Abstract
Background
Autotetraploid Carassius auratus (4n = 200, RRRR) (abbreviated as 4nRR) is derived from whole genome duplication of Carassius auratus red var. (2n = 100, RR) (abbreviated as RCC). Ribosome DNA (rDNA) is often used to study molecular evolution of repeated sequences because it has high copy number and special conserved coding regions in genomes. In this study, we analysed the sequences (5S, ITS1-5.8S-ITS2 region), structure, methylation level (NTS and IGS), and expression level (5S and 18S) of 5S and 45S ribosomal RNA (rRNA) genes in 4nRR and RCC in order to elucidate the effects of autotetraploidization on rDNA in fish.
Results
Results showed that there was high sequence similarity of 5S, 5.8S and ITS1 region between 4nRR and RCC. This study also identified two different types of ITS2 region in 4nRR and predicted the secondary structure of ITS2. It turns out that both secondary structures are functional. Compared with RCC, there was no significant difference in NTS (5S rRNA) methylation level, but the expression level of 5S rRNA was lower in 4nRR, indicating that methylation had little effect on the expression level in 4nRR. IGS (45S rRNA) was hypermethylated in 4nRR compared to RCC, but the expression of 18S rRNA gene was no significantly different from that in RCC, indicating that methylation regulation affected gene expression in 4nRR.
Conclusion
The above studies initially revealed the effects of autotetraploidization on the structure and function of 5S and 45S rRNA in Carassius auratus, and provided a theoretical support for the systematic study of the evolution pattern and characteristics of rDNA in vertebrates.
Similar content being viewed by others
Background
Polyploidy studies have reported about different aspects of life such as genome duplication, gene expression, and subsequent evolution [1, 2]. Polyploids can be classified into autopolyploids and allopolyploids. The former presents two or more homologous chromosomes in a homopolyploid which may contribute to the formation of polyvalent bodies during meiosis, whereas the latter predominantly forms bivalent pairings [3, 4]. It is worth noting that most polyploidy associated studies mainly focus on plants and less on animals. In our previous studies, we developed allotetraploid hybrids (4n = 148, RRBB) (abbreviated as 4nRB) from the first generation of Carassius auratus red var. (2n = 100, RCC) (♀) × Megalobrama amblycephala (2n = 48, BSB) (♂) hybrids [5]. In subsequent studies, abnormal chromosomal behavior during meiosis in allotetraploid hybrids (4nRB) led to the formation of autotetraploid sperms and autodiploid eggs, which eventually formed autotetraploid Carassius auratus (4nRR) [6, 7]. Previous researches mainly focused on allopolyploids, with only few autopolyploid studies. As the most prone to hybridization, the genomes of fish have been comprehensively studied, and thus they can be used to better understand the evolution of vertebrate cell genomes [8].
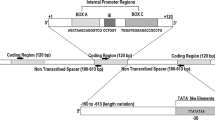
Ribosomal DNA (rDNA) is commonly used to study the molecular evolution of multigene families. In eukaryotes, rDNA genes are mainly divided into two categories: 5S rDNA and 45S rDNA repeats. rDNA encodes rRNA that represents a highly conserved gene product in all cells [9, 10]. Because of the predominance of its structure, rDNA often serves as a good resource for studying evolutionary events [4, 11]. In animals, 45S rDNA contains 18S, 5.8S, 28S, and spacers (IGS, ITS1 and ITS2), while the 5S rDNA gene is a unit consisting of a gene transcription region (120 bp) and a non-transcribed spacer (NTS). Previous studies of fish have reported that different types of 5S rDNA were due to differences in NTS (NTS I, NTS II, and NTS III) [4, 10, 12]. IGS is a transcriptional regulatory sequence of rDNA which modulates cellular processes [13, 14]. Previous analyses of rDNA repeats have mostly been carried out in invertebrates and plants. Therefore, information on 5S and 45S rDNA in vertebrates is scarce. One study reported that rRNA molecules must fold into secondary structures in order to function properly in ribosomes [15]. ITS2 provides useful biological information at a higher taxonomic level, even in all eukaryotes, because it has a conserved secondary structure [16]. Many gene promoter regions are rich in CpG, commonly known as CpG islands. Studies have shown that cytosine methylation in CpG dinucleotide guanosine 5' plays an important role in gene expression regulation [17, 18]. In this study, we analyzed the sequence, structure, methylation, and expression in 5S and 45S rRNA clusters between autotetraploid Carassius auratus (4nRR) and its parental species (RCC). From an evolutionary perspective, comparing the arrangement of the 5S and 45S rRNA in 4nRR and RCC makes sense because of their similar genomic compositions. Our results initially revealed the effects of autotetraploidization on 5S rRNA and 45S rRNA of Carassius auratus, and provided theoretical support for the systematic study of the evolution patterns and characteristics of rDNA in vertebrates.
Results
Expression sequence analysis of 5S rRNA coding region and ITS1-5.8S-ITS2 sequence
A total of 40 copies of the gene sequences were analyzed from 4nRR and RCC. Amplification of the 5S rRNA coding region in 4nRR and RCC produced a 120 bp band. BLASTn alignment of the sequences in 4nRR detected two types: one was identical to RCC (not shown in figure), and the other had high sequence identity (average similarity of 97.5%) with corresponding sequences from RCC, although there was a few base substitution changes (Fig. 1). Therefore, our preliminary analysis showed that the 5S rRNA coding region of 4nRR had high similarity with the corresponding parental species sequences (GenBank Accession Nos. MZ041022 and MZ041023).
It is well known that two specific sequences (called internal transcription spacers) separate the mature rRNA sequences: ITS1 (between 18S rRNA and 5.8S rRNA) and ITS2 (between 5.8S and 28S rRNA). We cloned and sequenced PCR products in order to compare the internal transcription region (ITS1-5.8S-ITS2) of 4nRR and RCC. For better comparison, the ITS region was divided into ITS1, 5.8S and ITS2 regions. BLASTn sequence alignments showed that the ITS1 and 5.8S rRNA of 4nRR had 100% similarity (Fig. 2) to RCC (ITS1: GenBank Accession Nos. MZ041015 and MZ041016; 5.8S: GenBank Accession Nos. MZ041020 and MZ041021). Nevertheless, we found two different types of ITS2 in 4nRR: type I (inherited from the parental species (RCC)) and type II (a newly formed type which was only expressed in tetraploid species) (Fig. 3) (GenBank Accession Nos. MZ041017-MZ041019). Figure 3 showed intraspecific variation of these sequences. Results indicated that type II ITS2 had obvious insertion, deletion and base substitution. These findings suggested that the ITS1 sequence was more conserved than ITS2.
Prediction of ITS2 secondary structure
ITS2 usually has four helices, but not all eukaryotes have the same number of helices. Studies have shown that only helix II and helix III are recognizable and essentially common in all organisms [16]. In this study, we predicted the secondary structure of ITS2 according to the two different sequences of type I and type II (Fig. 4). It turned out that both secondary structures were functional. The results showed that helix II (pyrimidine-pyrimidine) and helix III had high conservation in type I and type II of ITS2, especially the 5’ side of helix III (CCGGTGG).
Expression analysis of 5S and 18S rRNA
We compared the expression of 5S and 18S rRNA genes in 4nRR using quantitative real-time PCR with RCC acting as the control group (Fig. 4). Results showed that the amount of 5S rRNA transcriptional products in the liver tissues of 4nRR was significantly lower than that of RCC group (Fig. 5A; P < 0.05). However, there was no significant difference in the expression of 18S rRNA gene between RCC and 4nRR (Fig. 5B; P > 0.05). These results suggested that the effects of polyploidy on the expression levels of 5S and 18S rRNA genes were not consistent.
Methylation-specific PCR of NTS (5S rRNA) and IGS (45S rRNA)
The results only showed differences of methylation level in NTS II because the 5S arrays of NTS I and NTS III had the same levels of methylation in RCC and 4nRR (Fig. 6) (GenBank Accession Nos. MZ041027- MZ041032). However, there was no significant methylation difference of NTS II between RCC and 4nRR (85% and 92.5%, respectively) (P > 0.05). Figure 7 showed analysis of the IGS methylation status of 45S rRNA in liver tissues (GenBank Accession Nos. MZ041024-MZ041026). Our results indicated that there were two different types of IGS (4nRR I and 4nRR II) in 4nRR. Furthermore, 4nRR I had a similar methylation level with RCC (P > 0.05), while 4nRR II had a higher methylation level than RCC (P < 0.05). In general, the IGS methylation status of 4nRR was hypermethylated and the degree of IGS methylation was negatively correlated with the relative expression of genes.
Discussion
5S and 45S rDNA genes play a critical role in ribosome folding and functionality [9]. Studies have shown that the ITS region is a useful genetic marker for the analysis of intraspecific variation [19,20,21]. Our results indicated that the coding region of 5S rRNA gene, 5.8S rRNA gene and ITS1 region sequences were almost conserved in 4nRR. A previous study reported that the 5S rRNA gene (transcribed by RNA polymerase III) contained an internal control region (ICR) that acted as the promoter for the gene [22]. Generally, variation in 5S rDNA occurs in the NTS region, but the coding region remains unchanged [23, 24]. It has been reported that the ITS region (45S rDNA) participates in proper processing of ribosomal RNA sequences and forming mature functional rRNA subunits [25]. Thus, based on these results, autotetraploidization has no significant effect on the organization of 5.8S rRNA and ITS1 region. The sequences and structures are consistent, so 5.8S rRNA and ITS1 perform the same function in RCC and 4nRR. With regard to the ITS2 region, a comparison between RCC and 4nRR indicated that there were two types of ITS2 region in 4nRR. These mutations can be attributed to the weak selection pressure on any single copy of the gene, thereby allowing a degree of variation in the gene region [26, 27]. In addition, hybridization is accompanied by genome changes in order to overcome threats to its survival [28].
The ITS2 secondary structures presented in this study is consistent with other ITS2 structure predictions. ITS2 usually has four helices, with helix II and helix III being recognizable in almost all organisms. Helix II is very short, does not have any branches, and has a pyrimidine–pyrimidine mismatch. On the other hand, helix III is usually longer than helix II and often has branches. Previous studies reported that the largest absolute sequence conserved region in the entire ITS2 was located on the 5’ side (YCGGTGGR) of helix III close to the tip [16, 29, 30]. Moreover, these conservative characteristics are preserved in type I and type II helices. The ITS2 conserved structural motifs are necessary for all aspects of ribosomal processing [31]. The helix I is highly similar in both types. Traditionally, helix IV is the most variable region in ITS2, thus, it is normal for the two types of 4nRR to be different, both secondary structures are functional [8, 32]. These differences may also reflect differences in the formation of mature functional ribosomes because there are many steps involved in the production of a mature rRNA gene [25].
Newly formed polyploids undergo extensive genomic changes after genome combination and replication [33]. Polyploidy significantly affects genome formation and other genetic aspects such as gene expression. We found that there were no significant differences in expression of the 18S rRNA gene between 4nRR and RCC. However, the 5S rRNA gene showed significant differences. Moreover, all the genes were doubled in autotetraploid fish compared to RCC. Theoretically, if each gene was normally expressed, the total gene volume would be much higher than that of the diploid parent. This supports the findings of a previous study which reported that the origin of polyploid lineages are not consistent at the ploidy level of gene expression, with regard to increase or decrease [34]. Previous studies have shown that the genomic DNA loci of autotetraploids differ from those of diploids [35]. However, these results did not explain whether the gene expression differences were caused by genomic DNA site changes or epigenetic silencing. For example, changes in DNA methylation, a common epigenetic phenomenon, can also regulate gene expression.
To verify whether the differences between RCC and 4nRR were dependent on the methylation status, we analyzed the NTS methylation patterns of the different 5S rRNA arrays of RCC and 4nRR using the genomic sequencing technique. Previous studies have associated cytosine methylation with the non-expression of a gene [36, 37]. The 5S rRNA clusters of NTS I and NTS III in RCC and 4nRR were all methylated and they showed no difference in methylation status. Furthermore, although the methylation levels of NTS II varied, there was no significant difference. In summary, the methylation level in all 5S rRNA sequences was similar. These results indicated that methylation may not affect the binding of transcription factors to 5S rDNA, nor did it regulate transcription of the 5S rRNA gene. Thus, it may have no significant effect on expression of 5S rRNA gene. IGS, as a variable part of 45S rDNA, usually contains enough variation to allow examination of genetic relationships between closely related species [14, 38, 39]. In the study, there were two types of IGS in 4nRR: type I was hypomethylated, while type II was typehypomethylated. The results ensured that the methylation level was consistent in 4nRR. Among them, there was no significant difference between type I and RCC, while type II showed significant differences and a higher methylation degree than RCC. The results showed that IGS methylation was negatively correlated with relative gene expression, and methylation inhibited the expression level to some extent. The emergence of two types of IGS can be attributed to the fact that the establishment of nucleolar dominance requires several generations of selection and screening during the homologous polyploidization process. It is possible that the inhibitory mechanism that controls nuclear dominance in hybrids also control the number of active 45S rRNA gene in pure breeds and may reflect the dose compensation mechanism [11, 40, 41]. However, regulation of the active 5S rRNA gene may be different. Our quantitative real-time PCR results indicated that the expression of 5S rRNA gene was low in all 4nRR individuals, while the expression level of 18S rRNA gene showed no significant difference between RCC and 4nRR. In our previous studies, we observed loss of chromosomal sites in the generation of 4nRR [35]. As regulatory regions, NTS and IGS regulate gene expression during the late stage according to methylation. This phenomenon might explain why the number of chromosomes in 4nRR increased but there was no positive increase in the expression level. In addition, 45S and 5S rRNA could not make many differences in number because they need to form the large and small subunits of the ribosome. Otherwise, the subunits would not be paired quantitatively.
rDNA is an important component of nuclear structure and mechanisms that maintain genomic integrity [42–44]. 5S rRNA and partitial ITS sequences are still conserved during the autotetraploidization, the study has revealed that the basic unity of rDNA sequences in 4nRR and RCC. One study reported that the high transcriptional and recombination rates of rDNA contributed to the diversity of the genome and formation of reproductive barriers [8]. Moreover, the repetitive nature of rDNA and other duplicated genes leads to a high degree of evolutionary dynamics [45, 46]. Therefore, this tetraploid lineage can be an attractive model for elucidating genomic changes associated with autotetraploidization. Our results will expand the understanding of homologous polyploidy effects on ribosomal DNA and have important significances for the evolutionary study of polyploid Carassius auratus. In addition, the information on the sequences and structures of 4nRR (5S and 45S rRNA) provides a reference for further studies on the evolution of rDNA in fish and other vertebrates.
Conclusion
By comparing and analyzing the sequences, structures, expression levels and methylation levels of ribosomal RNA genes (5S rRNA, 45S rRNA) in autotetraploid Carassius auratus (4nRR), we found that 5S rRNA, 5.8S rRNA and ITS1 were highly conserved, but autotetraploidization promoted the structural differentiation of ITS2 in 4nRR. The expression levels and methylation results showed that the methylation of the 5S rRNA regulation region did not regulate the expression of the gene, but the 45S rRNA regulation region affected the expression of 18S rRNA gene in 4nRR to some extent. Polyploidization is one of the main driving forces of biological evolution. The datas from this study provide some references for studying the evolution of ribosomal DNA in autopolyploid species.
Materials and methods
Materials
Experimental fishes were provided by the Engineering Center of Polyploid Fish Breeding of the National Education Ministry, Hunan Normal University.
Expression sequence and expression analysis of 5S rDNA
Our analyses involved sequencing of 30 clones for each accession. Genomic DNA was isolated from blood of all samples using genomic DNA extraction kit (Takara). PCR was then performed with a specific primer complementary to the 5S rRNA conserved coding region. The primers were synthesized according to the method described by Qin et al. [47]. Primer sequences were: GCTATGCCCGATCTCGTCTGA (5′-3′) and CAGGTTGGTATGGCCGTAAGC (5′-3′). The PCR program included 30 cycles of denaturation at 94 °C for 1 min, annealing at 59 °C for 35 s, and elongation at 72 °C for 35 s. Final extension was performed at 72 °C for 15 min. Moreover, RNA was extracted from liver tissues using Trizol reagent in accordance with the manufacturer’s instructions (Invitrogen, San Diego, CA). Next, the RNA was reverse transcribed to cDNA using the PrimeScript™ RT reagent kit (Perfect Real Time, Takara) with a gDNA eraser. The 5S rRNA gene-specific primer (5′-CAGGTTGGTATGGCCGTAAG-3′) was then used to amplify the first-strand cDNA.
Amplification products were analyzed using 1–1.2% agarose gel electrophoresis stained with ethidium bromide. The PCR products were then cloned, followed by selection of clones with inserts of the predicted length (203 bp) for sequencing. Next, Bioedit and ClustalW software was used to analyze the sequence homology and variation of the amplified fragments of 4nRR and RCC. To determine gene expression differences, quantitative real-time PCR (Prism 7500 sequence detection system, ABI) was used to analyze the expression level of the target genes. Relative gene expression was normalized to the expression of β-actin gene, an endogenous control.
Expression sequence (ITS1-5.8S-ITS2) and expression (18S) analysis of 45S rDNA
For amplification of ITS1-5.8S-ITS2, the following primers were used: 5′-AGTCGTAACAAGGTTTCCGTAGGTG-3′ and 3′-TTATGGCCGTGCTCTGGCTAT-5′ [11]. PCR was carried out using the conditions described above but with exception of the annealing temperature (57 °C). Moreover, the 18S rRNA gene-specific primer (5′-CATCTAAGGGCATCACAGAC-3′) was used to amplify the first-strand cDNA. Sequences and expression analysis were conducted according to a previously described protocol [48].
Secondary structure of ITS2 sequences
We conducted comparative sequence analysis to elucidate the secondary structure of ITS2 sequences. More information about species relatability and intraspeciality variation was obtained by examining the functional folding patterns or secondary structures of the rRNA regions of interest [8, 21]. We determined the structure with the lowest free energy and compared the secondary structure of ITS2 cloned by 4nRR.
Methylation-specific PCR
Using the common carp genome as a reference, we identified the spacer regions (NTS and IGS) of 5S and 45S rRNA genes in NCBI database. Sequences of the corresponding target NTS and IGS were retrieved from RCC genome (DDBJ/EMBL/GenBank Accession No. PRJNA289059) and 4nRR genome (unpublished), respectively. Genomic DNA was extracted from liver tissues using Sangon Animal Genomic DNA extraction kit (n = 3 fishes per treatment). Next, the extracted DNA was treated according to the methylcoded bisulfite conversion kit protocol (Thermo Fisher). Gene-specific primers for NTS (NTS I, NTS II, and NTS III) and IGS (Table 1) were designed using Primer 5.0 software. PCR products were ligated, transformed, and sequenced. Finally, sequences obtained from methylation results were retrieved using BiQ analyzer.
Availability of data and materials
All data generated or analysed during this study were included in this published article. The sequence for these libraries have been uploaded to the NCBI Sequence Read Archive site (http://www.ncbi.nlm.nih.gov/sra/; accession nos.): ITS1 (GenBank Accession Nos. MZ041015 and MZ041016); ITS2 (GenBank Accession Nos. MZ041017-MZ041019); 5.8S (GenBank Accession Nos. MZ041020 and MZ041021); 5S (GenBank Accession Nos. MZ041022 and MZ041023); IGS (GenBank Accession Nos. MZ041024-MZ041026); NTS (GenBank Accession Nos. MZ041027- MZ041032). All data have been stored in the NCBI database, and will postpone the release on April 24, 2022.
Abbreviations
- RCC:
-
Carassius auratus Red var.
- BSB:
-
Megalobrama amblycephala
- 4nRB:
-
Allotetraploid hybrids
- 4nRR:
-
Autotetraploid Carassius auratus
- NTS:
-
Non-transcribed spacer
- IGS:
-
Internal transcribed spacer
- rDNA:
-
Ribosomal DNA
- rRNA:
-
Ribosomal RNA
References
Adams KL, Wendel JF. Polyploidy and genome evolution in plants. Curr Opin Plant Biol. 2005;8(2):135–41. https://doi.org/10.1016/j.pbi.2005.01.001.
Hegarty M, Coate J, Sherman-Broyles S, Abbott R, Hiscock S, Doyle J. Lessons from natural and artificial polyploids in higher plants. Cytogenet Genome Res. 2013;140(2–4):204–25. https://doi.org/10.1159/000353361.
Comai L. The advantages and disadvantages of being polyploid. Nat Rev Genet. 2005;6(11):836–46. https://doi.org/10.1038/nrg1711.
Qin QB, Liu QW, Wang CQ, Cao L, Zhou YW, Qin H, Zhao C, Liu SJ. Molecular organization and chromosomal localization analysis of 5S rDNA clusters in autotetraploids derived from Carassius auratus red var. (♀) × Megalobrama amblycephala (♂). Front Genetics. 2019;10:437. https://doi.org/10.3389/fgene.2019.00437.
Qin QB, Wang YD, Wang J, Dai J, Liu Y, Liu SJ. Abnormal chromosome behavior during meiosis in the allotetraploid of Carassius auratus red var. (♀)× Megalobrama amblycephala (♂). BMC Genet. 2014;15(1):95–95. https://doi.org/10.1186/s12863-014-0095-6.
Qin QB, Wang YD, Wang J, Dai J, Xiao J, Hu FZ, Luo KK, Tao M, Zhang C, Liu Y, Liu SJ. The autotetraploid fish derived from hybridization of Carassius auratus red var. (female)× Megalobrama amblycephala (male). Biol Reprod. 2014;91(4):93. https://doi.org/10.1095/biolreprod.114.122283.
Qin QB, Wang J, Dai J, Wang YD, Liu Y, Liu SJ. Induced all-female autotriploidy in the allotetraploids of Carassius auratus red var. (♀)× Megalobrama amblycephala (♂). Mar Biotechnol. 2015;17(5):604–12.
Symonová R, Howell WM. Vertebrate genome evolution in the light of fish cytogenomics and rDNAomics. Genes. 2018;9(2):96. https://doi.org/10.3390/genes9020096.
Pinhal D, Yoshimura TS, Araki CS, Martins C. The 5S rDNA family evolves through concerted and birth-and-death evolution in fish genomes: an example from freshwater stingrays. BMC Evol Biol. 2011. https://doi.org/10.1186/1471-2148-11-151.
Long EO, Dawid IB. Repeated genes in eukaryotes. Annu Rev Biochem. 1980;49(1):727–64. https://doi.org/10.1146/annurev.bi.49.070180.003455.
Cao L, Qin QB, Xiao Q, Yin HT, Wen J, Liu QW, Huang X, Huo YY, Tao M, Zhang C, Luo KK, Liu SJ. Nucleolar dominance in a tetraploidy hybrid lineage derived from Carassius auratus red var. (♀) × Megalobrama amblycephala (♂). Front Genetics. 2018;9:386. https://doi.org/10.3389/fgene.2018.00386.
Korn LJ, Brown DD. Nucleotide sequence of Xenopus borealis oocyte 5S DNA: comparison of sequences that flank several related eucaryotic genes. Cell. 1978;15(4):1145–56. https://doi.org/10.1016/0092-8674(78)90042-9.
Ruffini CM, Gelati MT, Cremonini R, Frediani M. The intergenic spacer region of the rDNA in Haplopappus gracilis (Nutt.) Gray. Protoplasma. 2013;250(3):683–9. https://doi.org/10.1007/s00709-012-0441-3.
Fernández M, Polanco C, Ruiz ML, de la Vega PM. A comparative study of the structure of the rDNA intergenic spacer of Lens culinaris Medik, and other legume species. Genome. 2000;43(4):597–603. https://doi.org/10.1139/g00-022.
Noller HF. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53(1):119–62. https://doi.org/10.1146/annurev.bi.53.070184.001003.
Coleman AW. Pan-eukaryote ITS2 homologies revealed by RNA secondary structure. Nucleic Acids Res. 2007;35(10):3322–9. https://doi.org/10.1093/nar/gkm233.
Bird A. The essentials of DNA methylation. Cell. 1992;70(1):5–8. https://doi.org/10.1016/0092-8674(92)90526-I.
Herman JG, Graff JR, Myöhänen S, et al. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci. 1996;93(18):9821–6.
Kahn AB. Aspects of the molecular phylogeny of three species of the wild rice genus, Zizania, based on nuclear rDNA sequences. Southwest Texas State University, 1996.
Lee S, Wen J. A phylogenetic analysis of Prunus and the Amygdaloideae (Rosaceae) using ITS sequences of nuclear ribosomal DNA. Am J Bot. 2001;88(1):150–60. https://doi.org/10.2307/2657135.
Paskewitz SM, Wesson DM, Collins FH. The internal transcribed spacers of ribosomal DNA in five members of the Anopheles gambiae species complex. Insect Mol Biol. 1994;2(4):247–57. https://doi.org/10.1111/j.1365-2583.1994.tb00144.x.
Hallenberg C, Nederby-Nielsen J, Frederiksen S. Characterization of 5S rRNA genes from mouse. Gene. 1994;142(2):291–5. https://doi.org/10.1016/0378-1119(94)90277-1.
Martins C, Galetti PM. Two 5S rDNA arrays in neotropical fish species: is it a general rule for fishes. Genetica. 2001;111(1):439–46. https://doi.org/10.1023/A:1013799516717.
Martins C, Wasko AP, Oliveira C, Porto-Foresti F, Parise-Maltempi PP, Wright JM, Foresti F. Dynamics of 5S rDNA in the tilapia (Oreochromis niloticus) genome: repeat units, inverted sequences, pseudogenes and chromosome loci. Cytogenet Genome Res. 2002;98(1):78–85. https://doi.org/10.1159/000068542.
Johansen T, Repolho T, Hellebo A, Raae AJ. Strict conservation of the ITS regions of the ribosomal RNA genes in Atlantic cod (Gadus morhua L.) full length research paper. DNA Seq. 2006;17(2):107–14. https://doi.org/10.1080/10425170600624701.
Kellogg EA, Appels R. Intraspecific and interspecific variation in 5S RNA genes are decoupled in diploid wheat relatives. Genetics. 1995;140(1):325–43. https://doi.org/10.1101/gad.9.9.1137.
Allaby RG, Brown TA. Network analysis provides insights into evolution of 5S rDNA arrays in Triticum and Aegilops. Genetics. 2001;157(3):1331–41. https://doi.org/10.1093/genetics/157.3.1331.
McClintock B. The significance of responses of the genome to challenge. Science. 1984;226(4676):792–801. https://doi.org/10.1126/science.15739260.
Joseph N. Ribosomal internal transcribed spacer 2 (ITS2) exhibits a common core of secondary structure in vertebrates and yeast. Nucleic Acids Res. 1999;27(23):4533–40. https://doi.org/10.1093/nar/27.23.4533.
Coleman AW, Vacquier VD. Exploring the phylogenetic utility of ITS sequences for animals: a test case for abalone (Haliotis). J Mol Evol. 2002;54(2):246–57. https://doi.org/10.1007/s00239-001-0006-0.
Keller A, Schleicher T, Schultz J, Tobias M, Dandekar T, Wolf M. 5.8S–28S rRNA interaction and HMM-based ITS2 annotation. Gene. 2009;430(1–2):50–7. https://doi.org/10.1016/j.gene.2008.10.012.
Young I, Coleman AW. The advantages of the ITS2 region of the nuclear rDNA cistron for analysis of phylogenetic relationships of insects: a Drosophila example. Mol Phylogenet Evol. 2004;30(1):236–42. https://doi.org/10.1016/S1055-7903(03)00178-7.
Madlung A, Wendel JF. Genetic and epigenetic aspects of polyploid evolution in plants. Cytogenet Genome Res. 2013;140(2–4):270–85. https://doi.org/10.1159/000351430.
Church SA, Spaulding EJ. Gene expression in a wild autopolyploid sunflower series. J Hered. 2009;100(4):491–5. https://doi.org/10.1093/jhered/esp008.
Qin QB, Cao L, Wang YD, Ren L, Liu QW, Zhou YW, Wang CQ, Qin H, Zhao C, Liu SJ. Rapid genomic and genetic changes in the first generation of autotetraploid lineages derived from distant hybridization of Carassius auratus red Var. (♀)× Megalobrama amblycephala (♂). Mar Biotechnol. 2019;21(2):139–49. https://doi.org/10.1007/s10126-018-9859-8.
Flavell RB, ODell M, Thompson WF, Vincentz M, Barker RF. The differential expression of ribosomal RNA genes. Philos Trans R Society London B Biol Sci. 1986;314(1166):385–97. https://doi.org/10.1098/rstb.1986.0060.
Razin A, Riggs AD. DNA methylation and gene function. Science. 1980;210(4470):604–10. https://doi.org/10.1126/science.6254144.
de Penteado MIO, García P, de la Vega PM. Genetic variability and mating system in three species of the genus Centrosema. J Heredity. 1996;87(2):124–30. https://doi.org/10.1093/oxfordjournals.jhered.a022967.
Nickrent DL, Patrick JA. The nuclear ribosomal DNA intergenic spacers of wild and cultivated soybean have low variation and cryptic subrepeats. Genome. 1998;41(2):183–92. https://doi.org/10.1139/g98-001.
Wallace H, Langridge WHR. Differential amphiplasty and the control of ribosomal RNA synthesis. Heredity. 1971;27(1):1–13. https://doi.org/10.1038/hdy.1971.66.
Pikaard CS. Nucleolar dominance and silencing of transcription. Trends Plant Sci. 1999;4(12):478–83. https://doi.org/10.1016/S1360-1385(99)01501-0.
Guetg C, Santoro R. Formation of nuclear heterochromatin: the nucleolar point of view. Epigenetics. 2012;7(8):811–4. https://doi.org/10.4161/epi.21072.
Tsekrekou M, Stratigi K, Chatzinikolaou G. The nucleolus: in genome maintenance and repair. Int J Mol Sci. 2017;18(7):1411. https://doi.org/10.3390/ijms18071411.
Grummt I. The nucleolus-guardian of cellular homeostasis and genome integrity. Chromosoma. 2013;122(6):487–97. https://doi.org/10.1007/s00412-013-0430-0.
Terencio ML, Schneider CH, Gross MC, do Carmo EJ, Nogaroto V, de Almeida MC, Artoni RF, Vicari MR, Feldberg E. Repetitive sequences: the hidden diversity of heterochromatin in prochilodontid fish. Comp Cytogenet. 2015. https://doi.org/10.3897/compcytogen.v9i4.5299.
Charlesworth B, Sniegowski P, Stephan W. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature. 1994;371(6494):215–20. https://doi.org/10.1038/371215a0.
Qin QB, He W, Liu SJ, Wang J, Xiao J, Liu Y. Analysis of 5S rDNA organization and variation in polyploid hybrids from crosses of different fish subfamilies. J Exp Zool B Mol Dev Evol. 2010;314(5):403–11. https://doi.org/10.1002/jez.b.21346.
Cao L, Zhao C, Wang CQ, Qin H, Qin QB, Tao M, Zhang C, Zhao RR, Liu SJ. Evolutionary dynamics of 18S and 5S rDNA in autotriploid Carassius auratus. Gene. 2020;737: 144433. https://doi.org/10.1016/j.gene.2020.144433.
Acknowledgements
We thank many researchers for maintaining and nursing autotetraploid fish for many years. Liu, Qin and Zhang contributed to the conception and designed the study. All authors read and approved the final manuscript.
Funding
This work was supported by National Key Research and Development Program of China (2020YFD0900100), National Natural Science Foundation of China (Grant No. 31730098, U19A2040), Earmarked Fund for China Agriculture Research System (Grant No. CARS-45), Natural Science Foundation for Distinguished Young Scholars of Hunan Province (Grant No. 2017JJ1022).
Author information
Authors and Affiliations
Author notes
Chun Zhao and Yuxin Zhang are contributed equally
Contributions
SL and QQ have designed of the work. CZ has contributed to this study for the design, in executing experiments and in writing manuscript. YZ, HQ, CW and XH have made substantial contributions to the acquisition and analysis of data. LY, TY, XX and XL have substantively revised the work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by Ethics Committee of Hunan Normal University, all methods were carried out in accordance with relevant guidelines and regulations. This study was carried out in compliance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, C., Zhang, Y., Qin, H. et al. Organization and expression analysis of 5S and 45S ribosomal DNA clusters in autotetraploid Carassius auratus. BMC Ecol Evo 21, 201 (2021). https://doi.org/10.1186/s12862-021-01918-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12862-021-01918-2