolates from pigs with pneumonia in Korea. BMC Vet Res. 2019;15(1):1–8.
Tan HY, Nagoor NH, Sekaran SD. Cloning, expression and protective capacity of 37 kDa outer membrane protein gene (ompH) of Pasteurella multocida serotype B:2. Trop Biomed. 2010;27(3):430–41.
Wu J-R, Shien J-H, Shieh HK, Chen C-F, Chang P-C. Protective immunity conferred by recombinant Pasteurella multocida lipoprotein E (PlpE). Vaccine. 2007;25(21):4140–8.
Hatfaludi T, Al-Hasani K, Gong L, Boyce JD, Ford M, Wilkie IW, Quinsey N, Dunstone MA, Hoke DE, Adler B: Screening of 71 P. multocida proteins for protective efficacy in a fowl cholera infection model and characterization of the protective antigen PlpE. PloS One 2012, 7(7):1–11.
Singh AP, Singh S, Ranjan R, Gupta SK, Singh VP, Sharma B: Molecular heterogeneity of plpE gene in Indian isolates of Pasteurella multocida and expression of recombinant PlpE in vaccine strain of P. multocida serotype B: 2. J Vet Sci 2010, 11(3):227–233.
Mostaan S, Ghasemzadeh A, Ehsani P, Sardari S, Shokrgozar MA, Abolhassani M, Nikbakht Brujeni G. In silico analysis of Pasteurella multocida PlpE protein epitopes as novel subunit vaccine candidates. Iran Biomed J. 2021;25(1):41–6.
He F, Zhao Z, Wu X, Duan L, Li N, Fang R, Li P, Peng Y. Transcriptomic Analysis of High- and Low-Virulence Bovine Pasteurella multocida in vitro and in vivo. Front Vet Sci. 2021;8:1–12, 616774. https://doi.org/10.3389/fvets.2021.616774.
T EK, Leeanan R, Pannoi S, Anuntasomboon P, Thongkamkoon P, Thamchaipenet A: OmpA protein sequence-based typing and virulence-associated gene profiles of Pasteurella multocidaisolates associated with bovine haemorrhagic septicaemia and porcine pneumonic pasteurellosis in Thailand. BMC Vet Res 2017, 13(1):243
Townsend KM, Frost AJ, Lee CW, Papadimitriou JM, Dawkins HJ. Development of PCR assays for species- and type-specific identification of Pasteurella multocida isolates. J Clin Microbiol. 1998;36(4):1096–100.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20.
Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–77.
Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–9.
Jolley K, Bray J, Maiden M: Open-access bacterial population genomics: BIGSdb software, the PubMLST. org website and their applications. Wellcome Open Res. 2018; 3(124):1–20.
Arndt D, Grant JR, Marcu A, Sajed T, Pon A, Liang Y, Wishart DS. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44(W1):W16-21.
Carattoli A, Zankari E, Garcia-Fernandez A, Voldby Larsen M, Lund O, Villa L, Moller Aarestrup F, Hasman H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58(7):3895–903.
Naz K, Naz A, Ashraf ST, Rizwan M, Ahmad J, Baumbach J, Ali A. PanRV: Pangenome-reverse vaccinology approach for identifications of potential vaccine candidates in microbial pangenome. BMC Bioinform. 2019;20:1–10.
Kaas RS, Leekitcharoenphon P, Aarestrup FM, Lund O. Solving the Problem of Comparing Whole Bacterial Genomes across Different Sequencing Platforms. PLoS One. 2014;9(8): e104984.
Price MN, Dehal PS, Arkin AP. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5(3): e9490.
Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, et al. The RAST Server: Rapid Annotations using Subsystems Technology. BMC Genomics. 2008;9(1):75.
Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014;42(D1):D206–14.
Alcock BP, Raphenya AR, Lau TTY, Tsang KK, Bouchard M, Edalatmand A, Huynh W, Nguyen AV, Cheng AA, Liu S, et al. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020;48(D1):D517–25.
Alikhan N-F, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12(1):402.
Acknowledgements
Not applicable
Funding
This work was supported in part by the Higher Education Commission (HEC) of Pakistan grant No. NRPU-7254, awarded to Moazur Rahman.
Author information
Authors and Affiliations
Contributions
SM performed the experiments, formal analysis, and data collection, and wrote the original manuscript; NU processed and analyzed the WGS data, and wrote the original manuscript; MFUH performed data analysis, and was involved in reviewing and editing the manuscript; WR supervised the study, provided resources, and was involved in reviewing-editing the manuscript; MI conceptualized the study and contributed in reviewing and editing the manuscript; AA was involved in data analysis, and reviewing-editing the manuscript; MR conceptualized and supervised the study, was involved in funding acquisition and resources, as well as reviewing and editing the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Ethics Committee of the National Institute for Biotechnology and Genetic Engineering (IEC-NIBGE), Faisalabad, Pakistan, and the research work on experimental animals was performed according to the guidelines and protocols established by the NIBGE Animal House Committee (NAHC) and in accordance with ARRIVE guidelines for the animal experiments.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. Molecular characterization of P. multocida isolates (PM1, PM2 and PM3). (A) The amplified product of kmt1 gene using Pasteurella species-specific primers. (B) amplified product of 6B gene using HS causing P. multocida B:2 type-specific primers. Figure S2. Core-genome SNPs-based phylogenetic relationship of the sequenced strains (PM1, PM2, and PM3) with 9 other Pakistani P. multocida strains (PVAcc, V1, TX1, Islm, Karachi, BUKK, Faisal, ATTK, and Pesh) and a reference strain Pm70.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mahboob, S., Ullah, N., Farhan Ul Haque, M. et al. Genomic characterization and comparative genomic analysis of HS-associated Pasteurella multocida serotype B:2 strains from Pakistan. BMC Genomics 24, 546 (2023). https://doi.org/10.1186/s12864-023-09626-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09626-5