Abstract
Background
As a type of calmodulin binding protein, CAMTAs are widely involved in vegetative and reproductive processes as well as various hormonal and stress responses in plants. To study the functions of CAMTA genes in tea plants, we investigated bioinformatics analysis and performed qRT-PCR analysis of the CAMTA gene family by using the genomes of ‘ShuChaZao’ tea plant cultivar.
Results
In this study, 6 CsCAMTAs were identified from tea plant genome. Bioinformatics analysis results showed that all CsCAMTAs contained six highly conserved functional domains. Tissue-specific analysis results found that CsCAMTAs played great roles in mediating tea plant aging and flowering periods. Under hormone and abiotic stress conditions, most CsCAMTAs were upregulated at different time points under different treatment conditions. In addition, the expression levels of CsCAMTA1/3/4/6 were higher in cold-resistant cultivar ‘LongJing43’ than in the cold-susceptible cultivar ‘DaMianBai’ at cold acclimation stage, while CsCAMTA2/5 showed higher expression levels in ‘DaMianBai’ than in ‘LongJing43’ during entire cold acclimation periods.
Conclusions
In brief, the present results revealed that CsCAMTAs played great roles in tea plant growth, development and stress responses, which laid the foundation for deeply exploring their molecular regulation mechanisms.
Similar content being viewed by others
Introduction
The divalent ions of calcium (Ca2+) is an universal secondary messenger, which served as a core sensor and regulator of plants in dealing with growth, development and various environment stimuli [1,2,3,4]. Until now, there are three important calcium sensors, including calmodulins/calmodulin-like proteins (CaMs/CMLs), calcineurin B-like proteins (CBLs) and calcium-dependent protein kinases (CDPKs) have been identified in plants [5]. Among them, CaMs are regarded as main calcium sensors in the process of calcium signal transduction, which can sense the change of calcium concentration and participate in numerous of physiological activities by regulating downstream target proteins in plant. It has been demonstrated that more than 90 types of transcription factors, including CAMTAs (CaM-binding transcription activators), bZIPs (basic leucine zipper), MYBs (myeloblastosis), NACs (NAM/ATAF/CUC) and WRKYs (WRKYGQK), etc., were reported as downstream target TFs that regulated by CaMs [6,7,8,9]. Among them, CAMTAs, also called signal responsive (SR) proteins or ethylene-induced CaM-binding proteins (EICBP), are referred as central CaM-binding proteins (CBPs), which have been confirmed to mediate entire life cycles of multicellular eukaryotes from plants to humans [10,11,12]. In plant, it has been clear that CAMTAs contain six conserved functional domains, including nuclear localization signals (NLS) function in targeting protein into nucleus, CG-1 domain (CG-1) implicated in DNA binding [11, 12], TIG domain implicated in nonspecific DNA interactions [13], ankyrin (ANK) repeats involved in protein–protein interaction [14, 15], IQ motifs (IQXXXRGXXXR) combined with CaM [16], and calcium dependent CaM binding domain(CaMBD)contributed to the combination of Ca2+-loaded CaM to CAMTAs [17]. In addition to functional domains, two cis-acting elements, (G/A/C) CGCG (C/G/T) and (A/C) CGTGT, have been identified as specific CAMTA-binding sites in plants [12, 17, 18].
Currently, lots of CAMTAs genes have been identified from different plant species, such as 6 AtCAMTAs from Arabidopsis [11], 10 VvCAMTAs from grape [19], 9 ZmCAMTAs from maize [18], 7 SlCAMTAs from tomato [20], 5 MaCAMTAs from banana [21], 7 MsCAMTAs from alfalfa [22], 9 LuCAMTAs from flax [23], 9 CsCAMTAs from citrus [24]. Among them, numerous CAMTAs have been shown to play great roles in the regulation of plant growth and development, hormones, biotic and abiotic stress responses, especially in low temperature responses [12, 15, 25, 26]. Under cold condition, the increased Ca2+ contents could promote the combination of CAMTAs with ‘CCGAC’ cis-acting element, and then induce the expressions of many downstream genes, thus rapidly respond to cold stress and enhance cold adaptability and tolerance of plants [24, 27]. In Arabidopsis, the spatio-temporal expressions of all 6 AtCAMTAs were rapidly and differentially influenced by various hormones, biotic and abiotic stresses [3]. Galon et al. (2010) reported that AtCAMTA1-3 were referred as negative regulators of auxin, which correlated to red light and high light responses, while AtCAMTA4-6 were functioned as positive regulators to regulate auxin signaling and homeostasis [28]. Besides, AtCAMTA1 transcripts were triggered by exogenous auxin with a cell-specific manner, mutation of AtCAMTA1 stunted root and rosette leaves development, meanwhile, camta1 showed higher sensitivity to drought stress with poor water use efficiency (WUE), low photosystem II efficiency, declined in relative water content (RWC) and reduced survivability [29]. As a negative regulator of plant immunity, AtCAMTA3 could inhibit enhanced disease susceptibility 1 (EDS1) transcripts by interacting with its promoter, while the mutation of AtCAMTA3 could stimulate EDS1 transcripts and improve salicylic acid accumulation, and thus enhance disease resistance of camta3 mutants [23]. Even so, it has also reported that AtCAMTA3 could positively mediate the freezing tolerance of Arabidopsis through binding to the CG-1 DNA-binding sites in the promoters of core binding factor (CBFs) [30]. Further research found that CAMTA1-3 could synergistically induce the highest expressions of CBF1-3 after 2 h of 4 °C chilling treatment, following lead to the up-regulation of more than 15% cold responsive genes in CBF independent pathway, and thus enhance the freezing tolerance of Arabidopsis [31]. Apart from 6 AtCAMTAs, the functions of many CAMTAs in plants also have been extensively explored. In citrus, the expressions of 8 CitCAMTAs genes were regulated by various stress and hormone treatments [24]. 7 SlSR/CAMTAs of tomato showed differential expressions during fruit development and ripening [20]. In wheat genome, about 584 genes were predicted to contain ACGCGG/CCGCGT cis-acting elements in their promoter regions, suggesting that these genes could be considered as potential target genes of TaCAMTAs, which mainly participated in RNA regulation, protein degradation, signaling transduction, biotic and abiotic stresses, hormone metabolism, and lipid metabolism [25]. Similarly, many stress-related cis-acting elements also presented in the promoter regions of some ZmCAMTA genes, suggesting that ZmCAMTAs widely involved in stress responses. Specifically, ZmCAMTAs transcripts were rapidly triggered by maize rough dwarf disease (RBSDV) infection, of which ZmCAMTA6/7a showed differential expressions between disease-tolerant and disease-sensitive cultivars [18].
Tea plant (Camellia sinensis) is a type of evergreen woody plants, which is mainly distributed in tropical and subtropical regions of the Northern hemisphere. Generally, tea plant is suitable for acid soil (pH4.5–6.5), high moisture, and normal temperature conditions. However, with the frequent occurrence of extreme climates, such as freezing, cold spell in spring, drought and heat etc., the growth, tea production and quality are seriously retarded in recent years. Therefore, more and more researchers are focusing on how to improve the stress-resistance of tea plants, of which the molecular mechanisms in responding to environmental stimuli are the main research areas. Currently, lots of studies have demonstrated that calcium signaling plays critical role in dealing with various stresses in tea plant, and multiples genes (e.g. CsCBLs, CsCDPKs, CsCIPKs and CsCMLs) involved in calcium signaling were up-regulated under stress conditions [26, 32]. Based on the tea plant genome, many genes associated with calcium signaling perception and transduction have been comprehensively identified and further performed expression analysis under various stresses treatment conditions [32,33,34]. However, as the central CBPs in calcium signaling pathway, the functions of CAMTAs have not been extensively explored in tea plant. In the present study, we systematically performed genome-wide analysis of CAMTA genes and widely explored their tissue-specific and spatial–temporal expressions profiles in tea plant. These results will provide a solid theoretical foundation for intensive study on the role of calcium signal in stress responses of tea plant.
Methods
Plant materials and stress treatments
The one bud and two leaves, mature leaves, senescent leaves, flower buds, mature flowers, young fruits, young stems, mature stems and roots of ten-year-old clonal tea plant cultivar ‘ShuChaZao’ were sampled for tissue-specific analysis. Each tissue was performed three independent biological replicates, and all samples were quickly frozen in liquid nitrogen and stored at -80 °C until used.
The one-year-old clonal cuttings of ‘ShuChaZao’ were used to perform 3% H2O2 treatment. Before processing, all cuttings were moved into chamber for adjusting growth one week, and the culture conditions were as follows: temperature 25℃, 14 h light/10 h darkness, humidity 75%. For H2O2 treatments, the tea plants were sprayed with 3% H2O2, and the samples were collected at 0 h, 6 h, 12 h and 24 h. Three biological replicates were performed for each treatment, and all samples were frozen in liquid nitrogen and stored at -80 °C. The above mentioned tea plants were cultivated in the greenhouse of the Tea Research Institute of Qingdao Agricultural University (TRI, QAU, N36°33′, E120°4′).
The methods of cold, polyethylene glycol (PEG), NaCl, abscisic acid (ABA) and gibberellin (GA) treatments were performed as described by Wang et al. (2021) [35]. In brief, one-year-old clonal cutting seedlings of the ‘LongJing43’ cultivar with similar growth potential were used to process different treatments. 4 °C, PEG-6000 (10% (w/v)) and 250 mmol·L−1 NaCl were respectively used to imitate cold (CT), drought (DT) and salt (ST) treatment, and 100 μmol·L−1 ABA and 100 μmol·L−1 GA were sprayed onto the surfaces of tea leaves to imitate hormone treatments. Each treatment was proceeded 2 d and three independent biological replicates. The third and/or fourth mature leaves from the terminal bud were sampled at 0, 12, 24 and 48 h within treatment periods, and then all samples were quickly frozen in liquid nitrogen and stored at -80 °C until used. The above mentioned tea plants were cultivated in the greenhouse of the Tea Research Institute of Qingdao Agricultural University (TRI, QAU, N36°33′, E120°4′).
Eighteen-year-old of two tea plant cultivars, ‘LongJing43’ and ‘DaMianBai’, with different cold resistance as reported by Wang et al. (2019) [36], were used to perform cold acclimation (CA) analysis. The sampling method was performed as described by Qian et al. (2018) [37]. The above mentioned tea plants were cultivated at the Tea Research Institute of the Chinese Academy of Agricultural Sciences (TRI, CAAS, N30°10′, E120°5′).
Genome-wide identification of the CAMTA genes from tea plant genome
In order to obtain putative CAMTA genes, four Hidden Markov Models (HMM) files of CAMTA functional domains, including CG-1 domain (PF03859), IPT/TIG domain (PF01833), Ankyrin repeat (PF00023), and IQ domain (PF00612) were respectively downloaded from protein families (Pfam) database (http://pfam.xfam.org/) [38]. Subsequently, the HMM profiles were respectively performed blast search in the tea plant protein database of the ‘ShuChaZao’ cultivar as reported by Wei et al. (2018) by using HMMER 3.0 software [39]. Following, both the simple modular architecture research tool (SMART) server (http://smart.embl-heidelberg.de/) [40] and conserved domain database of national center for biotechnology information (NCBI) (https://www.ncbi.nlm.nih.gov/cdd/advanced) [41] were used to further ensure whether the obtained sequences contain the conserved CAMTA functional domains, such as the CG-1 domain, IQ motifs, Ank repeats, and IPT/TIG. Finally, those sequences that met the above conditions were reserved for subsequent analysis.
Bioinformatics analysis of CsCAMTAs in tea plant
The opening reading frame (ORF) lengths of CsCAMTAs were predicted by using the NCBI ORF finder website (https://www.ncbi.nlm.nih.gov/orffinder/). The molecular weights, theoretical pI, instability index and aliphatic index were predicted by using protein parameter (ProtParam) tool (http://web.expasy.org/protparam/) [42]. Signal peptides and transmembrane regions (TMHs) were respectively predicted with the Signal peptide (SignalP) server (http://www.cbs.dtu.dk/services/SignalP) [43] and the transmembrane protein topology with a hidden Markov model (TMHMM) Server v.2.0 (http://www.cbs.dtu.dk/services/TMHMM/) [44], and the plant multiple protein locations (PlantmPLoc) web server (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/) [45] was used to predict the sub-cellular location of CsCAMTAs.
Phylogenetic analysis of CsCAMTAs
In order to explore the evolutionary relationship of CAMTAs in different plant species, a total of 88 CAMTAs protein sequences (Table S1) originated from tea plant, Arabidopsis, Oryza sativa, Populus trichocarpa, Malus pumila, Triticum aestivum, Nicotiana tabacum and Zea mays were used to construct a phylogenetic tree based on the neighbor-joining method of MEGA 7.0 software [46]. The detailed parameters were as follows: 1000 repeated bootstrap tests, p-distance method and pairwise deletion treatment. Finally, the ITOL web server (https://itol.embl.de/) [47] was further used to beautify and generate the phylogenetic tree.
Chromosomal distribution, Ka/Ks ratios, and synteny analysis of CsCAMTAs
The chromosomal positions of CsCAMTAs, collinearity analysis within ‘ShuChaZao’ genome and the synteny analysis of ‘ShuChaZao’ cultivar associated with Arabidopsis, Oryza sativa, Zea may and another two tea plant cultivars (‘HuangDan’ and ‘TieGuanYin’) genomes were performed and visualized by using TBtools software as demonstrated by Chen et al. (2020) [48]. Besides, the synonymous substitution rate (Ks) values, nonsynonymous substitution rate (Ka) values and the ratios of Ka/Ks were also performed by using TBtools software [48]. The genomes of Arabidopsis, Oryza sativa, and Zea may were downloaded from NCBI web (https://www.ncbi.nlm.nih.gov/datasets/genomes/). The genomes of ‘HuangDan’ and ‘TieGuanYin’ cultivars were downloaded from national genomics data center (NGDC) (https://ngdc.cncb.ac.cn/) [49] by using accession number GWHAZTZ00000000 [50] and GWHASIV00000000 [51].
Gene structure, protein domain distribution and cis-acting element analysis
The coding sequences (CDSs) and the corresponding genomic sequences of CsCAMTAs were submitted into the gene structure display server 2.0 (GSDS2.0) website (http://gsds.cbi.pku.edu.cn/) [52] to predict their exon–intron structures. The SMART web server (http://smart.embl-heidelberg.de/) [40], classification of protein families (InterPro) database (https://www.ebi.ac.uk/interpro/search/sequence/) [53], Motif Scan database (https://myhits.sib.swiss/cgi-bin/motif_scan#GRAPHIC) [54] and CaMBD database (http://calcium.uhnres.utoronto.ca/ctdb/ctdb/home.html) was used to search the putative functional domains of CsCAMTAs. In order to understand the expression regulation factors of CsCAMTAs, 2000-bp upstream noncoding region sequence of the translation initiation site (ATG) in each CsCAMTA genome sequence was submitted into plant cis-acting regulatory element (PlantCARE) web server (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) [55] to predict putative cis-acting elements involved in responding to stresses and hormones. The results were further visualized in illustrator for the presentation and visualization of biological sequences (IBS) web server (http://ibs.biocuckoo.org/online.php) [56].
qRT-PCR analysis
RNA extraction kit (Bioflux, Hangzhou, China) and RT reagent Kit (Takara, Dalian, China) were respectively used to isolate total RNA and synthesize the first-strand cDNA following the corresponding instruction of kits. The qRT-PCR technique was performed as described by Wang et al. (2021) [35]. In brief, total of 20.0 μL reaction mix (10.0 μL SYBR Premix Ex Taq, 2 μL cDNA, 1.6 μL forward/reverse primers, and 6.4 μL distilled water) were amplified according to the following qRT-PCR programs: 95 °C, 15 s (step 1); 94 °C, 5 s following 60 °C, 30 s with 40 cycles (step 2); adding melting curve (step 3). A reference gene, polypyrimidine tract-binding protein (CsPTB) of tea plant [57] was used to quantify the relative expression levels of CsCAMTAs. The results were calculated by 2−ΔCt or 2−ΔΔCt method [58], and finally visualized as the mean values ± standard error (± SE). The qRT-PCR primers are listed in Table S2.
Results
Identification of CAMTA family genes in tea plants
Based on HMM files of CAMTA functional domains, and the conformation of the SMART server and the CD databases of NCBI, total of six putative CsCAMTAs, named as CsCAMTA1-6, were identified from ‘ShuChaZao’ tea plant cultivar genome. As bioinformatics analysis results showed that the ORF lengths of CsCAMTAs were varied from 2.772 kb to 3.294 kb, the amino acids lengths ranged from 924 to 1098 aa, the molecular weights (MW) range of 104.48–123.95 kDa, the theoretical isoelectric point (pIs) changed from 5.39 to 7.38, and all of them were predicted to be unstable proteins except for CsCAMTA4. Besides, all of them were predicted to contain no signal peptides and TMHs, and predicted to locate in nucleus (Table 1).
Phylogenetic analysis of CsCAMTAs
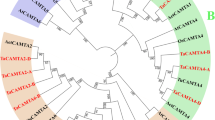
Phylogenetic analysis result showed that all 88 CAMTAs were grouped into three subfamilies, of which 4 CsCAMTAs were clustered into subfamily I (Fig. 1). Besides, 6 CsCAMTAs were clustered into 3 subgroups and showed closest relationship with NtCAMTAs except for CsCAMTA4. One 2:2 ortholog gene-pairs (CsCAMTA2 and CsCAMTA3/NtCAMTA2 and NtCAMTA3) with more than 88% bootstrap values were found between tea plant and tobacco. Furthermore, the 2:3 ortholog gene pairs (CsCAMTA1 and CsCAMTA6/NtCAMTA1, NtCAMTA4 and NtCAMTA7) with more than 92% bootstrap values were identified between tea plant and tobacco.
Phylogenetic analysis of CsCAMTAs and known CAMTAs of other plant species. A total of 88 CAMTA protein sequences from tea plant, Arabidopsis, rice, banana, poplar, apple, wheat, tobacco, and maize were used to construct phylogenetic tree. The amino acids sequences were listed in Table S1
Chromosomal distribution and synteny analysis of CsCAMTAs
To investigate the chromosomal distribution of CsCAMTAs, the CDS sequences of CsCAMTAs were matched on ‘ShuChaZao’ genome by means of TBtools software. As shown in Fig. 2A and Table S3, CsCAMTA1-3, 5 were respectively distributed on Chr9, Chr6, Chr10, and Chr2, while CsCAMTA4/6 were co-distributed on Chr5. The similar distributions were also respectively found in the genomes of ‘HuangDan’ and ‘TieGuanYin’ cultivars with the exception of CsCAMTA5 (Fig. S1).
Chromosomal distribution and synteny analysis of CsCAMTAs. A The chromosomal distribution of CsCAMTAs in ‘ShuChaZao’ genome. B Collinearity analysis of CsCAMTAs within ‘ShuChaZao’ genome. C The interspecies synteny analysis of CsCAMTAs in ‘ShuChaZao’ associated with Arabidopsis, Oryza sativa, Zea mays, and ‘HuangDan’ and ‘TieGuanYin’ cultivars
In addition to chromosomal distribution, we also explored the collinearity relationships of CsCAMTAs within the genome of ‘ShuChaZao’ cultivar. As Fig. 2B and Table S4 showed that a same chromosomal distribution result was also obtained by collinearity analysis in the genome of ‘ShuChaZao’ cultivar. Besides, two segmental duplication events (CsCAMTA1/6 and CsCAMTA2/3) were identified in the ‘ShuChaZao’ tea plant cultivar genome with the exception of CsCAMTA4/5. On the other hand, the Ka/Ks ratios of these CsCAMTAs were calculated, and we found that the Ka/Ks ratios of 17 pairs of CsCAMTAs were all lower than 1 (Table S5), which suggested that all CsCAMTAs underwent purification selection during evolution periods.
To further insight into the evolutionary relationships of CsCAMTAs, five comparative syntenic maps of ‘ShuChaZao’ tea plant cultivar genome associated with Arabidopsis genome, Oryza sativa genome, Zea may genome and another two tea plant cultivar genomes (‘HuangDan’ and ‘TieGuanYin’) were constructed respectively. As shown in Fig. 2C and Table S6, both CsCAMTA2 and CsCAMTA3 possess common orthologous genes in Arabidopsis (AtCAMTA4, AT1G67310), rice (OsCAMTA7, LOC4327253), ‘HuangDan’ (HD.03G0000940.t1 and HD.11G0000910.t1) and ‘TieGuanYin’ (TGY050530.t1 and TGY050530.t1). Besides, CsCAMTA3 possesses another orthologous gene in rice (OsCAMTA2, LOC4335664) and Zea mays (ZmCAMTA4, LOC103642708). CsCAMTA4 possesses one orthologous gene in ‘HuangDan’ (HD.08G0000210.t1) and ‘TieGuanYin’ (TGY050137.t1), respectively. CsCAMTA5 possesses one orthologous gene in Arabidopsis (AtCAMTA3, AT2G22300), and two orthologous genes in ‘HuangDan’ (HD.01G0023840.t1 and HD.04G0022130.t1) and ‘TieGuanYin’ (TGY007127.t1 and TGY014016.t1) respectively. Similarly, both CsCAMTA1 and CsCAMTA6 also possess one common orthologous gene in Arabidopsis (AtCAMTA6, AT3G16940), and two orthologous genes in ‘HuangDan’ (HD.08G0000960.t1 and HD.10G0029650.t1) and ‘TieGuanYin’ (TGY049973.t1 and TGY077391.t1) respectively. Moreover, CsCAMTA1 possesses another orthologous gene in Arabidopsis (AtCAMTA5, AT4G16150). These results were corresponded to the phylogenetic analysis result, where we found the orthologous gene pairs were grouped into same branches.
Cis-acting elements and exon–intron structures analysis of CsCAMTAs
To investigate the regulatory mechanisms of CsCAMTAs in response to various stresses and hormones, the cis-acting elements in 2000 bp promoter sequence of each CsCAMTAs were predicted. As shown in Fig. 3A, the distribution, number and type of cis-acting elements of CsCAMTAs are varied among each other, and many myeloblastosis (MYB) and myelocytomatosis (MYC) binding sites contained in the promoter region of each CsCAMTA. Besides, CsCAMTAs possess many light response cis-acting elements in their promoter regions. In addition, many stress-responsive elements, such as low-temperature responsiveness element (LTR), abscisic acid responsiveness element (ABRE), anaerobic induction element (ARE), salicylic acid responsiveness element (SA), MYB binding site involved in drought-inducibility element (MBS), methyl jasmonate responsiveness element (MeJA), auxin responsiveness element (AUX), defense and stress responsiveness element (DSRE) were enriched in the promoters of CsCAMTAs. For example, LTR elements were enriched in the promoter regions of CsCAMTA2-5, ABRE elements were enriched in the promoter regions of CsCAMTA3-6, MBS elements were enriched in the promoter regions of CsCAMTA2/5/6. These results demonstrated that each CsCAMTA plays an important role in coping with diurnal changes, hormones, and abiotic stresses.
The cis-acting elements in promoters of CsCAMTAs and exon–intron structures of CsCAMTAs. A cis-acting elements in promoters of CsCAMTAs. 2000-bp upstream noncoding region sequences of CsCAMTAs were used to predict cis-acting elements, and different colored blocks represent different elements. B The exon–intron structures of CsCAMTAs. Green boxes represent untranslated upstream/downstream regions, yellow boxes represent exons, and lines indicate introns
GSDS 2.0 was used to explore the structural diversity of CsCAMTAs. As Fig. 3B shown, the exon–intron distribution patterns of CsCAMTAs gene family are varied in terms of intron length and exon number. Among them, CsCAMTA2/3/4 possess same numbers of exons and introns, including 12 exons and 11 introns. Besides, both CsCAMTA1 and CsCAMTA5 contain 13 exons and 12 introns, while CsCAMTA6 contains 11 exons and 10 introns.
Motifs and protein domain compositions of CAMTAs
MEME tool was used to comprehend the motif conserveness among all 6 CsCAMTAs. Correspondingly, CsCAMTAs found to be highly conserved, and all of them contain motif 1–12 (Fig. 4A). However, motif 14 is not contained in CsCAMTA3, and motif 15 is just contained in CsCAMTA2/3. In addition, both CsCAMTA1 and CsCAMTA6 contain 2 motif 13, while CsCAMTA2/3/4/5 only contain 1 motif 13 respectively.
To further dissect the functions of CsCAMTAs, the conserved domains of each CsCAMTA were analyzed by the SMART server and the CD databases of NCBI. As shown in Fig. 4B, all of CsCAMTAs possess 6 conserved domains, including NLS, CG-1, ANK repeats, IQ motifs, TIG and CaMBD domain (Fig. 4B). However, the numbers of IQ motifs and ANK repeats were varied from each other, among of which CsCAMTA1/2/3/6 contain 2 IQ motifs respectively, and CsCAMTA4/5 just contain 1 IQ motif. Besides, 3 ANK repeats were contained in CsCAMTA4, 2 ANK repeats were contained in CsCAMTA2/5, and 1 ANK repeat was contained in CsCAMTA1/3/6 respectively. Moreover, we found the conserved CG-1 located in motif 1/9/12, and TIG, CaMBD and NLS domains respectively located in motif 4/10/5 were all contained in CsCAMTAs. These results demonstrated that each of CsCAMTA may be targeted by different CaMs or served as different types of binding proteins.
Tissue-specific analysis of CsCAMTAs in different tea plant tissues
The tissue-specific of CsCAMTAs were detected in 9 different tissues of ‘ShuChaZao’ cultivar. As Fig. 5 shown, the transcription abundance of each CsCAMTA was varied across the various tissues. Among them, CsCAMTA2/3/5/6 showed high transcription abundances in all detected tea plant tissues, and the transcription abundances of these genes were higher in senescent leaves, flowers and roots than that in the other tea plant tissues. Similarly, CsCAMTA1/4 also showed higher expression levels in senescent leaves and flower than that in the other tea plant tissues. In brief, our results demonstrated that CsCAMTAs mediated entire vegetative and reproductive progress of tea plant, especially in aging and flowering periods.
Expression analysis of CsCAMTAs under various abiotic stress conditions
To elucidate the spatial–temporal expression patterns of CsCAMTAs under various abiotic stress conditions, the transcription abundances of CsCAMTAs were detected. As shown in Fig. 6, CsCAMTAs were differentially expressed under different stress conditions. Specifically, all of CsCAMTAs were remarkably induced by CT, and their expression levels were more than twofold higher than those at 0 h of CT. In particular, the expression level of CsCAMTA4 was more than 30-fold higher after 12 h of CT, and the expression levels of CsCAMTA1/2 were also more than 30-fold higher after 1 d of CT. Similarly, the expressions of all CsCAMTAs were also up-regulated by DT at different treatment time points. Among them, CsCAMTA2 was significantly up-regulated by DT, which showed more than fourfold high expressions throughout entire DT period, especially more than 14-fold higher after 12 h of DT than those at 0 h of DT. Meanwhile, the expression level of CsCAMTA3 was induced more than twofold higher after 2 d of DT treatment. In contrast, the expressions of all CsCAMTAs were slightly influenced by NT, of which the expressions of CsCAMTA1/3/4 were reduced, but the other genes were induced by NT in some degrees at different processing time points. A similar result was also obtained by H2O2 treatment, where we found CsCAMTA1-4 transcripts were decreased, while CsCAMTA5 and CsCAMTA6 transcripts were increased within 1 d of H2O2 treatment, except for 6 h.
Expression patterns of CsCAMTAs under various abiotic stresses conditions. Samples at 0 h were set as control, and the data were calculated by using 2–ΔΔCt method. The red and green colors represent higher and lower expression levels, respectively. The colorbar was presented on the upper-left of the heat map
Expression analysis of CsCAMTAs under hormone treatment conditions
To explore the roles of CsCAMTAs in responding to hormone treatments, their expressions were analyzed under ABA and GA treatment conditions. Under ABA treatment condition, we found that the expressions of CsCAMTA1/4 were down-regulated firstly within 12 h of ABA treatment, and then up-regulated with the ABA treatment prolonged. In particular, CsCAMTA1/4 transcripts respectively showed more than 2- and sixfold higher after 2 d of ABA treatment than those at 0 h of ABA treatment (Fig. 7). In contrary, CsCAMTA5/6 were up-regulated firstly within 12 h of ABA treatment, and then down-regulated with the ABA treatment prolonged. In addition, CsCAMTA2 showed nearly twofold higher expressions within 2 d of ABA treatment, while CsCAMTA3 transcripts seemed to be not affected by ABA treatment. In contrary to ABA treatment, the expressions of CsCAMTAs were slightly influenced by GA treatment. Among them, CsCAMTA1/4 transcripts decreased within 12 h of GA treatment, but increased after 1 d of GA treatment, while CsCAMTA3/6 transcripts decreased within 2 d of GA treatment. Besides, the expressions of CsCAMTA2 were slightly induced within 1 d of GA treatment, and then deduced until to 2 d of GA treatment. However, CsCAMTA5 was not affected by GA treatment (Fig. 7).
Expression patterns of CsCAMTAs under hormone treatments conditions. Samples at 0 h were set as control, and the final results were calculated by using 2–ΔΔCt method. The red and green colors represent higher and lower expression levels, respectively. The colorbar was presented on the upper-left of the heat map
Expressions analysis of CsCAMTAs during CA periods
As above mentioned, due to CsCAMTAs transcripts were remarkably induced by cold treatment, we further explored their expressions patterns between the cold-resistant cultivar ‘LongJing43’ and the cold-susceptible cultivar ‘DaMianBai’ under CA condition (Fig.S2). As Fig. 8 shown, the expression patterns of CsCAMTAs were varied from each other and also varied in these two tea plant cultivars during CA periods. In terms of ‘LongJing43’, with the exception of CsCAMTA6, the other genes were up-regulated with the temperature decreased from November 14th to December 13th (CA stage), and then the expressions recovered to normal levels with the temperature raised from February 20th to March 19th (de-CA stage). Similarly, CsCAMTA1/2/5/6 transcripts increased at CA stage, while recovered to normal levels at de-CA stage in ‘DaMianBai’. In addition, the expression levels of CsCAMTA1/3/4/6 were higher in ‘LongJing43’ than in ‘DaMianBai’ at CA stage, while CsCAMTA2/5 showed higher expression levels in ‘DaMianBai’ than in ‘LongJing43’ during entire CA periods, suggesting that the differential expression patterns of CsCAMTAs may be positively contributed to the cold resistance of tea plant. However, the correlation between the expressions of CsCAMTAs and the cold resistance of tea plant needs to be further explored in future.
Discussion
CsCAMTAs possess similar biological characteristics with other CAMTAs of various plant species
As a type of signal responsive proteins or ethylene-induced CaM-binding proteins, CAMTAs are known as the largest and best characterized CaM-binding TFs [3]. Currently, numerous of CAMTAs have been identified from more than 20 kinds of plant species, such as maize [18], bananas [21], wheat [25], flax [27], Arabidopsis [59], etc. Further research found that CAMTAs were constituted with 6 highly conserved functional domains across the species, including nuclear localization signals (NLS), CG-1 domain, TIG domain, CaMBD, ANK repeat and IQ motif [12]. As well known that CG-1 domain was contributed to binding DNA directly and activating transcription, TIG was associated with the interaction to TFs through nonspecific DNA binding, ANK was function to protein–protein interaction, and IQ motif was correlated to the binding of CaM and CaM-like proteins [12]. In this study, basing on the HMM models of CG-1 domain, IPT/TIG domain, Ankyrin repeat, and IQ domain, total of 6 CsCAMTAs were identified from tea plant genome, and all of them shared closet relationship with NtCAMTAs. Besides, each CsCAMTA possesses the above mentioned functional domains. In particular, the NLS was detected in CG-1 domains of all CsCAMTAs, which further confirmed the nucleus localization of CsCAMTAs. However, the numbers of ANK repeats and IQ motifs were varied among these CsCAMTAs, suggesting that CsCAMTAs might interact with different numbers of proteins or form different numbers of heteromeric (or homomeric) complexes through their ANK domains, meanwhile, CsCAMTAs might bind to different numbers of CaM or CaM-like proteins. Similarly, many CAMTAs in other species, such as FaCAMTA [60], DzCAMTAs [61], LuCAMTAs [27], and MuCAMTAs [21], etc. had also been demonstrated to perform similar functions. In terms of the gene structures of CAMTAs, many studies have found a fixed number of introns and exons existed in CAMTAs gene family members. In this study, the introns numbers of CsCAMTAs were varied from 10 to 12, which was similar to the genes structures of CAMTAs in Arabidopsis [59], maize [18] and tomato [20], respectively. This result was also similar to the result of phylogenetic tree, suggesting that CAMTAs are relatively conserved among different species in the permanent evolution.
Gene duplication events, including tandem replication and segment duplication, are the main pathways that involved in the expansion of gene family members. Tandem replication events have also been demonstrated to contribute to improving the stress-resistance of plants in dealing with various environment stresses [62]. In our study, the tandem replication events were not found in CsCAMTAs gene family, but two segment duplication events were identified, which suggested that segment duplication events might be the major pattern for the expansion of CsCAMTAs genes family in tea plant. The similar results have also found in banana [21], Durio zibethinus [48], and Cucurbita moschata and Cucurbita maxima [51], where they found that almost all of the identified CAMTAs were located on different chromosomes in different species. At present, there is no study on the collinearity analysis of CAMTAs between different species. Based on the results of the synteny analysis in our study, we found different numbers of orthologous gene pairs of CsCAMTAs were identified between different species. Similar to our results, many gene families, such as CsNACs [63], CsMYBs [64], CsACSs [65], and FtHsfs [66] and so on, have also been found possess different numbers of orthologous gene pairs between different species though synteny analysis, which suggested the divergence evolutionary existed in different species.
CsCAMTAs mediate vegetative and reproductive progress of tea plant
Numerous of studies have showed that CAMTAs are widely involved in the plant vegetative and reproductive processes, especially in leaf senescence, flowering and fruit development. As demonstrated by Yang et al. (2012) [20], 7 SlSR/CAMTAs mediated fruit development and ripening of tomato, and SlSRs differently expressed in different tomato tissues, different fruit development stages and in a tomato ripening mutant (rin). Most notably, the transcription abundances of SlSR2 were too low to detect at the mature green and breaker stages, while SlSR3L and SlSR4 expressed highly in fruit tissues. Besides, SlSRs were rapidly induced by ethylene treatment in mature green stage fruit, of which the expressions of SlSR1 increased about fourfold higher after 2 h of ethylene treatment, which indicated that SlSRs served as early ethylene responsive genes mediate fruit ripening through ethylene-dependent pathway [20]. A similar result was also obtained by Yang et al. (2000) [67], where they found a CAMTA gene, NtER1, transcripts were higher in fully opened flowers, senescing flowers, senescent leaves than that in immature, fully mature leaves and buds. In addition, NtER1 transcripts were rapidly induced after 15 min of exposure to ethylene in tobacco flowers at different development stages, suggesting NtER1 was an early ethylene-up-regulated gene [67]. In Cucurbita maxima and Cucurbita moschata, all CmoCAMTAs and CmaCAMTAs showed higher expression levels in roots than that in stem, leaf, and fruit tissues, meanwhile, the expression levels of CmaCAMTA1-6 were higher in fruit than that in leaf, implicating that CmaCAMTAs mediated fruit development [68]. As CAMTAs served as the downstream targets of CaM, in order to confirm whether calcium/calmodulin signaling participated in fruit ripening, Yang et al. (2015) further analyzed the expressions of SlCaMs during fruit development and ripening, and thus they found all SlCaMs had a peak expression pattern at 10–30 days after anthesis and at turning/pink stages, respectively [22]. Besides, SlCaMs, especially SlCaM2, were also stimulated by ethylene treatment. In addition, SlCaM2 overexpressed transiently in mature green fruit could delay ripening, while retarding SlCaM2 expression would promote ripening, which indicated that SlCaM2 could be a major regulator involved in the modulation of fruit ripening [69]. These results further confirmed the functions of Ca2+-CaM-CAMTAs complexes in dealing with plant vegetative and reproductive processes. At present, there have 5 Calmodulin-like (CML) proteins (CsCMLs), been isolated and functionally characterized in tea plant. Expression analysis results showed that CsCML16/18–1 presented remarkable expression levels in flowers than in other tissues, suggesting that CsCMLs possess tissue-specific expression in tea plant [70]. Correspondingly, we found that 6 CsCAMTAs were expressed differentially in various tea plant tissues, of which the highest expression levels were detected in senescent leaves, flower and root than that in other tissues, indicating that CsCAMTAs were developmentally regulated and acted as triggers for senescence and death. However, whether the similar expression patterns exist in CsCaMs family genes in different tea plant organisms still needs to be further studied in future, as the gene numbers, tissue-specific and spatial–temporal expression patterns of CsCaMs have not been explored until now. Besides, as a type of EICBPs, whether CsCAMTAs served as ethylene responsive genes to mediate leaf senescence also needs to be further studied.
CsCAMTAs involved in various abiotic stresses and hormones responses
Apart from mediating plant developmental biology, CAMTAs also play critical roles in regulating biotic and abiotic stresses responses, such as diseases, pests, drought, salt, low temperature etc. [29, 59]. For example, 6 AtCAMTAs have been reported to be quickly and differentially stimulated by various stresses and hormones [11, 29, 71, 72]. Similarly, 9 ZmCAMTAs, 9 CiCAMTAs, and 15 TaCAMTAs were respectively stimulated by various hormones, abiotic and biotic stresses [18, 24, 25]. It is now known that CAMTAs-regulated genes depend on Ca2+ signals, the Ca2+-CaM-CAMTAs complexes could nonlinearly amplify different calcium signatures, and then the calcium signatures are decoded to produce specific CAMTA-regulated gene expression responses [73]. In recent years, the stress responses regulation mechanisms of CAMTAs have been partially elucidated in Arabidopsis based on the overexpression and mutation techniques. Under stress stimuli condition, CAMTAs regulate the gene expressions of downstream targets through specifically binding to the core motifs (A/C) CGCG (C/G/T) or (A/C) CGTGT contained in the promoter regions of lots stress-responsive genes. It is well known that the ICE-CBF-COR signaling pathway plays the leading role in enhancing cold tolerance of plants upon exposure to nonfreezing temperatures. In Arabidopsis, 3 AtCBFs could be induced within 15 min, and reached the highest level after 3 h of cold treatment when exposed to low temperature. Besides, AtCBF1-3 genes mediate 414 COR genes expressions, including 346 CBF-activated genes and 68 CBF-repressed genes when exposed to nonfreezing temperature, indicating that CBF genes play central role in CA [74]. Apart from CBF regulons, CAMTAs have been identified as transcription activators of CBFs in Arabidopsis, which specifically binding to conserved DNA motif 2 (CM2, vCGCGb) in the promoter of CBF2. Among them, CAMTA3 referred as a positive regulator, mutation of AtCAMTA3 resulted in approximately 50% reduction of CBF2 expression, and a much higher extent were observed in double camta1 camta3 mutant plants, which indicated that CAMTA proteins play positive roles in cold acclimation [59]. Further research found that AtCAMTA1 and AtCAMTA2 cooperated with AtCAMTA3 induced the expressions of CBF1, CBF2 and CBF3, following upregulated the expression of approximately 15% genes that independent CBF pathway after exposed to 4 °C for 24 h, and thus resulted in enhancing plant freezing tolerance [31]. Moreover, Kidokoro et al. (2017) found that AtCAMTA3/5 could induce the expression of DREB1B under rapid temperature reduction condition [75]. Meanwhile, AtCAMTA3/5 sustained this effect throughout the day and night, in contrast to CIRCADIAN CLOCK ASSOCIATED1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY), which only promoted the upregulation of DREB1B only during the day [75]. In terms of tea plant, it has also revealed that calcium signaling pathway plays critical role in improving cold tolerance during CA condition [26]. In our research, we found the expression levels of all CsCAMTAs were significantly induced by cold treatment. Meanwhile, CsCAMTAs showed differential expressions between cold-resistant and cold-susceptible tea plant cultivars during CA periods. At the same time, the expressions of CsCBFs genes in tea plant have also been confirmed to up-regulated by low temperature. Recently, 6 CsCBFs genes have been isolated from the chromosome-level genome of tea plant [76]. As Hu et al. (2020) demonstrated that the yeast cells containing 5 pGBKT7-CsCBFs (pGBKT7-CsCBF2-6) recombinant plasmids grew well on the selection media and positive for α-galactosidase activity respectively, which indicated that CsCBF2-6 possess transcriptional activity [76].
Expression analysis results found that the transcriptions of CsCBFs were differentially regulated by various abiotic stresses and hormone treatments. Notably, these CsCBFs genes were markedly up-regulated, and the expression levels of CsCBF1-3 were up-regulated more than 100-fold or even 1000-fold within 1 d of cold treatment. In addition, overexpression of CsCBF3 could enhance the cold tolerance of transgenic Arabidopsis potentially through an ABA-independent pathway [76]. In the present study, we also searched the putative core CsCAMTAs binding motifs, (A/C) CGCG (C/G/T) or (A/C) CGTGT, in the promoters of CsCBF1-6. As shown in Table S7, each of CsCBFs contains different numbers of (A/C) CGCG (C/G/T) and (A/C) CGTGT motifs, which indicated that CsCAMTAs are served as a type of transcription activators of CsCBFs, which could specifically bind to the conserved (A/C) CGCG (C/G/T) or (A/C) CGTGT motifs in the promoters of CsCBFs to regulate their expressions, and thus mediate the cold response of tea plant.
In plants, CAMTAs also involved in response to drought and salinity. As Pandey et al. (2013) demonstrated that AtCAMTA1 mediated drought responses in Arabidopsis [29]. Microarray analysis found that the expressions of many genes involved in DNA methylation, stress response, apoptosis, photosynthesis and osmotic balance were greatly altered in camta1 mutants under drought conditions. Specifically, several stress responsive genes, including RD26, Early-responsive to dehydration 7 (ERD7), Ras-related protein (RAB18), Lipid protein (LTPs), Clod related protein (COR78), CBF1, Heat shock proteins (HSPs) etc., were positively regulated by AtCAMTA1, and the conserved (A/C) CGCG (C/G/T) or (A/C) CGTGT motifs enriched in the promoters regions of these genes, suggesting that AtCAMTA1 mediated drought recovery mainly through regulating the expressions of AP2-EREBP transcription factors and depending on ABA signaling pathway [29]. In addition, overexpression of GmCAMTA12 in Arabidopsis and soybean respectively enhanced drought tolerance of transgenic lines. Under drought stress condition, the expression of AtAnnexin5, calmodulin binding heat shock protein (AtCaMHSP), At2G433110 and AtWRKY14 were up-regulated in transgenic Arabidopsis. Similarly, the expressions of elongator complex (GmELO), nucleic acids binding (GmNAB) and phospholipase A1-IId (GmPLA1-IId) were significantly up-regulated in transgenic soybean hairy roots when exposed to drought stress condition, and the conserved (A/C) CGCG (C/G/T) or (A/C) CGTGT motifs were also enriched in the promoter regions of these genes [77]. For tea plant, it has been known that the application of exogenous ABA could promote drought resistance of tea plant, suggesting that drought response of tea plant relies on ABA signaling pathway [78, 79]. Under drought stress condition, 12 TF families members (bZIP, NAC, squamosa promoter-binding protein-like (SPL), APETALA2/Ethylene-responsive element binding proteins (AP2/EREBP), Basic helix loop helix (bHLH), etc.) and numbers of genes involved in ABA biosynthesis and signaling (9-cis-epoxycarotenoid dioxygenase 1 (NCED1), NCED4, pyrabactin resistance 1-like 4 (PYL4), PYL8, PP2C1-6, sucrose non-fermenting1-related protein kinase 2.2 (SnRK2.2), SnRK2.3, SnRK2.5, SnRK2.6, etc.), carbohydrate metabolism (UDP-glucose pyrophosphorylase (UDPGase), sucrose-phosphate synthase (SPS), trehalose phosphate synthase (TPS), trehalose phosphatases (TPP), mannose-6-phosphate reductase (M6PR), and mannose-1 phosphate phosphatase (M1PP) were up-regulated [80]. In this study, we found all CsCAMTAs were up-regulated by DT at different treatment time points, which indicated that CsCAMTAs collaborates with other TFs family members to participate in regulating drought response of tea plant. However, the regulation mechanism of CsCAMTAs needs to be further explored.
Recently, Shkolnik et al. (2015) found that AtCAMTA6 contributed to controlling Na+ homeostasis in germinating seedlings of Arabidopsis through ABA-dependent and-independent signaling pathways [72]. However, another CAMTA gene, AtCAMTA3, was reported as a negative regulator of salt tolerance by directly repressing salt-responsive genes transcripts, mutation of AtCAMTA3 resulted in higher salt tolerance in camta3 mutants than the wide type and complemented line [81]. Yuan et al. (2021) found that all CmoCAMTAs in the leaf vein were remarkably induced, while all CmoCAMTAs in leaf mesophyll were inhibited by salt stress [68]. In terms of tea plant, transcriptomic analysis revealed that many TFs genes (e.g. bZIP, HD-Zip, APETALA2/ethylene-responsive factor (AP2/ERF), WRKY, NAC, MYB, bHLH and zinc finger-TFs) and many genes involved in Ca2+ signal transduction (e.g. CaM4, CDPK7/3/15/16, and CML18/20/49), ABA signaling pathway (e.g. type 2C protein phosphatase (PP2C) 2/3/12/27/14/51/54/60), and mitogen-activated protein kinase (MAPK) cascades pathway (MAPK kinase (MAPKK) 2/4/5) were differentially expressed in tea plant under slat stress condition. In Ca2+ signal transduction pathway, 3 CaMs/CMLs genes (CML20, CML18, and CML49) were up-regulated, whereas CaM4 was down-regulated by salt stress [82]. At present study, partial of CsCAMTAs were slightly induced by NT, which indicated that Ca2+-CaM-CAMTAs complexes also play a role in salt response of tea plant. However, the specific downstream targets of CsCAMTAs need to be further explored.
At present, it has been clear that the expressions of many CAMTAs in different plant species could be stimulated by various hormones, such as auxin, ABA, ethylene, methyl jasmonate (MeJA), and SA, etc. [18, 24, 25, 31]. For example, CAMTA1-3 repressed the expressions of isochorismate synthase 1 (ICS1), CBP60g and SAR deficient 1 (SARD1), and thus inhibited SA biosynthesis in Arabidopsis under warm temperature condition [31]. In the present study, we found the expressions of 5 CsCAMTAs (CsCAMTA1/2/4/5/6) were induced or reduced by exogenous ABA at different treatment time points. Meanwhile, GA treatment also slightly affected the expressions of 5 CsCAMTAs (CsCAMTA1-4/6) within 2 d of treatment. Correspondingly to these results, many hormone-related cis-acting elements were enriched in the promoter regions of CsCAMTAs, suggesting that the expressions of CsCAMTAs were regulated by hormone signaling pathways.
Conclusion
Total of 6 CsCAMTAs genes were identified from tea plant genome of ‘ShuChaZao’ cultivar. Each CsCAMTA was predicted to contain 6 conserved functional domains and located in nucleus. All CsCAMTAs showed closest relationship with NtCAMTAs except for CsCAMTA4. CsCAMTAs may be mediated tea plant vegetative and reproductive processes, especially in aging and flowering periods. Besides, CsCAMTAs were widely involved in various abiotic stresses and hormones responses, among of which CsCAMTAs may be contributed to improve cold tolerance of tea plant depending on CBF signaling pathway. In addition, CsCAMTAs were differentially expressed between cold-resistant cultivar ‘LongJing43’ and cold-susceptible cultivar ‘DaMianBai’ during cold acclimation periods, suggesting that CsCAMTAs may be served as potential molecular markers for screening tea plant germplasms with cold resistance. Overall, the present study provided theoretical support for deeply exploring the regulation mechanisms of CsCAMTAs in tea plants.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in this article and the additional files. The nucleotide and protein sequences of CAMTA-related genes in Arabidopsis, Oryza sativa, Populus trichocarpa, Malus pumila, Triticum aestivum, Nicotiana tabacum and Zea mays are available in Phytozome v13 database (JGI, https://phytozome.jgi.doe.gov/pz/portal.html).
References
Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annu Rev Plant Biol. 2010;61:593–620. https://doi.org/10.1146/annurev-arplant-070109-104628.
Kudla J, Batistic O, Hashimoto K. Calcium signals: the lead currency of plant information processing. Plant Cell. 2010;22(3):541–63. https://doi.org/10.1105/tpc.109.072686.
Iqbal Z, Shariq Iqbal M, Singh SP, Buaboocha T. Ca(2+)/Calmodulin complex triggers CAMTA transcriptional machinery under stress in plants: signaling cascade and molecular regulation. Front Plant Sci. 2020;11: 598327. https://doi.org/10.3389/fpls.2020.598327.
Sanders D, Brownlee C, Harper JF. Communicating with calcium. Plant Cell. 1999;11(4):691. https://doi.org/10.2307/3870893.
Hashimoto K, Kudla J. Calcium decoding mechanisms in plants. Biochimie. 2011;93(12):2054–9. https://doi.org/10.1016/j.biochi.2011.05.019.
Du L, Poovaiah BW. A novel family of Ca2+/calmodulin-binding proteins involved in transcriptional regulation: interaction with Fsh/Ring3 class transcription activators. Plant Mol Biol. 2004;54(4):549–69. https://doi.org/10.1023/B:PLAN.0000038269.98972.bb.
Kim Y, Gilmour SJ, Chao L, Park S, Thomashow MF. Arabidopsis CAMTA transcription factors regulate pipecolic acid biosynthesis and priming of immunity genes. Mol Plant. 2020;13(1):157–68. https://doi.org/10.1016/j.molp.2019.11.001.
Lorenzo O. Bzip edgetic mutations: at the frontier of plant metabolism, development and stress trade-off. J Exp Bot. 2019;70(20):5517–20. https://doi.org/10.1093/jxb/erz298.
Zhu D, Hou L, Xiao P, Guo Y, Deyholos MK, Liu X. VvWRKY30, a grape WRKY transcription factor, plays a positive regulatory role under salinity stress. Plant Sci. 2019;280:132–42. https://doi.org/10.1016/j.plantsci.2018.03.018.
Bouche N, Scharlat A, Snedden W, Bouchez D, Fromm H. A novel family of calmodulin-binding transcription activators in multicellular organisms. J Biol Chem. 2002;277(24):21851–61. https://doi.org/10.1074/jbc.M200268200.
Yang T, Poovaiah BW. A calmodulin-binding/CGCG box DNA-binding protein family involved in multiple signaling pathways in plants. J Biol Chem. 2002;277(47):45049–58. https://doi.org/10.1074/jbc.M207941200.
Finkler A, Ashery-Padan R, Fromm H. CAMTAs: calmodulin-binding transcription activators from plants to human. FEBS Lett. 2007;581(21):3893–8. https://doi.org/10.1016/j.febslet.2007.07.051.
Aravind L, Koonin EV. Gleaning non-trivial structural, functional and evolutionary information about proteins by iterative database searches. J Mol Biol. 1999;287(5):1023–40. https://doi.org/10.1006/jmbi.1999.2653.
Rubtsov AM, Lopina OD. Ankyrins. FEBS Lett. 2000;482(1–2):1–5. https://doi.org/10.1016/S0014-5793(00)01924-4.
Sedgwick SG, Smerdon SJ. The ankyrin repeat: a diversity of interactions on a common structural framework. Trends Biochem Sci. 1999;24(8):311–6. https://doi.org/10.1016/s0968-0004(99)01426-7.
Kaplan B, Davydov O, Knight H, Galon Y, Knight MR, Fluhr R, Fromm H. Rapid transcriptome changes induced by cytosolic Ca2+ transients reveal ABRE-related sequences as Ca2+-responsive cis elements in Arabidopsis. Plant Cell. 2006;18(10):2733–48. https://doi.org/10.1105/tpc.106.042713.
Reddy AS, Reddy VS, Golovkin M. A calmodulin binding protein from Arabidopsis is induced by ethylene and contains a DNA-binding motif. Biochem Biophys Res Commun. 2000;279(3):762–9. https://doi.org/10.1006/bbrc.2000.4032.
Yue R, Lu C, Sun T, Peng T, Han X, Qi J, Yan S, Tie S. Identification and expression profiling analysis of calmodulin-binding transcription activator genes in maize (Zea mays L.) under abiotic and biotic stresses. Front Plant Sci. 2015;6:576. https://doi.org/10.3389/fpls.2015.00576.
Shangguan L, Wang X, Leng X, Liu D, Ren G, Tao R, Zhang C, Fang J. Identification and bioinformatic analysis of signal responsive/calmodulin-binding transcription activators gene models in Vitis vinifera. Mol Biol Rep. 2014;41(5):2937–49. https://doi.org/10.1007/s11033-014-3150-5.
Yang T, Peng H, Whitaker BD, Conway WS. Characterization of a calcium/calmodulin-regulated SR/CAMTA gene family during tomato fruit development and ripening. BMC Plant Biol. 2012;12:19. https://doi.org/10.1186/1471-2229-12-19.
Meer L, Mumtaz S, Labbo AM, Khan MJ, Sadiq I. Genome-wide identification and expression analysis of calmodulin-binding transcription activator genes in banana under drought stress. Sci Hortic. 2019;244:10–4. https://doi.org/10.1016/j.scienta.2018.09.022.
Yang Y, Sun T, Xu L, Pi E, Wang S, Wang H, Shen C. Genome-wide identification of CAMTA gene family members in medicago truncatula and their expression during root nodule symbiosis and hormone treatments. Front Plant Sci. 2015;6:459. https://doi.org/10.3389/fpls.2015.00459.
Du L, Ali GS, Simons KA, Hou J, Yang T, Reddy AS, Poovaiah BW. Ca(2+)/calmodulin regulates salicylic-acid-mediated plant immunity. Nature. 2009;457(7233):1154–8. https://doi.org/10.1038/nature07612.
Zhang J, Pan XT, Ge T, Yi SL, Xie RJ. Genome-wide identification of citrus CAMTA genes and their expression analysis under stress and hormone treatments. J Hortic Sci Biotechnol. 2018;94(3):331–40. https://doi.org/10.1080/14620316.2018.1504631.
Yang F, Dong FS, Hu FH, Liu YW, Chai JF, Zhao H, Lv MY, Zhou S. Genome-wide identification and expression analysis of the calmodulin-binding transcription activator (CAMTA) gene family in wheat (Triticum aestivum L.). BMC Genet. 2020;21(1):105. https://doi.org/10.1186/s12863-020-00916-5.
Wang XC, Zhao QY, Ma CL, Zhang ZH, Cao HL, Kong YM, Yue C, Hao XY, Chen L, Ma JQ, et al. Global transcriptome profiles of Camellia sinensis during cold acclimation. BMC Genomics. 2013;14:415. https://doi.org/10.1186/1471-2164-14-415.
Ali E, Raza MA, Cai M, Hussain N, Shahzad AN, Hussain M, Ali M, Bukhari SAH, Sun P. Calmodulin-binding transcription activator (CAMTA) genes family: Genome-wide survey and phylogenetic analysis in flax (Linum usitatissimum). PLoS ONE. 2020;15(7): e0236454. https://doi.org/10.1371/journal.pone.0236454.
Galon Y, Snir O, Fromm H. How calmodulin binding transcription activators (CAMTAs) mediate auxin responses. Plant Signal Behav. 2010;5(10):1311–4. https://doi.org/10.4161/psb.5.10.13158.
Pandey N, Ranjan A, Pant P, Tripathi RK, Ateek F, Pandey HP, Patre UV, Sawant SV. CAMTA 1 regulates drought responses in Arabidopsis thaliana. BMC Genomics. 2013;14:216. https://doi.org/10.1186/1471-2164-14-216.
Thomashow MF. Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiol. 2010;154(2):571–7. https://doi.org/10.1104/pp.110.161794.
Kim Y, Park S, Gilmour SJ, Thomashow MF. Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant J. 2013;75(3):364–76. https://doi.org/10.1111/tpj.12205.
Wang L, Feng X, Yao L, Ding C, Lei L, Hao X, Li N, Zeng J, Yang Y, Wang X. Characterization of CBL-CIPK signaling complexes and their involvement in cold response in tea plant. Plant Physiol Biochem. 2020;154:195–203. https://doi.org/10.1016/j.plaphy.2020.06.005.
Ding C, Lei L, Yao L, Wang L, Hao X, Li N, Wang Y, Yin P, Guo G, Yang Y, et al. The involvements of calcium-dependent protein kinases and catechins in tea plant [Camellia sinensis (L.) O. Kuntze] cold responses. Plant Physiol Biochem. 2019;143:190–202. https://doi.org/10.1016/j.plaphy.2019.09.005.
Liu H, Wang YX, Li H, Teng RM, Wang Y, Zhuang J. Genome-Wide identification and expression analysis of calcineurin b-like protein and calcineurin b-like protein-interacting protein kinase family genes in tea plant. DNA Cell Biol. 2019;38(8):824–39. https://doi.org/10.1089/dna.2019.4697.
Wang H, Ding Z, Gou M, Hu J, Wang Y, Wang L, Wang Y, Di T, Zhang X, Hao X, et al. Genome-wide identification, characterization, and expression analysis of tea plant autophagy-related genes (CsARGs) demonstrates that they play diverse roles during development and under abiotic stress. BMC Genomics. 2021;22(1):121. https://doi.org/10.1186/s12864-021-07419-2.
Wang L, Yao LN, Hao XY, Li NN, Wang YC, Ding CQ, Lei L, Qian WJ, Zeng JM, Yang YJ, et al. Transcriptional and physiological analyses reveal the association of ROS metabolism with cold tolerance in tea plant. Environ Exp Bot. 2019;160:45–58. https://doi.org/10.1016/j.envexpbot.2018.11.011.
Qian W, Xiao B, Wang L, Hao X, Yue C, Cao H, Wang Y, Li N, Yu Y, Zeng J, et al. CsINV5, a tea vacuolar invertase gene enhances cold tolerance in transgenic Arabidopsis. BMC Plant Biol. 2018;18(1):228. https://doi.org/10.1186/s12870-018-1456-5.
Mistry J, Chuguransky S, Williams L, Qureshi M, Salazar GA, Sonnhammer ELL, Tosatto SCE, Paladin L, Raj S, Richardson LJ, et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021;49(D1):D412–9. https://doi.org/10.1093/nar/gkaa913.
Wei C, Yang H, Wang S, Zhao J, Liu C, Gao L, Xia E, Lu Y, Tai Y, She G, et al. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc Natl Acad Sci U S A. 2018;115(18):E4151–8. https://doi.org/10.1073/pnas.1719622115.
Letunic I, Khedkar S, Bork P. SMART: recent updates, new developments and status in 2020. Nucleic Acids Res. 2021;49(D1):D458–60. https://doi.org/10.1093/nar/gkaa937.
Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Marchler GH, Song JS, et al. CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res. 2020;48(D1):D265–8. https://doi.org/10.1093/nar/gkz991.
Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, Appel RD, Hochstrasser DF. Protein identification and analysis tools in the ExPASy server. Methods in molecular biology (Clifton, NJ). 1999;112:531–52. https://doi.org/10.1385/1-59259-584-7:531.
Almagro Armenteros JJ, Tsirigos KD, Sonderby CK, Petersen TN, Winther O, Brunak S, von Heijne G, Nielsen H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol. 2019;37(4):420–3. https://doi.org/10.1038/s41587-019-0036-z.
Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305(3):567–80. https://doi.org/10.1006/jmbi.2000.4315.
Chou KC, Shen HB. Plant-mPLoc: a top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE. 2010;5(6): e11335. https://doi.org/10.1371/journal.pone.0011335.
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–4. https://doi.org/10.1093/molbev/msw054.
Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(W1):W293–6. https://doi.org/10.1093/nar/gkab301.
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–202. https://doi.org/10.1016/j.molp.2020.06.009.
Xue YB, Bao YM, Zhang Z, Zhao WM, Xiao JF, He SM, Zhang GQ, Li Y, Zhao GP, Chen RS, et al. Database resources of the national genomics data center, China National Center for Bioinformation in 2022. Nucleic Acids Res. 2022; 50(1):27–38. https://doi.org/10.1093/nar/gkab951.
Wang P, Yu J, Jin S, Chen S, Yue C, Wang W, Gao S, Cao H, Zheng Y, Gu M, et al. Genetic basis of high aroma and stress tolerance in the oolong tea cultivar genome. Horticulture Research. 2021;8(1):107. https://doi.org/10.1038/s41438-021-00542-x.
Zhang X, Chen S, Shi L, Gong D, Zhang S, Zhao Q, Zhan D, Vasseur L, Wang Y, Yu J, et al. Haplotype-resolved genome assembly provides insights into evolutionary history of the tea plant Camellia sinensis. Nat Genet. 2021;53(8):1250–9. https://doi.org/10.1038/s41588-021-00895-y.
Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31(8):1296–7. https://doi.org/10.1093/bioinformatics/btu817.
Blum M, Chang HY, Chuguransky S, Grego T, Kandasaamy S, Mitchell A, Nuka G, Paysan-Lafosse T, Qureshi M, Raj S, et al. The InterPro protein families and domains database: 20 years on. Nucleic Acids Res. 2021;49(D1):D344–54. https://doi.org/10.1093/nar/gkaa977.
Duvaud S, Gabella C, Lisacek F, Stockinger H, Ioannidis V, Durinx C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021;49(W1):W216–27. https://doi.org/10.1093/nar/gkab225.
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Peer YVD, Rouzé P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–7. https://doi.org/10.1093/nar/30.1.325.
Liu W, Xie Y, Ma J, Luo X, Nie P, Zuo Z, Lahrmann U, Zhao Q, Zheng Y, Zhao Y, et al. IBS: an illustrator for the presentation and visualization of biological sequences. Bioinformatics. 2015;31(20):3359–61. https://doi.org/10.1093/bioinformatics/btv362.
Hao X, Horvath DP, Chao WS, Yang Y, Wang X, Xiao B. Identification and evaluation of reliable reference genes for quantitative real-time PCR analysis in tea plant (Camellia sinensis (L.) O. Kuntze). Int J Mol Sci. 2014;15(12):22155–72. https://doi.org/10.3390/ijms151222155.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–8. https://doi.org/10.1006/meth.2001.1262.
Doherty CJ, Van Buskirk HA, Myers SJ, Thomashow MF. Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell. 2009;21(3):972–84. https://doi.org/10.1105/tpc.108.063958.
Leng XP, Han J, Wang XM, Zhao MZ, Sun X, Wang C, Fang JG. Characterization of a calmodulin-binding transcription factor from strawberry (Fragaria × ananassa). The Plant Genome. 2015;8(2):1–12. https://doi.org/10.3835/plantgenome2014.08.0039.
Iqbal Z, Iqbal MS, Sangpong L, Khaksar G, Sirikantaramas S, Buaboocha T. Comprehensive genome-wide analysis of calmodulin-binding transcription activator (CAMTA) in durio zibethinus and identification of fruit ripening-associated DzCAMTAs. BMC Genomics. 2021;22(1):743. https://doi.org/10.1186/s12864-021-08022-1.
Schilling S, Kennedy A, Pan S, Jermiin LS, Melzer R. Genome-wide analysis of MIKC-type MADS-box genes in wheat: pervasive duplications, functional conservation and putative neofunctionalization. New Phytol. 2020;225(1):511–29. https://doi.org/10.1111/nph.16122.
Liu M, Ma Z, Sun W, Huang L, Wu Q, Tang Z, Bu T, Li C, Chen H. Genome-wide analysis of the NAC transcription factor family in Tartary buckwheat (Fagopyrum tataricum). BMC Genomics. 2019;20(1):113. https://doi.org/10.1186/s12864-019-5500-0.
Chen X, Wang P, Gu M, Lin X, Hou B, Zheng Y, Sun Y, Jin S, Ye N. R2R3-MYB transcription factor family in tea plant (Camellia sinensis): Genome-wide characterization, phylogeny, chromosome location, structure and expression patterns. Genomics. 2021;113(3):1565–78. https://doi.org/10.1016/j.ygeno.2021.03.033.
Sun LF, Nasrullah, Ke FZ, Nie ZP, Xu JG, Huang X, Sun JH, Wang P. Genome-wide identification and transcript analysis during fruit ripening of ACS gene family in sweet orange (Citrus sinensis). Scientia Horticulturae. 2022;294:110786. https://doi.org/10.1016/j.scienta.2021.110786.
Liu M, Huang Q, Sun W, Ma Z, Huang L, Wu Q, Tang Z, Bu T, Li C, Chen H. Genome-wide investigation of the heat shock transcription factor (Hsf) gene family in Tartary buckwheat (Fagopyrum tataricum). BMC Genomics. 2019;20(1):871. https://doi.org/10.1186/s12864-019-6205-0.
Yang T, Poovaiah BW. An early ethylene up-regulated gene encoding a calmodulin-binding protein involved in plant senescence and death. J Biol Chem. 2000;275(49):38467–73. https://doi.org/10.1074/jbc.M003566200.
Yuan J, Shen C, Chen B, Shen A, Li X. Genome-Wide characterization and expression analysis of CAMTA gene family under salt stress in cucurbita moschata and cucurbita maxima. Front Genet. 2021;12: 647339. https://doi.org/10.3389/fgene.2021.647339.
Yang T, Peng H, Bauchan GR. Functional analysis of tomato calmodulin gene family during fruit development and ripening. Hortic Res. 2014;1:14057. https://doi.org/10.1038/hortres.2014.57.
Ma Q, Zhou Q, Chen C, Cui Q, Zhao Y, Wang K, Arkorful E, Chen X, Sun K, Li X. Isolation and expression analysis of CsCML genes in response to abiotic stresses in the tea plant (Camellia sinensis). Sci Rep. 2019;9(1):8211. https://doi.org/10.1038/s41598-019-44681-7.
Kim YS, An C, Park S, Gilmour SJ, Wang L, Renna L, Brandizzi F, Grumet R, Thomashow MF. CAMTA-mediated regulation of salicylic acid immunity pathway genes in Arabidopsis exposed to low temperature and pathogen infection. Plant Cell. 2017;29(10):2465–77. https://doi.org/10.1105/tpc.16.00865.
Shkolnik D, Finkler A, Pasmanik-Chor M, Fromm H. Calmodulin-binding transcription activator 6: a key regulator of Na(+) homeostasis during germination. Plant Physiol. 2019;180(2):1101–18. https://doi.org/10.1104/pp.19.00119.
Liu J, Whalley HJ, Knight MR. Combining modelling and experimental approaches to explain how calcium signatures are decoded by calmodulin-binding transcription activators (CAMTAs) to produce specific gene expression responses. New Phytol. 2015;208(1):174–87. https://doi.org/10.1111/nph.13428.
Zhao C, Zhang Z, Xie S, Si T, Li Y, Zhu JK. Mutational evidence for the critical role of CBF transcription factors in cold acclimation in Arabidopsis. Plant Physiol. 2016;171(4):2744–59. https://doi.org/10.1104/pp.16.00533.
Kidokoro S, Yoneda K, Takasaki H, Takahashi F, Shinozaki K, Yamaguchi-Shinozaki K. Different cold-signaling pathways function in the responses to rapid and gradual decreases in temperature. Plant Cell. 2017;29(4):760–74. https://doi.org/10.1105/tpc.16.00669.
Hu Z, Ban Q, Hao J, Zhu X, Cheng Y, Mao J, Lin M, Xia E, Li Y. Genome-Wide characterization of the C-repeat binding factor (CBF) gene family involved in the response to abiotic stresses in tea plant (Camellia sinensis). Front Plant Sci. 2020;11:921. https://doi.org/10.3389/fpls.2020.00921.
Noman M, Jameel A, Qiang WD, Ahmad N, Li HY. Overexpression of GmCAMTA12 enhanced drought tolerance in Arabidopsis and soybean. Int J Mol Sci. 2019;20(19):4849. https://doi.org/10.3390/ijms20194849.
Gai Z, Wang Y, Ding Y, Qian W, Qiu C, Xie H, Sun L, Jiang Z, Ma Q, Wang L, et al. Exogenous abscisic acid induces the lipid and flavonoid metabolism of tea plants under drought stress. Sci Rep. 2020;10(1):12275. https://doi.org/10.1038/s41598-020-69080-1.
Zhu X, Liao J, Xia X, Xiong F, Li Y, Shen J, Wen B, Ma Y, Wang Y, Fang W. Physiological and iTRAQ-based proteomic analyses reveal the function of exogenous gamma-aminobutyric acid (GABA) in improving tea plant (Camellia sinensis L.) tolerance at cold temperature. BMC Plant Biology. 2019;19(1):43. https://doi.org/10.1186/s12870-019-1646-9.
Liu SC, Jin JQ, Ma JQ, Yao MZ, Ma CL, Li CF, Ding ZT, Chen L. Transcriptomic analysis of tea plant responding to drought stress and recovery. PLoS ONE. 2016;11(1): e0147306. https://doi.org/10.1371/journal.pone.0147306.
Prasad K, Abdel-Hameed AAE, Xing D, Reddy ASN. Global gene expression analysis using RNA-seq uncovered a new role for SR1/CAMTA3 transcription factor in salt stress. Sci Rep. 2016;6:27021. https://doi.org/10.1038/srep27021.
Wan SQ, Wang WD, Zhou TS, Zhang YH, Chen JF, Xiao B, Yang YJ, Yu YB. Transcriptomic analysis reveals the molecular mechanisms of Camellia sinensis in response to salt stress. Plant Growth Regul. 2018;84(3):481–92. https://doi.org/10.1007/s10725-017-0354-4.
Acknowledgements
Not applicable.
Funding
This work was supported by the Sub Project of Shandong Agricultural Elite Variety Project (Grant No. 2321401), the National Natural Science Foundation of China (Grant No. 32272767 and 31800588), and the School Fund Project of Qingdao Agricultural University (Grant No. 1118025), The Innovation and Entrepreneurship Training Programs for College Students of Shandong Province (S202110435062), The Innovation and Entrepreneurship Training Program for College Students in Qingdao Agricultural University.
Author information
Authors and Affiliations
Contributions
QW conceived and designed the experiments and analyzed data. LB, HS and ZY performed experiments, and wrote original draft preparation. WY, LX, WH, FK, HJ and DZ collected samples and edited draft. All authors contributed to the acquisition of data, interpretation of results and critical discussion and approved the final version of the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The seedlings of tea plant cultivar ‘ShuChaZao’ were purchased from Nanjing Yarun Tea Co., LTD. (Nanjing, China), and then cultivated in the greenhouse of the Tea Research Institute of Qingdao Agricultural University (TRI, QAU, N36°33′, E120°4′). This article does not contain any studies with animals or humans performed by any of the authors. This study complies with institutional, national and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Fig. S1. The chromosomal distributions of CsCAMTAs in ‘HuangDan’ and ‘TieGuanYin’ cultivars. A: The chromosomal distribution of CsCAMTAs in ‘HuangDan’ genome. B: The chromosomal distribution of CsCAMTAs in ‘TieGuanYin’ genome.
Additional file 2:
Fig. S2. The diurnal dynamic changes of temperature from November 2018 to March 2019 in Hangzhou.
Additional file 3:
Table S1. All sequences used to construct phylogenetic tree.
Additional file 4:
Table S2. Primers information used in qRT-PCR detection.
Additional file 5:Table S3.
The chromosomal distribution positions in chromosomes of three tea plant cultivars.
Additional file 6:
Table S4. The synteny analysis results of CsCAMTAs within ‘ShuChaZao’ genome.
Additional file 7: Table S5.
The Ka-Ks ratios of CsCAMTAs.
Additional file 8:
Table S6. The synteny analysis results of CsCAMTAs between different species.
Additional file 9:
Table S7. 2000-bp promoter sequences of CsCBFs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, B., He, S., Zheng, Y. et al. Genome-wide identification and expression analysis of the calmodulin-binding transcription activator (CAMTA) family genes in tea plant. BMC Genomics 23, 667 (2022). https://doi.org/10.1186/s12864-022-08894-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-022-08894-x