Abstract
Background
Skin lesions and dermatoses in cattle are often associated with infections due to bacteria, fungi or environmental risk factors. Dermatoses with genetic etiology have been described in cattle. Among these rare disorders, there are primary congenital dermatoses that are associated with inherited nutritional deficiencies, such as bovine hereditary zinc deficiency or zinc deficiency-like syndrome. This study presents three cases of Holstein cattle with congenital skin lesions observed on a single farm that resemble zinc deficiency-like syndrome. Close clinical and pathological examinations took place in two cases. Pedigree analysis indicated autosomal recessive inheritance and whole-genome sequencing of both affected calves was performed.
Results
The two calves showed retarded growth and suffered from severe ulcerative dermatitis with hyperkeratosis, alopecia furunculosis and subcutaneous abscess formation. Blood analysis showed correspondent leukocytosis with neutrophilia whereas minerals, macro- and micronutrients were within the reference ranges. Variant calling and filtering against the 1000 Bull Genomes variant catalogue resulted in the detection of a single homozygous protein-changing variant exclusively present in both sequenced genomes. This single-nucleotide deletion in exon 3 of IL17RA on bovine chromosome 5 was predicted to have a deleterious impact on the encoded protein due to a frameshift leading to a truncated gene product. Genotyping of the affected cattle family confirmed recessive inheritance.
Conclusions
A loss-of-function mutation of the IL17RA transmembrane protein could be identified as most likely pathogenic variant for the psoriasis-like skin alterations observed in the two affected Holstein calves. In man, rare recessive diseases associated with IL17RA include immunodeficiency 51 and chronic mucocutaneous candidiasis. This supports the observed immunodeficiency of the presented cases. This study reports the first naturally occurring IL17RA-associated animal model.
Similar content being viewed by others
Background
An intact coat and skin is of utmost importance for the protection of the body from environmental impacts, including infections and thermal challenges. Clinical dermatological conditions vary in their dimensions, from alopecia, hypo- and hyperkeratosis, coat discoloration, seborrhoea to pruritus. Often several symptoms occur simultaneously. Dermatoses are reported frequently and can be due to a variety of causes. The aetiology of most skin disorders is based on a combination of genetic and environmental factors representing multifactorial or complex diseases. Nevertheless, some are determined exclusively by the environment, e.g. due to infections with pathogens belonging to bacteria, viruses, fungi and parasites [1,2,3,4], intoxication [5] and dysfunctional housing [6]. Skin lesions, e.g. due to injuries are at great risk for secondary infections. Generally, they can lead to a reduced animal welfare, performance and therefore to a loss of the economic value of an animal. In cattle, the most common and frequently endemic skin disorder in Central Europe is due to infection with dermatophytes [7]. More rarely, dermatoses occur that are determined exclusively by genetic factors, such as e.g. epidermolysis bullosa [8], or that are associated with inherited disorders leading to nutritional deficiencies of e.g. vitamin A and C, zinc, copper, or fatty acids due to malabsorption [9]. Genetic diseases of the skin are called genodermatoses and typically follow a monogenic mode of inheritance, which means that the genotype at a single gene determines whether the trait is expressed or not [10]. A hallmark of genodermatoses is familial clustering of cases and recently molecular veterinary genetics has made significant advances in the analysis of hereditary dermatoses [10].
A known genetic disorder segregating in the Holstein breed and leading to recurrent bacterial infections, delayed wound healing and retarded growth is the bovine leukocyte adhesion defect (BLAD; OMIA 000595–9913) [11, 12]. This defect is due to the p.Asp128Gly missense variant in the ITGB2 gene [11]. The clinical symptoms induced by this variant are lesions and inflammations on oral mucous membranes and teeth, along with chronic pneumonia and diarrhoea [12].
Another autosomal monogenic recessive condition primarily seen in Holstein and Fleckvieh dairy cattle with psoriasis-like skin lesions in young calves was suspected to be the consequence of a secondary zinc deficiency [13]. It was assumed that a congenital gastrointestinal zinc malabsorption leads to an impaired function of the immune system, growth retardation and severe skin alterations (acrodermatitis). In Holstein cattle this disorder is known as bovine hereditary zinc deficiency (BHZD, OMIA 000593–9913) for a long time and reported to be associated with a recessively inherited splice-site variant in the bovine SLC39A4 gene [14]. The altered SLC39A4 protein is described to lack two motifs, which lie in adjacent transmembrane domains involved in the construction of a pore responsible for the transport of zinc. Therefore the reported SLC39A4 variant is most likely responsible for the impaired zinc absorption in this disorder [14]. The identification of another pathogenic variant associated with the so-called zinc deficiency-like (ZDL) syndrome (OMIA 001935–9913), a highly similar genodermatosis occurring in Fleckvieh cattle indicated obvious locus heterogeneity for this entity of disorders. Clinically a massive hyperkeratosis in combination with secondary microbial infections were observed in the ZDL-affected animals carrying two copies of the unravelled nonsense mutation in bovine PLD4 [15]. In addition, all BHZD as well as ZDL cases were also underdeveloped in body size and weight and had a history of recurring diarrhoea and pneumonia [13,14,15,16]. Although there is a striking similarity in the phenotypic appearance of these two disorders, there are no clues for a functional connection between PLD4, an uncharacterized member of the family of phospholipid signalling enzymes, and zinc metabolism. As the ZDL-affected Fleckvieh calves did not respond to a dietary zinc supplementation, an impaired zinc metabolism is most likely not causing this disorder.
Herein we report the detailed phenotype of three half-sib purebred Holstein calves with severe congenital psoriasis-like skin alterations, retarded growth and an enhanced susceptibility to different infectious diseases such as bronchopneumonia. Clinical, genealogical, pathological and histopathological examination of the calves suggested a rare and recessive genodermatosis resembling ZDL. However, ZDL has not been observed in Holstein cattle until now. Our genetic analysis revealed a perfectly linked recessively inherited frameshift variant in the interleukin 17 receptor A (IL17RA) gene, which is most likely disease causing.
Results
Clinical findings
Initially, three calves were presented to the local veterinarian for clinical examination and therapy. One of the calves suffered from a severe bronchopneumonia and died shortly after presentation despite immediate therapy. The two other calves (3 and 5 months old) showed retarded growth and development even though they had been clinically inconspicuous within the first 4 to 6 weeks of their lives. Chronic skin lesions with signs of severe inflammation were present especially in skin folds, beneath the tail and at the umbilical region. Blood analysis including zinc supply, examinations for infections with bluetongue virus (BTV) or lumpy skin disease (LSD) resulted unsuspicious.
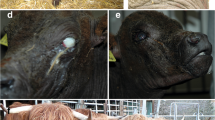
These two female calves were admitted to the Clinic for Ruminants (Internal medicine and Surgery) of the Justus-Liebig University Giessen for a deeper clinical investigation. The 5-months old calf will further be referred to as case 1 and the 3-months old calf as case 2. Both cases were lethargic and slightly dehydrated and showed massive hyperkeratotic alterations of the skin with regional alopecia and signs of inflammation. Skin lesions were partly bleeding and a sanious odour of the skin could be noticed (Fig. 1). In case 1, the head was asymmetric due to severe swelling in the region of the left mandibular lymph nodes (Fig. 1a). It was a 20 × 20 × 10 cm large mass in front of the shoulder joint. This calf also showed inflammations at the inguinal skin fold. Case 2 mainly showed marked inflammation of the axillar skin (Fig. 1f) and at the inguinal skin fold (Fig. 1b and e) as well as small abscesses at the lower surface of the tale (Fig. 1) and jaw (Fig. 1d). The palpable lymph nodes were markedly swollen in this calf.
Features of the skin anomalies in case 1 (A) and case 2 (B-F). a Head with massive swelling at the left mandibular angle. Palpation of this mass displayed fluctuation and severe phlegmon of the skin. b Ventral view on the lesions in the inguinal skin. c Abscesses beneath the tail. Crusts of pus and incrusted faeces are present. d Lesions around the mouth. e Right inguinal skin fold with massive inflammation and ulceration. f Axillar skin lesions. Palpation of the inflamed skin resulted in instant bleeding
Results of the peripheral blood analysis regarding the standard parameters are summarized in additional file 1 for each calf individually. The numbers of leukocytes, segmented neutrophils and the lymphocytes deviated from the normal range, indicating inflammation. The value for glutathione peroxidase indicated a sufficient supply of selenium in both calves. Regarding the minerals, solely magnesium showed a considerably reduced serum concentration, while zinc showed a mildly increased concentration.
Furthermore, the microbiological analysis of skin biopsies and lung tissue samples are provided in additional file 2 for both calves. Comparing the levels of infection, a high content of specific bacteria was more often observed in case 2 than in case 1, and comparing the tissue types, the bacterial content on skin was slightly higher than in the lungs. No fungal pathogens were determined in skin or lung samples. Tests concerning bovine virus diarrhoea (BVD), bluetongue virus (BTV) and bovine poxvirus (BPV) resulted negative.
Necropsy
Both calves showed similar gross lesions and a deferred development with a body weight of 113 kg (case 1) and 95 kg (case 2). The dermis revealed an ulcerative dermatitis and pododermatitis with pustules, alopecia, furunculosis, subcutaneous abscess formation, and marked orthokeratotic hyperkeratosis (Fig. 1a-f). Mainly the areas of the axillar skin (Fig. 1f), the inguinal skin (Fig. 1b,e, Fig. 2a), the tale (Fig. 1c) and the mucocutaneous junctions were affected. Additionally, there was a severe epithelial hyperplasia and a moderate perivascular infiltration with lymphocytes, plasma cells, and macrophages (Fig. 2). Microscopically the massively inflamed skin lesions at the skin folds are visualized in Fig. 2b and c, where the chronic ulcerations with serocellular crusts are visible (Fig. 2a). The junctions show ulcerations of the epithelium with formed granulated tissue and the inflamed cells (Fig. 2b) and orthokeratotic hyperkeratosis with pustules were detected (Fig. 2c). Furthermore, the calves had multiple large abscesses in the cranial pulmonary lobes (Fig. 3a). They were accompanied by dystelectasis and a mild suppurative bronchopneumonia in the surrounding lung tissue (Fig. 3). There were subcutaneous masses in the region of the left mandibular lymph node and in front of the shoulder joint (Fig. 1a), and were identified as abscesses (Fig. 4).
Details of the inguinal skin lesions of case 2. a Macroscopic image of chronic ulcerations with serocellular crusts. b Microscopic view of severe ulceration of the epithelium (arrowheads) with formation of granulation tissue and necrosuppurative inflammation. Hematoxylin and Eosin, 5x, bar = 1 mm. c Microscopic view showing marked orthokeratotic hyperkeratosis (arrowhead). Hematoxylin and Eosin, 10x, bar = 500 μm
Genetic findings
On the farm, the sire of the three affected calves was used in a single breeding period for natural mating with a total of 30 heifers, all originating from a common natural service sire used at the farm in the years before (Fig. 5a). Besides the two affected female calves investigated in this study, 11 more female and 17 male calves were born. One of these additional female calves showed similar signs as observed in the two presented cases and died of pneumonia. However, in this additional case no close clinical examination was done and no sample is available. Neither the 17 males nor the other 10 female offspring showed comparable skin alterations although it was not possible to follow-up the health status of the male calves after they left the farm with 2-weeks of age for fattening. Both, the sire of the affected calves and all 30 dams were apparently normal. The pedigree of the affected calves is in accordance with recessive inheritance. Genetic testing allowed to rule out bovine leukocyte adhesion deficiency (BLAD) as possible cause for the observed symptoms as both cases were tested negative by a commercial laboratory (IFN Schönow GMbH, Bernau).
IL17RA frameshift variant associated with the observed genodermatosis. a Pedigree including the three affected calves (shown in black). All thirteen available family members were genotyped for the IL17RA variant (genotypes presented below the individuals). b IGV screenshot showing the single-nucleotide deletion on chromosome 5 in the two sequenced cases. Note the indicated change in the deduced amino acid sequence below. A premature stop codon is predicted to occur after 62 altered residues. ($ indicates the first nucleotide of codon 59 belonging to IL17RA exon 2)
Adopting the assumption of a possible recessive mode of inheritance, whole-genome sequencing (WGS) followed by single-nucleotide variant (SNV) and small insertion and deletion (InDel) calling resulted in 23 private protein-changing variants (Additional file 3) beside 4754 private non-coding variants (Additional file 4). Due to the strong effect of the putative genetic defect, we hypothesized that a loss-of-function variant affecting the coding sequence of a gene most likely would be responsible for the disorder. They were found to be homozygous exclusively in the two affected animals and absent or occurring heterozygous in 396 control genomes that were sequenced in the course of other ongoing projects at the Institute of Genetics. In a second step 22 variants out of these 23 protein-changing variants were found with both possible genotypes (heterozygous and homozygous) in the 3103 control genomes from the 1000 Bull Genomes project (Additional file 3) [17]. The single remaining protein-changing variant in the interleukin 17 receptor A (IL17RA) gene, which was absent from all controls was predicted to have a deleterious impact on the encoded protein. This single nucleotide deletion in exon 3 of IL17RA (chr5: g.108813251delC; Fig. 5b) leads to a frameshift (XM_015460734.2: c.180delC), which is predicted to replace 61 amino acids and to truncate a significant part (~ 85%) of the C-terminus of the protein (XP_015316220.2: p.(Cys61AlafsTer62)).
We genotyped the single-nucleotide deletion in exon 3 of IL17RA in both available cases, as well as in eleven non-affected relatives (Fig. 5a) by Sanger sequencing. This confirmed the homozygous state of the variant in the two affected calves, whereas both dams of the affected calves were heterozygous for the IL17RA deletion, as well as two half-sibs and two maternal sisters (Fig. 5a). All further tested relatives were homozygous wild type.
Discussion
Interestingly our study revealed interleukin 17 receptor A (IL17RA) as a new gene involved in a recessively inherited genodermatosis of Holstein cattle. The herein described clinical findings resemble strongly a disorder known in Fleckvieh cattle as zinc deficiency-like (ZDL) syndrome associated with a PLD4 missense variant [15]. Both herein described cases and the ZDL-affected calves show an increasing distribution and severity of skin lesions over time, where the first lesions appeared on the muzzle. Further common characteristics are the occurrence of pneumonia and the inability to response to oral zinc supplementation [16]. However, the PLD4 variant and the previously reported SLC39A4-associated zinc deficiency in Holstein cattle (BHZD) could be ruled out due to their clinical differences. While the dermatological changes associated with the PLD4 non-sense variant are described as a crusting dermatitis [16], the herein described cases show open wounds that scarcely heal. BHZD affected calves show a zinc deficiency, which can be treated by oral application of zinc [13]. This therapy was applied to the herein presented cases but did not lead to any clinical improvement and thereby a disorder like BHZD could be ruled out. Furthermore, bovine leukocyte adhesion deficiency (BLAD) was excluded through genetic testing. Finally, all known disease-causing variants were confirmed to not be present in the described cases by analysing the WGS data.
To our knowledge, the IL17RA gene had not yet been associated with similar disorders in domestic animals. Variants in human IL17RA were reported to cause autosomal recessively inherited immunodeficiency 51 (OMIM 605461) and affected individuals show inborn susceptibility to several infections and especially to chronic mucocutaneous candidiasis [18, 19]. The pathogenic variants associated with immunodeficiency 51 include missense variants as well as non-sense variants and lead to a total loss-of-function of IL17RA [19, 20]. Pro-inflammatory cytokines such as interleukin 17 and 22, as well as their receptors play a central role in acute and chronic inflammatory responses [21, 22]. Interleukin 17 (IL-17) is especially known to be of importance at mucosal and barrier surfaces and plays a possible role in the pathogenesis of autoimmunity [23]. The most common member of the IL-17 family is IL-17A and the receptor complex it signalling threw the heterodimer complex formed by the receptors IL-17RA and IL-17RC [23,24,25]. Furthermore, IL-17 had been associated with a wide range of diseases including inflammation ranging from arthritis, psoriasis, spondylitis, Crohn’s disease, multiple sclerosis, cardio vascular diseases and a variety of disorders of the lung [23]. The involvement of IL-17 and its receptor IL17RA has been shown in respect to psoriasis and psoriatic arthritis in humans and mice [26,27,28,29,30].
Interestingly, the herein described bovine cases homozygous for the IL17RA loss-of-function variant did not suffer from fungal but from multi-bacterial infections. This points to differences in the immune defence against fungal and bacterial infections between humans/mice and cattle.
This IL17RA-associated semi lethal genodermatosis, leading to psoriasis-like skin alterations and immunodeficiency resembling zinc deficiency-like syndrome in Holstein cattle, was not reported before. Based on the absence of the disease-causing IL17RA variant in the global cohort of whole-genome sequenced cattle, which includes several hundred Holstein sires used in artificial insemination, the frequency of the mutant allele in the international Holstein population is probably very low. Nonetheless, our findings allow for the first time a targeted monitoring of the prevalence of this most likely pathogenic variant in the local German population and the avoidance of further risk matings.
Conclusion
To the best of our knowledge, a similar IL17RA-associated genodermatosis showing chronic skin alterations correlated with an inherited immunodeficiency has not been described until now in cattle. Moreover, this is the first report of a most likely pathogenic variant in IL17RA in a domestic animal species.
Methods
Animals
In a single German Holstein breeding flock three calves which were sired by the same bull showed clinical signs suspicious for a disease similar to ZDL or BHZD. While one calf perished of a heavy lung inflammation during the first weeks of life the other two calves were chronically suffering from psoriasis-like skin alterations and showed reduced growth. Therefore, in this study clinical and pathological examinations were conducted on the two affected calves and the genetic background. Blood samples from eleven healthy relatives were collected. Unfortunately, the sire was already slaughtered when the study was executed. In addition, we collected blood of two dams, the paternal grandmother, the maternal great-grandmother, four paternal half-sibs and three paternal half-sibs of the dams (Fig. 5a).
Clinical analyses
Clinical examination was conducted on two affected calves. This includes a general description of their health status. Further, all important peripheral blood parameters were analysed. Blood analyses were performed using EDTA, serum and lithium-heparin samples from each of the affected calves. After necropsy microbiological analysis of the skin and lung tissue had been performed.
Whole-genome sequencing
In order to investigate the genetic architecture of the disorder, genomic DNA was extracted from EDTA blood samples of case 1 and 2 and used for whole-genome sequencing (WGS). Therefore, individual PCR-free fragment libraries, which were sequenced for 150 bp paired-end reads were prepared. Both cases were sequenced on the Illumina NovaSeq6000 resulting in a read depth of approximately 17.2x and 20.8x for the first and the second case, respectively. The WGS data was mapped to the latest reference genome ARS-UCD1.2 [31] and called for small nucleotide variants (SNVs) and small insertions and deletions (InDels). We followed the workflow proposed by the 1000 Bull Genomes Project (run 7) [17, 32] to process the raw data into binary alignment map (BAM) and genomic variant call format (GVCF) files. Further, the individual GVCF files were merged to one large variant call format (VCF) file by using CombineGVCFs and CatVariants of GATK v3.8 [33]. This file was produced together with another 396 genomes available at the Institute of Genetics of the University of Bern and thereby includes 398 genomes (Additional file 5) of a variety of 23 cattle breeds and few crossbred animals. For this VCF file SNVs and InDels were called using GenotypeGVCF of GATK v3.8 [33]. Furthermore, quality labels based on the best practice recommendations in GATK using the VariantFiltration of GATK v3.8 [33] were given for each variant. Finally, SnpEff v4.3 [34] was used to functionally annotated each variants effect, based on the NCBI Annotation Release 106 [35]. This resulted in the final VCF file, which includes all 398 individuals’ variants and their functional annotation.
We filtered the final VCF file based on the assumption that the disorder is a rare recessive disorder. Thereby, only the two affected animals need to be carriers of a possible candidate variant but no other of the 396 control genomes (Additional file 5). This approach is supported by the fact, that there are no other sequenced German Holstein animals in the data set. In order to validate the allele frequency of the variant and exclude variants that occur in other breeds all remaining variants were compared to the VCF file from the 1000 Bull Genomes project (run 7) [17]. This reference data includes 3103 cattle genomes which include 937 individuals from the Holstein breed from all around the globe. Integrative Genomic Viewer (IGV) software [36] was used for visual inspection of the remaining candidate variants.
Genotyping of the candidate variants
Forward primer 5′-GTCATGGCCTGACTGTGAAG-3′ and reverse primer 5′-GTCCACTCGATGTGAACCAC-3′ were designed with the software Primer3 [37] to produce a 243 bp fragment including the IL17RA variant. Sanger sequencing of the resulting PCR product was performed by a service laboratory (LGC Genomics, Berlin). The obtained sequences were analysed with ChromasPro 1.22 software (Technelysium Pty Ltd., South Brisbane) and compared with the relevant genome region of the latest reference genome ARS-UCD1.2 [31].
Availability of data and materials
Whole-genome sequencing data can be accessed on the European Nucleotide Archive with the project ID PRJEB18113 (sample accessions: SAMEA5714977; SAMEA5714978). Further publicly available data used in this study can be found in this repository with the indicated sample IDs in the additional file 4.
Abbreviations
- BAM:
-
Binary alignment map
- BHZD:
-
Bovine hereditary zinc deficiency
- BLAD:
-
Bovine leukocyte adhesion deficiency
- BPV:
-
Bovine poxvirus
- BTV:
-
Bluetongue virus
- BVD:
-
Bovine virus diarrhoea
- GVCF:
-
Genomic variant call format
- InDel:
-
Small insertion and deletion
- LSD:
-
Lumpy skin disease
- SNV:
-
Single-nucleotide variant
- VCF:
-
Variant call format
- WGS:
-
Whole-genome sequencing
- ZDL:
-
Zinc deficiency-like
References
Alsaaod M, Locher I, Jores J, Grimm P, Brodard I, Steiner A, et al. Detection of specific Treponema species and Dichelobacter nodosus from digital dermatitis (Mortellaro's disease) lesions in Swiss cattle. Schweiz Arch Tierheilkd. 2019;161(4):207–15.
Bianchi MV, Silveira S, Mosena ACS, de Souza SO, Konradt G, Canal CW, et al. Pathological and virological features of skin lesions caused by BVDV in cattle. Braz J Microbiol. 2019;50(1):271–7.
Beard PM. Lumpy skin disease: a direct threat to Europe. Vet Rec. 2016;178(22):557–8.
Marin RE, Houston R, Omanska-Klusek A, Alcaraz A, Garcia JP, Uzal FA. Pathology and diagnosis of proliferative and ulcerative dermatitis associated with Tunga penetrans infestation in cattle. J Vet Diagn Investig. 2015;27(1):80–5.
Coppock RW, Mostrom MS, Simon J, McKenna DJ, Jacobsen B, Szlachta HL. Cutaneous ergotism in a herd of dairy calves. J Am Vet Med Assoc. 1989;194(4):549–51.
Kielland C, Ruud LE, Zanella AJ, Osteras O. Prevalence and risk factors for skin lesions on legs of dairy cattle housed in freestalls in Norway. J Dairy Sci. 2009;92(11):5487–96.
Chermette R, Ferreiro L, Guillot J. Dermatophytoses in animals. Mycopathologia. 2008;166(5–6):385–405.
Medeiros GX, Riet-Correa F. Epidermolysis bullosa in animals: a review. Vet Dermatol. 2015;26(1):3–13 e1-2.
Hensel P. Nutrition and skin diseases in veterinary medicine. Clin Dermatol. 2010;28(6):686–93.
Leeb T, Müller EJ, Roosje P, Welle M. Genetic testing in veterinary dermatology. Vet Dermatol. 2017;28(1):4–e1.
Shuster DE, Kehrli ME, Ackermann MR, Gilbert RO. Identification and prevalence of a genetic defect that causes leukocyte adhesion deficiency in Holstein cattle. Proc Natl Acad Sci. 1992 Oct 1;89(19):9225–9.
Nagahata H. Bovine leukocyte adhesion deficiency (BLAD): a review. J Vet Med Sci. 2004;66(12):1475–82.
Machen M, Montgomery T, Holland R, Braselton E, Dunstan R, Brewer G, et al. Bovine hereditary zinc deficiency: lethal trait a 46. J Vet Diagn Investig. 1996;8(2):219–27.
Yuzbasiyan-Gurkan V, Bartlett E. Identification of a unique splice site variant in SLC39A4 in bovine hereditary zinc deficiency, lethal trait A46: an animal model of acrodermatitis enteropathica. Genomics. 2006;88(4):521–6.
Jung S, Pausch H, Langenmayer MC, Schwarzenbacher H, Majzoub-Altweck M, Gollnick NS, et al. A nonsense mutation in PLD4 is associated with a zinc deficiency-like syndrome in Fleckvieh cattle. BMC Genomics. 2014;15:623.
Langenmayer MC, Jung S, Majzoub-Altweck M, Trefz FM, Seifert C, Knubben-Schweizer G, et al. Zinc deficiency-like syndrome in Fleckvieh calves: clinical and pathological findings and differentiation from bovine hereditary zinc deficiency. J Vet Intern Med. 2018;32(2):853–9.
Hayes BJ, Daetwyler HD. 1000 bull genomes project to map simple and complex genetic traits in cattle: applications and outcomes. Annu Rev Anim Biosci. 2019;7:89–102.
Ling Y, Puel A. IL-17 and infections. Actas Dermosifiliogr. 2014;105:34–40.
Lévy R, Okada S, Béziat V, Moriya K, Liu C, Chai LY, et al. Genetic, immunological, and clinical features of patients with bacterial and fungal infections due to inherited IL-17RA deficiency. Proc Natl Acad Sci. 2016;113(51):E8277–E85.
Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332(6025):65–8.
de Masson A, Bouaziz JD, Battistella M, Bagot M, Bensussan A. Immunopathology of psoriasis: from bench to bedside. Med Sci. 2016;32(3):253–9.
Gu C, Wu L, Li X. IL-17 family: cytokines, receptors and signaling. Cytokine. 2013;64(2):477–85.
Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763–76.
Abusleme L, Moutsopoulos NM. IL-17: overview and role in oral immunity and microbiome. Oral Dis. 2017;23(7):854–65.
Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–67.
Adamopoulos IE, Suzuki E, Chao CC, Gorman D, Adda S, Maverakis E, et al. IL-17A gene transfer induces bone loss and epidermal hyperplasia associated with psoriatic arthritis. Ann Rheum Dis. 2015;74(6):1284–92.
Batalla A, Coto E, Gonzalez-Lara L, Gonzalez-Fernandez D, Gomez J, Aranguren TF, et al. Association between single nucleotide polymorphisms IL17RA rs4819554 and IL17E rs79877597 and psoriasis in a Spanish cohort. J Dermatol Sci. 2015;80(2):111–5.
Floudas A, Saunders SP, Moran T, Schwartz C, Hams E, Fitzgerald DC, et al. IL-17 receptor a maintains and protects the skin barrier to prevent allergic skin inflammation. J Immunol. 2017;199(2):707–17.
Li Y, Golden JB, Camhi MI, Zhang X, Fritz Y, Diaconu D, et al. Protection from psoriasis-related thrombosis after inhibition of IL-23 or IL-17A. J Invest Dermatol. 2018;138(2):310–5.
Su Y, Huang J, Zhao X, Lu H, Wang W, Yang XO, et al. Interleukin-17 receptor D constitutes an alternative receptor for interleukin-17A important in psoriasis-like skin inflammation. Sci Immunol. 2019;4(36).
NCBI Assembly - ARS-UCD1.2 reference sequence. National Center for Biotechnology Information, U.S. National Library of Medicine. 2018. https://www.ncbi.nlm.nih.gov/assembly/GCF_002263795.1. Accessed 21 Aug 2018.
1000 Bull Genomes Project. http://www.1000bullgenomes.com/. (2019) Accessed 27 Sept 2019.
DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491–8.
Cingolani P, Platts A, Wang le L, Coon M, Nguyen T, Wang L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. FlY. 2012;6(2):80–92.
NCBI Genome - Bos taurus Annotation Release 106. National Center for Biotechnology Information, U.S. National Library of Medicine. 2018. https://www.ncbi.nlm.nih.gov/genome/annotation_euk/Bos_taurus/106/. Accessed 21 Aug 2018.
Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–6.
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012;40(15):e115.
Acknowledgements
The authors are grateful to the cattle breeder for submission of the affected animals. We acknowledge the assistance of the Next Generation Sequencing Platform of the University of Bern for performing the whole-genome re-sequencing experiments. Furthermore, we thank the Interfaculty Bioinformatics Unit of the University of Bern for providing high performance computing infrastructure.
Funding
This study was partially funded by the Swiss National Science Foundation.
Author information
Authors and Affiliations
Contributions
MS conducted the clinical examinations and clinical laboratory analysis. IH performed the bioinformatics of the whole-genome sequencing data and drafted the manuscript. MHO ruled out infectious and heritable differential diagnosis, collected samples on farm, advised the farmer to submit the animals to the veterinary clinic of the Justus-Liebig University and contributed pedigree information. MHE and LMR performed the pathological examinations. SM, the veterinarian attending the farm, examined and treated the affected calves on farm and was the first to suspect a potential inherited disease. CD planned and supervised the whole-genome sequencing analysis. GL conducted pedigree analysis and genotyping of potential causal variants in relatives of the affected calves and in unrelated animals. MS, GL and CD coordinated the study and contributed to the writing of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study did not require official or institutional ethical approval as it was not experimental but part of clinical and pathological veterinary diagnostics. The animals were handled according to good ethical standards and German legislation (German Animal Welfare Act from 15th May 2006, last amended on 20th November 2019). A formal ethics approval for the euthanasia of the affected calves was not needed as this complies with §4 of the German Animal Welfare Act. The affected calves were euthanized by intravenous injection of an overdose of sodium pentobarbital (500 mg/mL), performed by MS. The aim was to identify the cause of the congenital disorder and thereby improve the animal welfare situation on the farm. Written consent was obtained from the cattle owner.
Consent for publication
Not applicable.
Competing interests
The authors declare that they no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Results of peripheral blood analyses.
Additional file 2.
Results of microbiological analyses in lung and skin samples. Note that the analyses could not display any mycological agents.
Additional file 3.
Twenty-three shared homozygous protein-changing variants in both sequenced cases. The frequency of the corresponding genotypes in the variant catalogue of the 1000 Bulls Genome project are given.
Additional file 4.
4754 shared homozygous non-coding variants in both sequenced cases.
Additional file 5.
EBI accession numbers of all publicly available genome sequences.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Häfliger, I.M., Sickinger, M., Holsteg, M. et al. An IL17RA frameshift variant in a Holstein cattle family with psoriasis-like skin alterations and immunodeficiency. BMC Genet 21, 55 (2020). https://doi.org/10.1186/s12863-020-00860-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12863-020-00860-4