Abstract
Background
Puccinellia tenuiflora, a forage grass, is considered a model halophyte given its strong tolerance for multiple stress conditions and its close genetic relationship with cereals. This halophyte has enormous values for improving our understanding of salinity tolerance mechanisms. The genetic information of P. tenuiflora also is a potential resource that can be used for improving the salinity tolerance of cereals.
Results
Here, we sequenced and assembled the P. tenuiflora genome (2n = 14) through the combined strategy of Illumina, PacBio, and 10× genomic technique. We generated 43.2× PacBio long reads, 123.87× 10× genomic reads, and 312.6× Illumina reads. Finally, we assembled 2638 scaffolds with a total size of 1.107 Gb, contig N50 of 117 kb, and scaffold N50 of 950 kb. We predicted 39,725 protein-coding genes, and identified 692 tRNAs, 68 rRNAs, 702 snRNAs, 1376 microRNAs, and 691 Mb transposable elements.
Conclusions
We deposited the genome sequence in NCBI and the Genome Warehouse in National Genomics Data Center. Our work may improve current understanding of plant salinity tolerance, and provides extensive genetic resources necessary for improving the salinity and drought tolerance of cereals.
Similar content being viewed by others
Background
Salinity stress affects over 6% of the global land area and is a severe problem that limits agriculture [1, 2]. Halophytes are remarkable plants that tolerate high salinity that would kill 99% of other plant species (glycophyte), and are applied to improve saline soil [3, 4]. Some extreme halophytes can survive salinity levels > 1000 mM NaCl, whereas glycophytes, such as rice and Arabidopsis, can only survive 50–100 mM NaCl [4, 5]. Most botanists believe that these salt-sensitive glycophytes may provide limited insights into mechanisms of salinity tolerance, and that extreme halophytes may have enormous values for improving our understanding of salinity tolerance mechanisms [4,5,6]. Given that many important crops are gramineous, understanding the salinity tolerance mechanisms of gramineous halophytes will be helpful in improving the salinity or drought tolerance of cereal crops. Although the genomes of several salinity-tolerant plant species have been reported [7,8,9,10], the genome of an extreme Gramineae halophyte is unavailable. Puccinellia tenuiflora (2n = 14) is a perennial halophyte of the Gramineae and is distributed in Asian and European grasslands [3, 11, 12]. It is a forage grass with high nutritional value and strong tolerance for multiple stress conditions, such as drought, disease, and chilling [3, 11, 12]. P. tenuiflora can survive at pH 10 and 900 mM NaCl [3, 11,12,13,14] and can grow normally and produce seeds under some extreme soil conditions (2–3% salt content and pH > 10) [14, 15]. Given these qualities, P. tenuiflora has been used to recover and exploit saline grasslands or croplands in northern China [14, 15]. A growing number of molecular studies have focused on P. tenuiflora [12, 16,17,18,19,20,21,22,23,24,25,26,27,28]. Currently, P. tenuiflora is recognized as a model halophyte [3, 12]. Unfortunately, the genomic sequence of P. tenuiflora is unavailable. Here, we provide first report on the P. tenuiflora genome. Our work may provide extensive genetic resources for improving the salinity or drought tolerance of cereals.
Construction and content
Evaluation of genome size
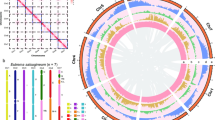
Taxonomy characteristics of Puccinellia tenuiflora are available at Flora of China (http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200026128). We surveyed the chromosome number of P. tenuiflora according to Kato et al. [29]. Total genomic DNA was extracted from fresh leaves. We used the conventional method to estimate the P. tenuiflora genome size. Briefly, we generated 49 Gb of high-quality short-insert Illumina reads to analyze the K-mer frequency of distribution [30]. Genome size was calculated using the following formula: Genome size = total K-mer number /K-mer depth [30, 31], in which K-mer depth is the peak value of K-mer distribution. The chromosome number of P. tenuiflora is 14 (Fig. 1). Our K-mer analysis showed that the genome size of extreme halophyte P. tenuiflora was 1.303 Gb (2n = 14) and the genome was complex, with 1.56% heterozygosity and 65.5% repeat content (Table 1).
Genome sequencing
Illumina paired-end (PE) libraries were constructed with short insert sizes of 250 and 450 bp. Illumina mate-pair (MP) libraries were constructed with insert sizes of 2, 5, and 10 k bp (Table 2). We generated 209.13 Gb of raw data by the PE libraries, and 197.38 Gb of raw data by the MP libraries. The Illumina libraries were sequenced on Illumina HiSeq XTen platform. We also sequenced 56.12 Gb of PacBio long reads and 161.03 Gb of 10× genomics barcoded reads (Table 2).
Genome assembly
Because the P. tenuiflora genome is highly complex and repeated, its genome was assembled by a combined strategy of PacBio (third-generation), 10× genomic technique, and Illumina Hiseq (second-generation). We generated 312.6× reads of Illumina, 43.2× read of PacBio and 123.87× reads of 10× genomic. First the PacBio sequences were corrected for errors. The accurate sequences of PacBio were assembled into primary contigs based on FALCON (Branch 3.1) [32] and FALCON-Unzip software (https://github.com/PacificBiosciences/FALCON_unzip). After treatment with FALCON-Unzip software, we corrected errors of these contigs using PacBio sequences based on quiver software [33] and using Illumina data based on pilon software [34], and finally obtaining consensus sequences of high quality. Next, we used Illumina long reads of 2, 5, and 10 kb to elongate and combine the preassembled contigs into scaffolds based on SSPACE software [35], and then used 10× genomics linked-reads to further elongate and combine the scaffolds based on 10× FragScaff software. Lastly, we used Purge Haplotigs software (https://bitbucket.org/mroachawri/purge_haplotigs/overview) to filter the redundant sequences caused by high heterozygosity. Finally, we assembled 2638 scaffolds with a total size of 1.107 Gb, contig N50 of 117 kb, and scaffold N50 of 950 kb (Table 3).
Genome annotation
Annotation of replicate sequences
Transposable elements (TEs) of the P. tenuiflora genome were annotated. We used two methods to find the TEs. The first method was RepeatMasker (version 3.3.0) to discover TEs in an integrated known replicate sequence library (Repbase 15.02) and the de novo replicate sequence library constructed by RepeatModeler (Version 1.0.5) [36, 37], RepeatScout [38], and LTR_FINDER [39]. The second method detected TEs in the P. tenuiflora genome using RepeatProteinMask by searching against the TE protein database [37]. We identified 691 Mb transposable elements (62.44% of the total sequence), including 580 Mb of LTR retrotransposons (52.43%) (Table 4).
Annotation of protein-coding genes
A combined strategy (de novo-, homolog-, and RNA-seq-based predictions) was used to annotate protein-coding genes in the P. tenuiflora genome using the following software: Augustus (version 3.0.2) [40, 41], Genescan (version 1.0) [42], Geneid [43], GlimmerHMM (version 3.0.2) [44], and SNAP [45]. The homologous sequences of six species (Zea mays, Sorghum bicolor, Brachypodium distachyon, Setaria italica, Arabidopsis thaliana, and Oryza sativa) were aligned against the repeat-masked P. tenuiflora genome with TBLASTN (E-value ≤10–5) [46], and then Genewise software 2.2.0 was used to predict the gene models [47]. Two strategies were used to assemble the RNA-seq reads to the unique transcripts. First, we mapped the RNA-seq reads to the P. tenuiflora genome with Tophat 2.0.8 [48] and Cufflinks 2.1.1 software [49] (http://cufflinks.cbcb.umd.edu/). Afterward, we used Trinity [50] to assemble the RNA-seq reads, and then used PASA [51] (http://pasapipeline.github.io/) to improve the structure of the assembled genes. We generated non-redundant gene sets using EVidenceModeler (EVM) [52] via integrating gene prediction results of all methods. Finally, the predicted genes were filtered by three criteria: coding region length of ≤50 amino acids; FPKM < 5; and supported only by de novo strategy. Functions of the protein-coding genes were annotated by BLASTP program (best hit with E-value ≤1E-05) against three public protein databases: TrEMBL [53], Swiss-Prot, and NR. The protein domains were analyzed by InterProScan software (4.8) via searching against InterPro databases 29.0 [54], and the GO term information was collected from the InterPro annotation results [55]. Moreover, we also conducted KEGG annotation for all genes [56].
On the basis of P. tenuiflora genomic sequences, we predicted 39,725 protein-coding genes (Tables 5). Of the 39,725 predicted protein-coding genes, the protein sequences of 39,470 genes (99.4%) were similar to sequences of known proteins and could be annotated (Table 6). The average gene length was 2818.5 bp, and the average CDS length was 1082.0 bp. The average exon number per gene was 4.2, with an average exon length of 260.5 bp and average intron length of 550.8 bp (Table 5).
Annotation of non-coding RNA
The tRNA genes were discovered with tRNAscan-SE software [57]. The rRNA, miRNA, and snRNA were predicted by INFERNAL software [58] against the Rfam database 9.1 [59]. We annotated non-coding RNA and identified 692 tRNAs, 68 rRNAs, 702 snRNAs, and 1376 microRNAs in the P. tenuiflora genome (Tables 4 and 7). The average lengths of microRNAs, tRNAs, rRNAs, and snRNAs were 124.89 bp, 75.27 bp, 207.79 bp, and 118.21 bp, respectively (Table 7). We deposited the genome sequence in the Genome Warehouse in National Genomics Data Center [60].
Assessment of genome quality
We assessed genome quality using the following methods: Burrow-Wheeler Aligner (BWA), Core Eukaryotic Genes Mapping Approach (CEGMA), and Benchmarking Universal Single-Copy Orthologs (BUSCO). First, in order to assess the quality of genome assembly, we aligned the high-quality Illumina short reads to the assembly using BWA (http://bio-bwa.sourceforge.net, parameters ‘-o 1 -i 15’) [61]. According to BWA method, 87.41% of raw reads were mapped to the genome with 93.34% coverage (Table 8). Next, we used CEGMA and BUSCO to estimate completeness of the assembly. CEGMA is a set of conserved protein families for a wide range of eukaryotes, and is used to identify exon–intron structures of these conserved protein families in a new genomic sequence [62]. CEGMA analysis revealed 223 out of 248 ultraconserved eukaryotic genes (89.9%) in the P. tenuiflora genome indicating integrity for the core genes in the assembly (Table 9). Moreover, completeness of the assembly also was assessed using BUSCO [63] combined with TBLASTN [46], Augustus (version 3.0.2) [40, 41], and HMMER (version 3.1b2) [64]. The BUSCO analysis showed that our assemblies contained 86.8% complete and 1.7% fragmented embryophyta orthologs, suggesting that the assembly quality was high (Table 10).
Utility and discussion
Description of database
The genome assembly of P. tenuiflora consisted of 14,036 contigs with a total size of 1.095 Gb. Finally, we assembled 2638 scaffolds with a total size of 1.107 Gb, contig N50 of 117 kb, and scaffold N50 of 950 kb. On the basis of P. tenuiflora genomic sequences, we predicted 39,725 protein-coding genes, and identified 692 tRNAs, 68 rRNAs, 702 snRNAs, 1376 microRNAs, and 691 Mb transposable elements. We assessed the quality and completeness of the assembled genome through BWA, CEGMA mapping, and BUSCO mapping (Tables 8, 9, 10). The results showed that our assembly had high quality. All raw data for genome assembly are deposited at NCBI. The genome sequence is deposited in the Genome Warehouse in National Genomics Data Center (https://bigd.big.ac.cn/gwh) (accession number GWHABHL00000000).
Significance of database
Halophytes belong to several families and are distributed among multiple clades; this broad distribution pattern suggests that the salinity tolerance mechanisms of halophytes have evolved numerous times or have multiple origins [2]. As a result, halophytes not only exhibit a wide range of salinity tolerance but have also evolved diverse molecular and physiological mechanisms for salinity tolerance [2]. This diversity complicates discovery of the salinity tolerance mechanisms of halophytes. To date, almost all known molecular mechanisms of salinity tolerance were characterized in glycophytes such as rice, wheat, and Arabidopsis [4,5,6]. Glycophytes only provide limited insights into mechanisms of salinity tolerance, and extreme halophytes may have enormous values for improving our understanding of salinity tolerance mechanisms. The genome sequence of extreme halophytes will unlock their molecular studies in salinity tolerance.
The Gramineae is an important plant group because it includes many important food crops, such as rice, wheat, maize, and barley. P. tenuiflora, an extreme Gramineae halophyte, is closely related to barley and wheat. Zhang et al. (2013) reported that P. tenuiflora can grow normally for 6 days under 900 mM NaCl and survive at pH 11 [23]. Wang et al. (2006) found that P. tenuiflora survived 670 mmol/L NaCl [13]. A growing number of molecular biology studies have focused on this species owing to its strong salinity tolerance and high genetic value for cereal improvement [16,17,18,19,20,21,22,23,24,25,26,27,28]. In the present study, we sequenced and assembled the P. tenuiflora genome (2n = 14, size 1.107 Gb). Our work may improve current understanding of salinity tolerance and provides genetic resources for cereal improvement.
Availability of data and materials
All raw data of genome sequencing are available at NCBI. Accession numbers for raw data of genome assembly are SRR7503009-SRR7503032, and SRP152905 and SRP239345 for transcriptional data. The genome sequence was deposited in the Genome Warehouse in National Genomics Data Center (https://bigd.big.ac.cn/gwh) [60], Beijing Institute of Genomics (BIG), Chinese Academy of Sciences, under accession number GWHABHL00000000 that is publicly accessible at https://bigd.big.ac.cn/search?dbId=gwh&q=GWHABHL00000000&page=1. Seeds of P. tenuiflora is available from the corresponsing author upon request.
Abbreviations
- BWA:
-
Burrow-Wheeler aligner
- CEGMA:
-
Core eukaryotic genes mapping approach
- BUSCO:
-
Benchmarking universal single-copy orthologs
References
Flowers TJ, Yeo AR. Breeding for salinity resistance in crop plants: where next. Aust J Plant Physiol. 1995;22(6):875–84.
Flowers TJ, Galal HK, Bromham L. Evolution of halophytes: multiple origins of salt tolerance in land plants. Funct Plant Biol. 2010;37(7):604–12.
Yan XF, Sun GR. Physiological Ecology Research of Puccinellia tenuiflora. Beijing: Science Press; 2000 p. 200.
Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytol. 2008;179(4):945–63.
Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59(1):651–81.
Flowers TJ. Physiology of halophytes. Plant Soil. 1985;89(1–3):41–56.
Wu HJ, Zhang Z, Wang JY, Oh DH, Dassanayake M, Liu B, et al. Insights into salt tolerance from the genome of Thellungiella salsuginea. Proc Natl Acad Sci U S A. 2012;109(30):12219–24.
Ma T, Wang J, Zhou G, Yue Z, Hu Q, Chen Y, et al. Genomic insights into salt adaptation in a desert poplar. Nat Commun. 2013;4(1):3797.
Guo L, Qiu J, Ye C, Jin G, Mao L, Zhang H, et al. Echinochloa crus-galli genome analysis provides insight into its adaptation and invasiveness as a weed. Nat Commun. 2017;8(1):1031.
Wang L, Ma G, Wang H, Chen C, Mu S, Wei Q, et al. A draft genome assembly of halophyte Suaeda aralocaspica, a plant that performs C4 photosynthesis within individual cells. GigaSci. 2019;8(9):giz116.
Zhao K, Song J, Feng G, Zhao M, Liu J. Species, types, distribution, and economic potential of halophytes in China. Plant Soil. 2011;342(1–2):495–509.
Meng X, Zhao Q, Jin Y, Yu J, Yin Z, Chen S, et al. Chilling-responsive mechanisms in halophyte Puccinellia tenuiflora seedlings revealed from proteomics analysis. J Proteome. 2016;143:365–81.
Wang X, Sun G, Wang J, Cao W, Liang J, Yu Z, et al. Relationships among MDA content, plasma membrane permeability and the chlorophyll fluorescence parameters of Puccinellia tenuiflora seedlings under NaCl stress. Acta Ecol Sin. 2006;26(1):122–9.
Xu A. Application of Puccinellia chinampoensis and Puccinellia tenuiflora in Western Jilin Province of China. China Grassl. 1990;2:62–5.
Xu H, Bao C, Ge C, Zhang P, Li L. Comparative study for two salt-tolerant herbages Puccinellia tenuiflora and Puccinellia chinampoensis. China Grassl. 1995;14:43–7.
Wang YC, Yang CP, Liu GF, Jiang J. Development of a cDNA microarray to identify gene expression of Puccinellia tenuiflora under saline-alkali stress. Plant Physiol Biochem. 2007;45(8):567–76.
Wang Y, Chu Y, Liu G, Wang MH, Jiang J, Hou Y, et al. Identification of expressed sequence tags in an alkali grass (Puccinellia tenuiflora) cDNA library. J Plant Physiol. 2007;164(1):78–89.
Liu H, Zhang XX, Takano T, Liu SK. Characterization of a PutCAX1 gene from Puccinellia tenuiflora that confers Ca2+ and Ba2+ tolerance in yeast. Biochem Biophys Res Commun. 2009;383(4):392–6.
Ardie SW, Xie L, Takahashi R, Liu SK, Takano T. Cloning of a high-affinity K+ transporter gene PutHKT2;1 from Puccinellia tenuiflora and its functional comparison with OsHKT2; 1 from rice in yeast and Arabidopsis. J Exp Bot. 2009;60(12):3491–502.
Ardie SW, Liu SK, Takano T. Expression of the AKT1-type K+ channel gene from Puccinellia tenuiflora, PutAKT1, enhances salt tolerance in Arabidopsis. Plant Cell Rep. 2010;29(8):865–74.
Ardie SW, Nishiuchi S, Liu SK, Takano T. Ectopic expression of the channel β subunits from Puccinellia tenuiflora (KPutB1) and rice (KOB1) alters K+/Na+ homeostasis of yeast and Arabidopsis. Mol Biotechnol. 2011;48(1):76–86.
Yu JJ, Chen SX, Zhao Q, Wang T, Yang CP, Diaz C, et al. Physiological and proteomic analysis of salinity tolerance in Puccinellia tenuiflora. J Proteome Res. 2011;10(9):3852–70.
Zhang X, Wei L, Wang Z, Wang T. Physiological and molecular features of Puccinellia tenuiflora tolerating salt and alkaline-salt stress. J Integr Plant Biol. 2013;55(3):262–76.
Yu JJ, Chen SX, Wang T, Sun GR, Dai SJ. Comparative proteomic analysis of Puccinellia tenuiflora leaves under Na2CO3 stress. Int J Mol Sci. 2013;14(1):1740–62.
Wang P, Cui YN, Gao L, Wang SM. Construction of RNAi expression vector of CYP86A gene in halophyte Puccinellia tenuiflora. Acta Pratacul Sin. 2017;26(6):105–10.
Zhang WD, Wang P, Bao Z, Ma Q, Duan LJ, Bao AK, et al. SOS1, HKT1;5, and NHX1 synergistically modulate Na+ homeostasis in the halophytic grass Puccinellia tenuiflora. Front Plant Sci. 2017;8:576.
Ye X, Wang H, Cao X, Jin X, Cui F, Bu Y, et al. Transcriptome profiling of Puccinellia tenuiflora during seed germination under a long-term saline-alkali stress. BMC Genomics. 2019;20(1):589.
Yin Z, Zhang H, Zhao Q, Yoo M-J, Zhu N, Yu J, et al. Physiological and comparative proteomic analyses of saline-alkali NaHCO3-responses in leaves of halophyte Puccinellia tenuiflora. Plant Soil. 2019;437(1–2):137–58.
Kato A, Lamb JC, Birchler JA, Wessler SR. Chromosome painting using repetitive DNA sequences as probes for somatic chromosome identification in maize. Proc Natl Acad Sci U S A. 2004;101(37):13554–9.
Li R, Fan W, Tian G, Zhu H, He L, Cai J, et al. The sequence and de novo assembly of the giant panda genome. Nature. 2010;463(7279):311–7.
Kim EB, Fang X, Fushan AA, Huang Z, Lobanov AV, Han L, et al. Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature. 2011;479(7372):223–7.
Chin C-S, Peluso P, Sedlazeck FJ, Nattestad M, Concepcion GT, Clum A, et al. Phased diploid genome assembly with single molecule real-time sequencing. Nat Methods. 2016;13(12):1050–4.
Chin C-S, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, et al. Nonhybrid, finished microbial genome assemblies from longread SMRT sequencing data. Nat Methods. 2013;10(6):563–9.
Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9(11):e112963.
Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics. 2011;27(4):578–9.
Smit AFA, Hubley R. RepeatModeler Open-1.0. 2008-2015. http://www.repeatmasker.org. Accessed 1 Feb 2015.
Chen N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr Protoc Bioinformatics. 2009. https://doi.org/10.1002/0471250953.bi0410s25.
Price AL, Jones NC, Pevzner PA. De novo identification of repeat families in large genomes. Bioinformatics. 2005;21(1):i351–8.
Xu Z, Wang H. LTR-FINDER: An efficient tool for the prediction of full-length LTR retrotransposons. Nucleic Acids Res. 2007;35(2_1):W265–8.
Stanke M, Waack S. Gene prediction with a hidden Markov model and a new intron submodel. Bioinformatics. 2003;19(2):ii215–25.
Stanke M, Schoffmann O, Morgenstern B, Waack S. Gene prediction in eukaryotes with a generalized hidden Markov model that uses hints from external sources. BMC Bioinform. 2006;7(1):62.
Salamov AA, Solovyev VV. Ab initio gene finding in Drosophila genomic DNA. Genome Res. 2000;10(4):516–22.
Parra G, Blanco E, Guigo R. GeneID in Drosophila. Genome Res. 2000;10(4):511–5.
Majoros WH, Pertea M, Salzberg SL. TigrScan and GlimmerHMM: two open-source ab initio eukaryotic gene-finders. Bioinformatics. 2004;20(16):2878–9.
Korf I. Gene finding in novel genomes. BMC Bioinform. 2004;5(1):59.
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–402.
Birney E, Clamp M, Durbin R. Genewise and genomewise. Genome Res. 2004;14(5):988–95.
Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–11.
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5):511–5.
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–52.
Haas BJ, Delcher AL, Mount SM, Wortman JR, Smith RK, Hannick LI, et al. Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucleic Acids Res. 2003;31(19):5654–66.
Haas BJ, Salzberg SL, Zhu W, Pertea M, Allen JE, Orvis J, et al. Automated eukaryotic gene structure annotation using evidence modeler and the program to assemble spliced alignments. Genome Biol. 2008;9(1):R7.
Bairoch A, Apweiler R. The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Res. 2000;28(1):45–8.
Mulder N, Apweiler R. InterPro and InterProScan: tools for protein sequence classification and comparison. Methods Mol Biol. 2007;396(2):59–70.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. Nat Genet. 2000;25(1):25–9.
Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25(5):955–64.
Nawrocki EP, Kolbe DL, Eddy SR. Infernal 1.0: inference of RNA alignments. Bioinformatics. 2009;25(10):1335–7.
Griffiths-Jones S, Moxon S, Marshall M, Khanna A, Eddy SR, Bateman A. Rfam: annotating non-coding RNAs in complete genomes. Nucleic Acids Res. 2005;33:D121–4.
BIG Data Center Members (BDCM). Database resources of the BIG Data Center in. Nucleic. Acids Res. 2019;47(D1):D8–D14.
Li H, Durbin R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics. 2009;25(14):1754–60.
Parra G, Bradnam K, Korf I. CEGMA: a pipeline to accurately annotate core genes in 2007; 23(9):1061–1067.
Simao FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–2.
Mistry J, Finn RD, Eddy SR, Bateman A, Punta M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013;41(12):e121.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 31971762, 31570328 and 31670218) and Fundamental Research Funds for the Central Universities (No. 2412019FZ026). Genome sequencing was supported by the National Natural Science Foundation of China (nos. 31971762, 31570328 and 31670218) and Fundamental Research Funds for the Central Universities (No. 2412019FZ026). The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Experiment design: RG and CY; experiment perform: RG, LZ, KZ, and CY; data analysis: RG, CY, LZ, KZ, and DG; manuscript writing: RG, LZ, KZ, and CY. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guo, R., Zhao, L., Zhang, K. et al. Genome of extreme halophyte Puccinellia tenuiflora. BMC Genomics 21, 311 (2020). https://doi.org/10.1186/s12864-020-6727-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-020-6727-5