Abstract
In recent years, some common themes in the development of sex-specific traits in different animal lineages have started to emerge since the discovery of the Dmrt (doublesex-mab3-related transcription factor gene) genes. Bivalves are characterized by a diversity of sexual systems, including simultaneous hermaphroditism, sequential hermaphroditism, and strict gonochorism. However, to date, no research has focused on the genome-wide characterization and analysis of Dmrt genes in bivalves. In this study, the identification and analysis of Dmrt genes in 15 bivalves were performed using bioinformatics methods. A total of 55 Dmrt genes were retrieved in the studied bivalve genomes. The number of Dmrt genes in different species ranged from 3 to 5. The phylogenetic tree showed that Dmrt genes in bivalves can be subdivided into 5 classes: the Dmrt2-like class, Dmrt3-like class, Dmrt4/5-like class, Dsx-like class, and scallop-specific Dmrt class. The Ka/Ks ratios suggested that all Dmrt classes underwent purifying selection pressure. Furthermore, the spatiotemporal expression of Dmrt genes in four bivalve species suggested that different Dmrt genes may have different functions, and scallop-specific Dmrt genes may play a key role in sex determination/differentiation. In general, this study provides a molecular basis for in-depth examination of the functions of Dmrt genes and phylogenomic analyses in bivalves.
Similar content being viewed by others
Introduction
The founding member of the Dmrt (double sex/male-abnormal-3 related transcription factor) genes was first formally identified in Drosophila, where the Dsx gene controls somatic sexual differentiation via alternatively spliced, male- and female-specific isoforms (dsxM and dsxF, respectively) [1]. Subsequently, different Dmrt members have been identified and proven to participate in the control of sex determination/differentiation in other organisms. For example, the Z-linked gene Dmrt1 is vital for male sex determination in chickens [1], and a W-linked Dmrt gene (DM-W) participates in primary ovary development in Xenopus laevis [2]. A similar phenomenon was also observed in aquatic animals. In the medaka Oryzias latipes, a Y-specific DMY gene, as a copy of autosome Dmrt1, was found to be the master sex-determining gene inducing male formation. In general, given the key role of the Dmrt gene in sex determination/differentiation, Dmrt genes have been intensively investigated [3,4,5].
The members of the Dmrt gene family in different organisms showed substantial differences. For instance, 8 Dmrt members have been found in several mammals [6, 7], and 7 members of the Dmrt gene family have been identified in many teleosts [8]. Only 4 Dmrt members have been identified in Drosophila melanogaster [9]. Little is known about Dmrt genes in aquatic invertebrates, and no comprehensive survey and analysis of Dmrt genes has been conducted in bivalves. Bivalves, including clams, oysters, mussels, and scallops, were characterized by a shell that was divided from front to rear into left and right valves. A diversity of sexual systems has been found in bivalves, including simultaneous hermaphroditism, sequential hermaphroditism, and strict gonochorism [10]. For example, Crassostrea virginica, Mizuhopecten yessoensis and Chlamys farreri are gonochoristic, while Argopecten purpuratus and Pecten maximus are hermaphroditic species. However, the role of Dmart genes in these quite diversified bivalves is not clear.
Previous studies have demonstrated that the Dmrt gene is associated with sex determination/differentiation in bivalves. For example, Dmrt is significantly differentially expressed between the ovaries and testes of scallops [11, 12], and RNAi of Dmrt can lead to the failure of gonadal differentiation in oysters [13]. However, many questions about the Dmrt gene in bivalves remain to be answered. For instance, how many types of Dmrt genes are present in bivalves? What are the potential functions of different types of Dmrt genes? With the genome decoding of many bivalves, it is now feasible to investigate genome-wide Dmrt genes. In the present study, genome-wide and comprehensive analyses of Dmrt genes were conducted. The findings from this study will lay a foundation for understanding the genomic organization and functional structure of the Dmrt genes in the available genomes of bivalves, which will be useful in the characterization of functional genomics.
Materials and methods
Identification of Dmrt sequences in bivalves
The Dmrt sequences in 15 bivalves were extracted using BLAST and HMM methods. First, the genomic sequences and annotation files of 15 species were downloaded from different databases (Supplementary Table S1). Second, BLAST (V2.11.0) [14] and HMMER (V3.2.1) [15] were used to search Dmrt sequences in each genome with the DM domain query (accession: PF00751) downloaded from Pfam (http://pfam.xfam.org/). The initial threshold expectation values for both the BLAST and HMMER searches were set to 1 × 10− 5 and 1.0, respectively. Third, all the candidate genes obtained by using BLAST and HMMER searches were merged, and redundant genes were removed. Finally, the nonredundant genes were checked for the presence of the DM domain according to an E-value (10− 5) by online SMART analysis [16]. When two or more transcripts were annotated for a gene from alternative splicing, the longest form with a DM domain was selected. The length of the amino acid sequence (AA), molecular weight (MW), isoelectric point (pI) and total average hydrophilicity (GRAVY) of Dmrt proteins were predicted using TBtools software (version 1.098) [17].
Phylogenetic analyses of the Dmrt gene family
A set of Dmrt protein sequences in different species was first obtained from the NCBI, JGI and UniProt databases (Supplementary Table S2). All retrieved Dmrt proteins and those identified in bivalves were used to perform phylogenetic analysis. Multiple sequence alignments of all Dmrt proteins were first generated using MAFFT v7.158b [18] with default parameters. Then, the phylogenetic tree was constructed using IQ-TREE v2.2.0 [19] with the option: -m MFP --bnni -B 1000 -T AUTO. The phylogenetic tree was visualized using iTOL (interactive tree of life) software [20] with the following settings: midpoint root and nonsorting leaf.
Sequence analyses and genomic distribution of Dmrts
The Batch SMART plug-in in TBtools software (version 1.098) [17] was used to identify the conserved domains of Dmrt genes, and the iTOL (interactive tree of life) online tool was used for visualization [20]. The general feature format (GFF3) file was used to retrieve the Dmrt gene structure and exon information. The conserved motifs of the Dmrt gene family were predicted using MEME [21] with the following parameters: maximum length of the conserved motif, 50; minimum length, 6, largest number, 20, and default values for other parameters. Conserved motifs and gene structure were visualized using TBtools software (version 1.098) [17].
Nonsynonymous (Ka) to synonymous substitution (Ks) ratio (Ka/ks) analysis
To understand the evolutionary rates of the Dmrt genes among scallops, a Bayesian inference approach for site-specific positive selection and purifying selection was used to estimate two types of substitution events by calculating the ratio of nonsynonymous (Ka) to synonymous substitutions (Ks) by the Selecton Server [22] with four evolutionary models (M5, M7, M8, and M8a).
Expression profiling of Dmrt in three bivalves
To understand the expression patterns of Dmrt genes in bivalves, publicly available RNA-seq data from A purpuratus, M. yessoensis, Mytilus coruscus, and Mercenaria mercenaria were downloaded from the NCBI SRA database (Supplementary Table S3). Raw RNA sequencing reads were trimmed using the NGStoolkit program [23] with the default parameters. Then, the reference genome was indexed, and the clean reads were mapped to the reference genome using HISAT2 [24]. After the resulting SAM files were converted into BAM files and sorted using SAMtools [25], the FPKM value of each gene was determined using StringTie v2.1.7 [26] based on the annotated gff file. Heatmaps of the gene expression levels were generated using the ggplot2 package in R software [27].
Results
Identification and characterization of Dmrt in bivalves
A total of 55 Dmrt genes were found in 15 bivalve genomes. The number of Dmrt genes in bivalve genomes ranged from 3 to 5. The amino acid sequences of all identified Dmrt genes are listed in Supplementary Table S4. The characteristics of all the identified proteins in 15 bivalves, including coding sequence length, number of amino acids, molecular weight, theoretical pI, instability index, aliphatic index, and grand average of hydropathicity (GRAVY), were predicted and are listed in Table 1. The results showed that the biophysical properties of different Dmrt proteins were relatively stable. AA length ranged from 164 to 3551, with a mean of 452.81. The molecular weight ranged from 19638.64 to 396231.09 Da, and the PI values ranged from 4.94 to 10.04. Additionally, all the Dmrt members have an instability index greater than 40.
Phylogenetic analysis of Dmrt genes
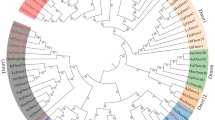
To explore the evolutionary history of Dmrt genes in bivalves, a phylogenetic analysis was performed using the DM-domain protein sequences from vertebrates and invertebrates. As shown in Fig. 1, the Dmrt proteins were clustered into 8 branches (Cluster I to Cluster VII), but bivalve DM-domain proteins were grouped into only five clusters, Cluster II, III, IV, V, and VI. The Cluster II, corresponding to Dmrt2, contains 7 bivalve DM-domain proteins. Cluster III corresponds to a Dmrt3 class and contains 15 bivalve Dmrt proteins. Twenty bivalve Dmrt proteins were grouped into the Dmrt4/5 class (Cluster IV). Interestingly, 5 scallop Dmrt proteins evolved into a separate cluster (Cluster V). In addition, 8 Dmrt proteins from 7 bivalves shared a close phylogenetic relationship with invertebrate Dsx genes.
Conserved domain, gene structure and motif analysis of Dmrt
A batch SMART search showed that all Dmrt genes included one to two DM domains, and some Dmrt genes contained Pfam:DMA and low_complexity_region (Fig. 2). Exon diversification among Dmrt genes is displayed in Fig. 3. Exon numbers of Dmrt genes in 15 bivalves ranged from 1 to 90. The exon numbers of Dmrt genes in Cluster II varied from 2 to 4. The majority of members in Clusters III and IV have two exons. In Cluster V, 4 Dmrt genes had three exons, and one Dmrt gene had 4 exons. All members in Cluster VI have no fewer than 4 exons. In addition, although all the predicted Dmrt proteins contain motif 1, the proteins in the same cluster also have similar motif structural features. For example, all members in Cluster V have the same motif structure and are quite different from the other clusters.
Selective pressure analysis
The Ka/Ks ratios of orthologous Dmrt genes among different clusters are shown in Table 2. The results indicated that the Ka/Ks ratios of the sequences from different Dmrt groups were significantly different. The highest Ka/Ks values in Cluster VI indicated a higher evolutionary rate within members of the Cluster IV. In contrast, the relatively low Ka/Ks values in Clusters IV and V implied a lower evolutionary rate or selective constraint within these groups. However, despite the differences in Ka/Ks values, all the estimated Ka/Ks values were substantially lower than 1, suggesting that the Dmrt sequences within each cluster are under purifying selection pressure.
Spatiotemporal expression profile in bivalves
RNA-seq datasets from different tissues and developmental stages of Dmrt genes in M. yessoensis were analyzed to investigate the expression patterns of different Dmrt genes. As shown in Fig. 4, the My|XP_021353714.1 and My|XP_021368788.1 in most developmental stages were not expressed (FPKM = 0) or expressed at low levels (FPKM < 1). From the trochophore stage, the expression of My|XP_021377273.1 showed a tendency to increase first followed by a decrease, while My|XP_021377274.1 exhibited high expression levels in juvenile stages. At the adult stage, the My|XP_021368788.1 and My|XP_021377273.1 were rarely expressed in all tissues, while the My|XP_021353714.1 and My|XP_021377274.1 showed specificity of high expression in male gonads and gills, respectively. Similar results can be learned in the hermaphrodite scallop A. purpuratus (Fig. 5). Ap|evm.model.scaffold_235.403 and Ap|evm.model.scaffold_95.77 were also specifically highly expressed in the testis and gill, respectively. The Ap|evm.model.scaffold_95.76 was rarely expressed in the studied tissues.
In M. mercenaria (Fig. 6), the Mme|XP_045157593.1 was expressed in multiple tissues, especially in gills. Similarly, the expression of Mme|XP_045159713.1 was also the highest in gills. However, the other Dmrt genes were not expressed (FPKM = 0) or were expressed at low levels (FPKM < 1) in all tissues.
The spatiotemporal expression profile of Dmrt genes in M. coruscus showed that no Dmrt genes were expressed (FPKM = 0) or they were expressed at low levels (FPKM < 1) in the trochophores, D-stage veliger, and umbo larvae (Fig. 7). In the pediveliger and juvenile stages, the Mc|CAC5360634.1 and Mc|CAC5397186.1 were still not expressed, while the Mc|CAC5398878.1 and Mc|CAC5404148.1 presented different levels of expression. At the adult stage, the Mc|CAC5398878.1, Mc|CAC5404148.1, and Mc|CAC5397186.1 were expressed in different tissues, while Mc|CAC5360634.1 was highly expressed in female gonads.
Discussion
Dmrt family genes have been considered sex-related genes because of their functions in sex determination/differentiation, testicular development, and embryo development [28, 29]. To date, genome-wide identification of Dmrt genes has been carried out in various animal groups but not in aquatic invertebrates [30,31,32]. In particular, a comprehensive survey and analysis of the Dmrt genes has not been conducted in bivalves. In this study, genome-wide identification of Dmrt genes was performed in 15 bivalve genomes. Three to five Dmrt genes have been identified in bivalve genomes. The number of Dmrt genes in bivalves was close to that of other invertebrates but lower than that observed in many teleosts. The difference in the number of Dmrt genes may be related to genome size and genomic duplication events [32]. Furthermore, the presence of different Dmrt genes in the animals seemed to be species specific. For example, Dmrt1 has only been identified in vertebrates, and Dmrt 6/7/8 is only present in mammals. In contrast, our results in this study showed that the Dmrt2-like, Dmrt3-like, Dmrt4/5-like and Dsx-like genes, but not the Dmrt1-like gene or Dmrt6/7/8-like gene, were identified in bivalves. Similar results were also found in Panarthropoda [33] and echinoderms [34].
Among the Dmrt genes identified in bivalves, some were identified in all the species studied, such as Dmrt3-like and Dmrt4/5-like genes, while the Dmrt2-like gene was only present in seven bivalves. Considering that Dmrt2 can be identified in the deuterostome [30, 34, 35], the origin and evolution of Dmrt2-like genes in bivalves should be studied further. Consistent with previous findings [30, 34, 35], the current phylogenetic analysis showed that Dmrt4 and Dmrt5 were clustered into a major branch, implying that these two types of genes originated from the same ancestor of Dmrt. The Dmrt4/5-like gene was identified in all bivalves and was duplicated in Mercenaria mercenaria, Cyclina sinensis, and Dreissena polymorpha. In addition, a Dsx-like gene class was found in the phylogenetic tree, although backed by low bootstrap values. This result may be related to little sequence conservation outside of the DM domains. Moreover, in this study, we identified a novel Dmrt class with unique exons and motifs that was phylogenetically distant from the other Dmrt members in scallops. Some novel Dmrt genes have also previously been identified in other aquatic invertebrates [34, 36, 37], suggesting that Dmrt genes in aquatic invertebrates may be distinct from those in other animals.
In the current study, the Dmrt4/5-like genes, including Ap|evm.model.scaffold_95.77, My|XP_021377274.1, Mc|CAC5398878.1, Mme|XP_045157593.1, and Mme|XP_045157593.1, were expressed in multiple tissues, especially in the gills of the four bivalves. Similar results can be found in other aquatic species. For instance, Dmrt4 is expressed only in the gills of Xiphophorus maculatus [38], and Dmrt4-like genes are highly expressed in the gills and mantle of Ruditapes philippinarum [39]. Previous reports have shown that Dmrt4 and Dmrt5 may be involved in neurogenesis. For example, in Xenopus, Dmrt4 and Dmrt5 are important regulators of olfactory placode neurogenesis [40, 41]. In Drosophila, Dmrt99B, an ortholog of Dmrt4/5, is required for initiating temporal patterning in medulla neuroblasts. Thus, it will be interesting to investigate whether Dmrt4/5 orthologs also play conserved roles in the neurogenesis of bivalves.
Previous studies have shown that the Dmrt2 gene participates in multiple biological processes, such as somitogenesis [42], oogenesis [43], and chondrocyte differentiation [44]. In the current study, the My|XP_021368788.1 was expressed in zygotes, 2–8 cells, and D-stage veliger, and the Mc|CAC5404148.1 was expressed from the umbo to juvenile stage. These results suggested that the Dmrt2-like genes might be involved in the early development of bivalves. To date, data on Dmrt3 genes are very limited. This gene has been confirmed to play a pivotal role in gonadal sex determination in fish [45] and spinal circuit function in mice (Andersson et al., 2012). The orthologs of Dmrt3 in the three bivalves showed different expression profiles, implying that these genes may perform different functions. However, it is worth noting that the Dmrt3-like (Mc|CAC5360634.1) gene is specifically expressed in the female gonad of M. coruscus. This result suggested that the Dmrt3-like gene may be an important gene involved in the ovary-determining pathway in M. coruscus. Moreover, the Dmrt3-like gene (Mg|VDI32052.1) in Mytilus galloprovincialis presents a unique gene structure, and the analysis based on multiple SRA datasets (Supplementary Table S3) did not detect the expression of this gene. Therefore, whether this gene is a pseudogene should be further verified.
In this study, the Dsx-like gene showed low expression in all tested stages and adult tissues of M. coruscus. Thus, the function of this type of gene remains to be further investigated. Interestingly, My|XP_021353714.1 was specifically expressed in the male gonad, suggesting that it may be an important gene involved in sex determination/differentiation. This result can also be learned in previous studies. For example, Li et al. (2016) showed that PyDMRT was testis-biased, and Li et al. (2018) suggested that the My|XP_021353714.1 (previously known as Dmrt1L) is a yang gene for determining the timing of sex differentiation in M. yessoensis [46]. Similar results were also found in Argopecten irradians [11], in which Dmrt1L showed male-biased expression in the gonad. In particular, the current study showed that the gene (Ap|evm.model.scaffold_235.403) from the hermaphrodite scallop A. purpuratus is also expressed specifically in the testis. Therefore, it is possible that the genes in Cluster V play pivotal roles in testis determination in scallops. In general, this study provides a molecular basis for Dmrt genes in bivalves.
Conclusions
In this study, Dmrt genes were identified and analyzed in 15 bivalves. A total of 55 Dmrt genes were identified, and the number of Dmrt genes in bivalves ranged from 3 to 5. The phylogenetic tree showed that all Dmrts from bivalves were classed into 5 clusters, corresponding to the Dmrt2-like class, Dmrt3-like class, Dmrt4/5-like class, Dsx-like class, and scallop-specific Dmrt class. Furthermore, the Ka/Ks ratios suggested that all the Dmrt clusters underwent purifying selection pressure. The spatiotemporal expression profile in bivalves suggested that different Dmrt genes may have different functions, and the scallop-specific Dmrt gene may play an important role in sex determination/differentiation. In general, this study provides a molecular basis for in-depth functional examination of Dmrt genes and phylogenomic analyses in bivalves.
Data availability
The datasets generated and/or analyzed during the current study are available in the NCBI repository [PRJNA259405, PRJNA428789, PRJEB35330, PRJNA578350, PRJNA533175, PRJNA638823, PRJNA785550, PRJEB58207, PRJNA740305, PRJEB35351, and PRJNA376014], [PERSISTENT WEB LINK OR ACCESSION NUMBER TO DATASETS], cfbase [http://mgb.ouc.edu.cn/cfbase/html/], GIGADB [http://www.gigadb.org/dataset/100419], dbSROG [http://soft.bioinfo-minzhao.org/srog/#], and DRYAD [https://datadryad.org/stash/dataset/doi:https://doi.org/10.5061/dryad.44j0zpcb5].
References
Smith CA, Roeszler KN, Ohnesorg T, Cummins DM, Farlie PG, Doran TJ, Sinclair AH. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature. 2009;461(7261):267–71.
Yoshimoto S, Okada E, Umemoto H, Tamura K, Uno Y, Nishida-Umehara C, Matsuda Y, Takamatsu N, Shiba T, Ito M. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proceedings of the National Academy of Sciences 2008, 105(7):2469–2474.
Kopp A. Dmrt genes in the development and evolution of sexual dimorphism. Trends Genet. 2012;28(4):175–84.
Zarkower D. DMRT genes in vertebrate gametogenesis. Curr Top Dev Biol. 2013;102:327–56.
Zhang T, Zarkower D. DMRT proteins and coordination of mammalian spermatogenesis. Stem cell research. 2017;24:195–202.
Ottolenghi C, Fellous M, Barbieri M, McElreavey K. Novel paralogy relations among human chromosomes support a link between the phylogeny of doublesex-related genes and the evolution of sex determination. Genomics. 2002;79(3):333–43.
Kim S, Kettlewell JR, Anderson RC, Bardwell VJ, Zarkower D. Sexually dimorphic expression of multiple doublesex-related genes in the embryonic mouse gonad. Gene Expr Patterns. 2003;3(1):77–82.
Rather MA, Dhandare BC. Genome-wide identification of doublesex and mab-3-Related transcription factor (DMRT) genes in nile tilapia (Oreochromis niloticus). Biotechnol Rep. 2019;24:e00398.
Volff J-N, Zarkower D, Bardwell VJ, Schartl M. Evolutionary dynamics of the DM domain gene family in metazoans. J Mol Evol. 2003;57(1):241–S249.
Breton S, Capt C, Guerra D, Stewart D. Sex-determining mechanisms in bivalves. Transitions between sexual systems 2018:165–92.
Wei H, Li W, Liu T, Li Y, Liu L, Shu Y, Zhang L, Wang S, Xing Q, Zhang L. Sexual development of the hermaphroditic scallop Argopecten irradians revealed by morphological, endocrine and molecular analysis. Front cell Dev biology. 2021;9:646754.
Li Y, Zhang L, Sun Y, Ma X, Wang J, Li R, Zhang M, Wang S, Hu X, Bao Z. Transcriptome sequencing and comparative analysis of ovary and testis identifies potential key sex-related genes and pathways in scallop Patinopecten yessoensis. Mar Biotechnol. 2016;18(4):453–65.
Sun D, Yu H, Li Q. Examination of the roles of Foxl2 and Dmrt1 in sex differentiation and gonadal development of oysters by using RNA interference. Aquaculture. 2022;548:737732.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–10.
Eddy SR. Profile hidden Markov models. Bioinf (Oxford England). 1998;14(9):755–63.
Schultz J, Copley RR, Doerks T, Ponting CP, Bork P. SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res. 2000;28(1):231–4.
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–202.
Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–80.
Nguyen L-T, Schmidt HA, Von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32(1):268–74.
Letunic I, Bork P. Interactive tree of life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019;47(W1):W256–9.
Bailey TL, Johnson J, Grant CE, Noble WS. The MEME suite. Nucleic Acids Res. 2015;43(W1):W39–W49.
Doron-Faigenboim A, Stern A, Mayrose I, Bacharach E, Pupko T. Selecton: a server for detecting evolutionary forces at a single amino-acid site. Bioinformatics. 2005;21(9):2101–3.
Patel RK, Jain M. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PLoS ONE. 2012;7(2):e30619.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–60.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–9.
Pertea M, Pertea GM, Antonescu CM, Chang T-C, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33(3):290–5.
Team RC. R: A language and environment for statistical computing. 2013.
Hong C-S, Park B-Y, Saint-Jeannet J-P. The function of dmrt genes in vertebrate development: it is not just about sex. Dev Biol. 2007;310(1):1–9.
Picard MA-L, Cosseau C, Mouahid G, Duval D, Grunau C, Toulza È, Allienne J-F, Boissier J. The roles of Dmrt (double sex/Male-abnormal-3 related transcription factor) genes in sex determination and differentiation mechanisms: Ubiquity and diversity across the animal kingdom. C R Biol. 2015;338(7):451–62.
Dong J, Li J, Hu J, Sun C, Tian Y, Li W, Yan N, Sun C, Sheng X, Yang S et al. Comparative Genomics Studies on the dmrt Gene Family in Fish. Front Genet 2020, 11.
Wan H, Zhong Z, Jiang Y, Zou P, Zhang Z, Wang Y. Genome-wide investigation of Dmrt gene family in large yellow croaker (Larimichthys crocea). Theriogenology. 2020;156:272–82.
Xu S, Zhang S, Zhang W, Liu H, Wang M, Zhong L, Bian W, Chen X. Genome-Wide Identification, Phylogeny, and Expression Profile of the Dmrt (Doublesex and Mab-3 Related Transcription Factor) Gene Family in Channel Catfish (Ictalurus punctatus). In: Front Genet vol. 13; 2022: 891204.
Panara V, Budd GE, Janssen R. Phylogenetic analysis and embryonic expression of panarthropod dmrt genes. Front Zool. 2019;16:23.
Wang Q, Cao T, Wang Y, Li X, Wang Y. Genome-wide identification and comparative analysis of dmrt genes in echinoderms. Sci Rep. 2023;13(1):7664.
Wexler JR, Plachetzki DC, Kopp A. Pan-metazoan phylogeny of the DMRT gene family: a framework for functional studies. Dev Genes Evol. 2014;224(3):175–81.
Peng B, Wan H, Zhang Z, Jia X, Liu C, Wang Y. A novel dmrt gene of crustacean: functional analysis of idmrt-2 gene in the male reproductive system from Scylla paramamosain. Gene. 2023;850:146922.
Abayed FAA, Manor R, Aflalo ED, Sagi A. Screening for dmrt genes from embryo to mature Macrobrachium rosenbergii prawns. Gen Comp Endocrinol. 2019;282:113205.
Veith A-M, Schäfer M, Klüver N, Schmidt C, Schultheis C, Schartl M, Winkler C, Volff J-N. Tissue-specific expression of dmrt genes in embryos and adults of the platyfish Xiphophorus maculatus. Zebrafish. 2006;3(3):325–37.
Wu Q, Tan Y, Wang J, Xie Q, Huo Z, Fang L, Yan X. Effect of estradiol stimulation on Dmrt gene expression in Manila clam Ruditapes philippinarum. J Dalian Ocean Univ. 2019;34(3):362–9.
Parlier D, Moers V, Van Campenhout C, Preillon J, Leclère L, Saulnier A, Sirakov M, Busengdal H, Kricha S, Marine J-C, et al. The Xenopus doublesex-related gene Dmrt5 is required for olfactory placode neurogenesis. Dev Biol. 2013;373(1):39–52.
Huang X, Hong C-S, O’Donnell M, Saint-Jeannet J-P. The doublesex-related gene, XDmrt4, is required for neurogenesis in the olfactory system. Proc Natl Acad Sci. 2005;102(32):11349–54.
Tingler M, Brugger A, Feistel K, Schweickert A. dmrt2 and myf5 link early somitogenesis to left-right Axis determination in Xenopus laevis. Front cell Dev biology 2022, 10.
Kasahara R, Yuzawa T, Fujii T, Aoki F, Suzuki MG. dmrt11E ortholog is a crucial factor for oogenesis of the domesticated silkworm, Bombyx mori. Insect Biochem Mol Biol. 2021;129:103517.
Ono K, Hata K, Nakamura E, Ishihara S, Kobayashi S, Nakanishi M, Yoshida M, Takahata Y, Murakami T, Takenoshita S, et al. Dmrt2 promotes transition of endochondral bone formation by linking Sox9 and Runx2. Commun Biol. 2021;4(1):326.
Ren Y, Mu Y, Zhao B, Gao Y, Dai X, Chu Z. dmrt3, nom1, abce1, and pkmyt1 play key roles in gonadal sex determination in Acrossocheilus fasciatus. Aquacult Int 2022.
Li R, Zhang L, Li W, Zhang Y, Li Y, Zhang M, Zhao L, Hu X, Wang S, Bao Z. FOXL2 and DMRT1L are yin and yang genes for determining timing of sex differentiation in the bivalve mollusk Patinopecten yessoensis. Front Physiol. 2018;9:1166.
Acknowledgements
This work was supported by the High-Performance Computing Platform of Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences.
Funding
This work was supported by the Open Project Program of Key Laboratory of Ecological Warning, Protection & Restoration for Bohai Sea, Ministry of Natural Resources [2022204], National Natural Science Foundation of China [31972791], the Earmarked Fund for Agriculture Seed Improvement Project of Shandong Province [2020LZGC016], the Earmarked Fund for Shandong Modern Agro-Industry Technology Research System [SDAIT-14], Natural Science Foundation of Shandong Province [ZR2021MC151], and Yantai Science and Technology Planning Project [2020MSGY074].
Author information
Authors and Affiliations
Contributions
Q.W. conducted the experiment and data processing. Q.W. and C.W. conceived and supervised the project. T.C. contributed to the data collection. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Each of the procedures that were used during this study was approved by the Key Laboratory of Coastal Biology and Biological Resources Utilization, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences prior to the initiation of the study and the Animal Management Regulations, revised on March 1, 2017, No. 676.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Q., Cao, T. & Wang, C. Genome-wide identification and expression analysis of Dmrt genes in bivalves. BMC Genomics 24, 457 (2023). https://doi.org/10.1186/s12864-023-09536-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09536-6