Abstract
Background
Neo-XY sex chromosome determination is a rare event in short horned grasshoppers, but it appears with unusual frequency in the Pamphagidae family. The neo-Y chromosomes found in several species appear to have undergone heterochromatinization and degradation, but this subject needs to be analyzed in other Pamphagidae species. We perform here karyotyping and molecular cytogenetic analyses in 12 Pamphagidae species from the center of biodiversity of this group in the previously-unstudied Anatolian plateau.
Results
The basal karyotype for the Pamphagidae family, consisting of 18 acrocentric autosomes and an acrocentric X chromosome (2n♂ = 19, X0; 2n♀ = 20, XX), was found only in G. adaliae. The karyotype of all other studied species consisted of 16 acrocentric autosomes and a neo-XY sex chromosome system (2n♂♀ = 18, neo-XX♀/neo-XY♂). Two different types of neo-Y chromosomes were found. One of them was typical for three species of the Glyphotmethis genus, and showed a neo-Y chromosome being similar in size to the XR arm of the neo-X, with the addition of two small subproximal interstitial C-blocks. The second type of the neo-Y chromosome was smaller and more heterochromatinized than the XR arm, and was typical for all Nocarodeini species studied. The chromosome distribution of C-positive regions and clusters of ribosomal DNA (rDNA) and telomeric repeats yielded additional information on evolution of these neo-XY systems.
Conclusion
Most Pamphagidae species in the Anatolian region were found to have neo-XY sex chromosome systems, belonging to two different evolutionary lineages, marked by independent X-autosome fusion events occurred within the Trinchinae and Pamphaginae subfamilies. The high density of species carrying neo-XY systems in the Anatolian region, and the different evolutionary stage for the two lineages found, one being older than the other, indicates that this region has a long history of neo-XY sex chromosome formation.
Similar content being viewed by others
Background
The karyotype structure of grasshoppers is very conservative. For instance, the modal male karyotype of Acrididae grasshoppers consists of 22 acrocentric autosomes and an acrocentric X chromosome. In Pyrgomorphidae and Pamphagidae, the modal karyotype consists of 18 acrocentric autosomes and an acrocentric X chromosome. The sex chromosome system in the vast majority of species is XX♀/XO♂ [1–4]. In the karyotype evolution of some species the X chromosome enters into centric fusion with an autosome, which leads to a neo-XX♀/neo-XY♂ sex chromosome system. In some species, this has resulted in new chromosome races [5–7] while in some other species neo-sex chromosomes have become a species karyoptypic feature (see review:[8]). At present, neo-sex chromosomes have been described in more than 100 species from different taxonomic groups of grasshoppers [1, 2, 8, 9]. However, it is rare for neo-sex chromosomes to characterize a group of closely related species. Furthermore, after chromosome fusion, the neo-sex chromosome usually does not exhibit further evolutionary change. This observation suggests that de novo originated neo-sex chromosomes are evolutionary dead ends which do not lead to species divergence [10]. For a long time, neotropical species belonging to the Melanoplinae subfamily of Acrididae grasshoppers were an exception to this rule. In this group, with a basal XX♀/XO♂ sex chromosome system, many species have a neo-XX♀/neo-XY♂ sex chromosome system. Furthermore, in some species, neo-Y chromosome-autosome fusion has resulted in a more advanced neo-X1X1X2X2♀/neo-X1X2Y♂ sex chromosome system [8, 9]. Cytogenetic studies of Pamphagidae grasshoppers in the Palearctic region indicates that this group is another example of sex chromosome evolution. The neo-XX♀/neo-XY♂ sex chromosome system was revealed in 11 of 43 studied species of the Pamphagidae family [11–22]. In one species, a neo-X1X1X2X2♀/neo-X1X2Y♂ sex chromosome system was described [22]. Species with neo-sex chromosomes belonged to different subfamilies, Trinchinae and Pamphaginae. What is more, within Trinchinae, neo-sex chromosomes were observed in 4 species of the Asiotmethis genus and in one closely related monotypic Atrichotmethis genus, while in Pamphaginae neo-sex chromosomes were observed in all studied species belonging to the Nocarodeini tribe [14, 18, 19, 22]. Neo-Y chromosomes in these groups exhibit different morphology indicating further intensive reorganization after formation, resulting in heterochromatinization and shrinkage of the neo-Y chromosome.
In early comparative studies of Pamphagidae grasshoppers, it was suggested that species with neo-sex chromosomes belong to monophyletic group. And species from Trinchinae subfamily, showing less heterochromatinized neo-Y chromosome, are an initial stage of more heteromochromatinized neo-Y chromosome in Nocarodeini species [19]. This suggestion was made on an assumption of low possibility of such rare event as neo-sex chromosome formation in different clades of one family.
These findings make Pamphagidae grasshoppers an interesting model for studying sex chromosome evolution. However, detailed cytogenetic analysis of grasshoppers’ karyotypes is hindered by lack of chromosome markers. Despite wide range of classical chromosome bandings used in cytogenetics, only C-banding, that reveals regions enriched with C-heterochromatin, can be used in grasshoppers. Molecular cytogenetic methods can overcome these difficulties through mapping molecular markers, such as repetitive or unique sequences on chromosomes. This approach has been successfully applied to different groups of grasshoppers including species of neotropical Melanoplinae subfamily with neo-sex chromosome systems [22–28]. Therefore, application of molecular cytogenetic methods as well as classical banding - is crucial for full understanding of sex chromosome evolution in yet unstudied species of this family.
Analysis of the geographical distribution of Pamphagidae grasshoppers showing neo-sex chromosomes indicated that some species of the Thrinchinae subfamily and all the Nocarodeini tribe (Pamphaginae subfamily) species studied occur in South Eastern Europe, Central Asia and the Caucasian Mountains [14, 18, 19, 22]. The number of species with neo-sex chromosomes was found to be high near the Western Asian region, yet this region remained cytogenetically unstudied. We thus focused our research on Pamphagidae grasshoppers from Western and Central Anatolia, to test the hypothesis that neo-sex chromosome formation may have taken place during the evolution of Pamphagidae grasshoppers in the Western Asian region.
Methods
Males of four species belonging to the Trinchinae subfamily and 8 species (two of them having two subspecies) from the Pamphaginae subfamily were collected during summer season 2014 in Western and Central Anatolia (Table 1). In addition to males, females of Oronothrotes furvus were captured and kept in a cage with moisturized sand for egg-pod laying. Chromosome preparations and C-banding were performed as described earlier [22].
An rDNA probe was generated through PCR of specific fragments of the 18S rRNA gene with specific primers (Table 2). Primers were designed in the PerlPremier software [29], using 18S rDNA consensus sequence from aligning 45S rDNA sequences of four sequences of different grasshoppers (gb|AY379758.1, gb|KM853211.1, gb|KF855839.1 and gb|JF792554.1) using the Mulalin software, (http://multalin.toulouse.inra.fr/multalin/) [30].
Three DNA fragments were amplified separately in 20 μl of reaction mix in 25 cycles of polymerase chain reaction (PCR) (initial denaturation 3 min 95 °C, cycles 1 min−95 °C, 40 s−58 °C, 1 min−72 °C, and final elongation 8 min−72 °C) from 40 ng of genomic DNA of Chorthippus biguttulus (Linnaeus, 1758). PCR mix contained 1 × Taq polymerase buffer, 2 mM MgCl2, 0.2 mM dNTP, 0.5 μM of each primer and 0.03 U/μl Taq DNA polymerase (Medigen, Novosibirsk, Russia). Labeling was performed in 25 additional cycles of PCR. The reaction mix was similar to that described above, but the concentration of dTTP was reduced to 0.15 mM and 0.05 mM Fluorescein-dUTP (Medigen, Novosibirsk, Russia) was added and the concentration of Taq DNA polymerase was increased to 0.06U/μl. The reaction mix from the previous step was diluted 100 fold and 1 μl of it was added to 19 μl of labeling reaction mix as a matrix. For hybridization, an equal amount of labeling reaction mix was mixed together and was used as a DNA probe.
Insect telomeric repeats (TTAGG)n were generated in non-template PCR with 5′-TAACCTAACCTAACCTAACC-3′ and 5′-TTAGGTTAGGTTAGGTTAGG-3′ primers according to standard protocol with modifications [31]. Labeling was performed with Tamra-dUTP (Medigen, Novosibirsk, Russia) in additional cycles of PCR, as described earlier [32].
Fluorescence in situ hybridization (FISH) was carried out as was described earlier [32, 33]. DAPI counterstaining was performed after FISH using Vectashield antifade containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector laboratories, USA) under cover glass which was then sealed with rubber cement.
Microscopic analysis was carried out at the Centre for Microscopy of Biological Subjects (Institute of Cytology and Genetics, Novosibirsk, Russia). Chromosomes were studied with an Axio Imager. M1 (Zeiss) fluorescence microscope equipped with filter sets #49, #46HE, #43HE (ZEISS), ProgRes MF (Meta Sistems) CCD camera. The ISIS5 software package (MetaSystems GmbH, Germany) was used for image capture and analysis.
The nomenclature of chromosomes suggested for Pamphagidae grasshoppers [12] was used for the description of chromosomes and karyotypes. According to this nomenclature, autosomes were numbered in order of decreasing size (1–9) and classified into three size groups: L – large, M – medium and S – small. In the species with neo-sex chromosomes they were named following White [34]. The arms of the submetacentric neo-X chromosome were referred to as XL and XR. The short arm (XL) corresponds to the former acrocentric X chromosome and the long arm (XR) to the translocated acrocentric autosome. The unfused autosome, homologous to the XR arm, remains acrocentric and is the neo-Y chromosome.
Results
The Pamphagidae grasshoppers from Western and Central Anatolia analyzed in the current study belonged to Trinchinae (four species) and Pamphaginae (Nocarodeini tribe, 8 species, two of them having two subspecies). These species had not hitherto been cytologically analyzed, and we provide here the first information on chromosome number, morphology and structure, using several techniques including Giemsa staining, C-banding and FISH for ribosomal DNA (rDNA) and telomeric repeats.
Karyotypes of studied species
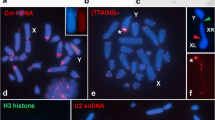
The Trinchinae species exhibited two types of karyotype. The first type was found only in Glyphotmethis adaliae and consisted of 18 acrocentric autosomes (L1–L4, M5–M8, S9) and a medium sized acrocentric X chromosome. The sex chromosome system was XX♀/X0♂. (Figure 1a, b). The second type of karyotype, found in Glyphotmethis dimorphus, Glyphotmethis efe, Glyphotmethis holtzi pulchripes, consisted of 16 autosomes (L1–L3, M4–M7, S8), a submetacentric neo-X chromosome and a large acrocentric neo-Y chromosome (Fig. 1 c–h). The sex chromosome system was neo-XX♀/neo-XY♂. The size of this neo-Y is equal to the XR arm of the neo-X chromosome, and during meiosis the neo-Y and XR arm formed a normal bivalent with 1–2 chiasmata.
C-banding (a,c,e,g) and fluorescence in situ hybridization (FISH) with both rDNA (green) and telomeric DNA (red) probes (b,d,f,h) in diakinesis/metaphase I for the following Glypotmethis species from Trinchinae subfamily: G. adaliae (a,b); G. dimorphus (c,d); G. efe (e,f); G. holtzi pukhripes (g,h). Red arrowheads indicate ITSs. The inset in the top right corner shows neo-X and neo-Y chromosomes in mitotic metaphase
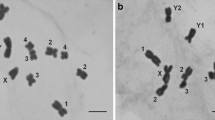
Large chromosomes in these three Trinchinae species were characterized by small short arms showing variation in size (Fig. 2a). In G. dimorphus and G. efe, the short arm in the L1 chromosome was polymorphic whereas in G. holtzi pulchripes all L1 chromosomes showed similar-sized short arms. In the L2 chromosome also showed a conspicuous short arm in G. dimorphus and G. holtzi pulchripes.
Features of karyotypes of species studied. a L1 and L2 chromosome pairs with polymorphic small second arms in species form Glyphotmetis genus; b Neo-X-neo-Y bivalent of Nocaracris sp. in early diplotene. Arrowheads indicate conjugation of terminal part of XL arm of neo-X chromosomes with two small dot-like B-chromosomes
The karyotypes of all Pamphaginae species analyzed here consisted of 16 acrocentric autosomes (three chromosome pairs being large acrocentrics (L1–L3), four pairs being medium acrocentrics (M4–M7) and one pair being small sized acrocentrics (S8)), a submetacentric neo-X chromosome and a medium sized acrocentric neo-Y chromosome. The size of the neo-Y chromosome was smaller than the XR arm and, during meiosis, the sex bivalents formed only one chiasma in the distal region of the neo-Y (Fig. 3). In one of the species studied (Nocaracris sp.) 1–4 dot-like B chromosomes were found in 6 out of the 7 specimens analyzed (Fig. 3e, f). In diplotene these chromosomes showed association with terminal part of XL arm (Fig. 2b).
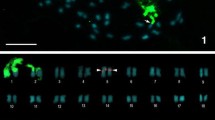
C-banding (a,c,e,g,i,k,m,o,q,s) and FISH using both rDNA (green) and telomeric (red) probes (b,d,f,h,j,l,n,p,r,t) of chromosomes in Nocarodeini tribe: Oronothrotes furvus (a,b); Paranocaracris cytripes cytripes (c,d); Nocaracris sp. (e,f) - arrowheads indicate dot-like B-chromosomes; P. cytripes idrisi (g,h); P. sureyanus (i,j); Paranocarodes fieberi anatoliensis (k,l); P. fieberi tolunai (m,n); P. straubei (o,p); P. turkmen (q,r); Pseudosavalania karabagi (s,t). Red arrowheads indicate ITSs (n and t)
C-heterochromatin variation
C-banding in the four Trinchinae species showed variation in size and location of C-heterochromatin between species. The C-banding pattern in G. adaliae, the only species showing the typical pamphagid karyotype (2n = 18 + X0), showed C-bands localized on pericentromeric regions of all chromosomes, with only an additional C-band on the distant end of the smallest chromosome (Fig. 1a).
The autosomes of G. dimorphus, G. holtzi pulchripes, and G. efe showed C-bands varying in number, size, and location, most being pericentromeric excepting a distal C-band in the L2 autosome. Pericentromeric C-bands in the autosomes of G. dimorphus were larger than those in G. holtzi pulchripes, and G. efe. In G. holtzi pulchripes, additional distal C-bands were found in the L1 and S8 chromosomes, and also an interstitial C-band in the L2 located close to the distal C-band.
The C-banding pattern in the neo-sex chromosome showed scarce variation in these three species. The size of the pericentromeric C-positive block of the neo-X chromosome was similar to those on the autosomes and was large in G. dimorphus and small in G. efe and G. holtzi pulkhripes. All three species showed a distal C-band on the XL arm, and G. holtzi pulkhripes additionally showed polymorphic subproximal and subdistal C-bands. The XR was C-negative excepta small distal C-band only in G. dimorphus. The neo-Y chromosome showed a small pericentric C-positive block and two small interstitial C-bands next to the pericentromeric region (Fig. 1c, e, g,). The neo-Y chromosome in G. dimorphus showed a small distal C-band Which was not observed in the two other species (Fig. 1c).
In the Pamphaginae species, C-positive bands were mostly located on pericentromeric regions, with some size differences among species. In addition to the pericentromeric C-bands, some interstitial and distal C-bands were observed (Figs. 3, and 4). For instance, O. furvus, P. cytripes cytripes, Nocaracris sp., P. sureyana, and P. fieberi tolunayi showed interstitial C-bands in the middle of the M6 chromosome. Likewise, O. furvus showed an additional interstitial C-band on the L2 chromosome. In some specimens of P. cytripes cytripes, additional interstitial C-bands were observed on proximal regions of the L1 and L2 chromosomes. Finally, distal C-bands were observed on five chromosome pairs in P. cytripes cytripes and on the L3 chromosome of P. sureyana. The dot-like B chromosomes revealed in Nocaracris sp. (Figure 3e, f) were C-positive, indicating their heterochromatic nature.
The neo-X chromosome in Nocarodeini species showed a small or medium sized pericentromeric C-band. In O. furvus and P. sureyana, a subproximal interstitial C-band was observed in the XL arm. Distal C-bands in the XL arm of the neo-X were identified in 9 species (O. furvus, P. cytripes cytripes, Nocaracris sp., P. cytripes idrisi, P. sureyana, P. pahlagonicus, P. turkmen, P. fieberi tolunayi, and P. fieberi anatoliensis). In all ten species, the neo-Y chromosome showed a large pericentromeric C-band and two or three large subproximal interstitial C-bands located close to each other. In highly condensed chromosomes, these blocks usually merge into one large C-positive region constituting almost half of the neo-Y chromosome (Fig. 3).
rDNA clusters in chromosomes of Pamphagidae grasshoppers
The number of rDNA clusters in species belonging to the Trinchinae subfamily varied from 4 to 7, with extensive variation in chromosome location (Figs. 1 and 4). Many rDNA clusters were located on pericentromeric and interstitial regions of large autosomes, the M6 chromosome and the XL arm, at any location. In most cases, every chromosome carried a single rDNA cluster, but two rDNA clusters were observed in a same chromosome arm in three Trinchinae species (G. adaliae, G. dimorphus and G. holtzi pulchripes) (Fig. 1 b, d, h). Furthermore, in G. dimorphus and G. holtzi pulchripes we found two pairs of autosomes containing two rDNA clusters in the same chromosome arm, although the precise position of rDNA clusters on these chromosomes differed. While in G. dimorphus all rDNA clusters were found on interstitial regions, in G. adaliae and G. holtzi pulchripes the locations of rDNA clusters were pericentromeric and interstitial.
In species belonging to the Nocarodeini tribe, within the Pamphaginae subfamily, the number of rDNA clusters varied from 2 to 6 (Figs. 3, 4). The level of variation in rDNA cluster location was also high; however, the four Paranocaracris species showed higher similarity in rDNA cluster location than the four Glyphotmethis species. Some of the conserved locations in Paranocaracris species were also observed in other Pamphaginae. For instance, O. furvus and Nocaracris sp. also showed rDNA clusters on L2 and M6 chromosomes, as well as on the proximal region of the XL arm (Fig. 3b, d, f, h, j), however the location on L2 being variable. In Nocaracris sp. and P. cytripes idrisi, two rDNA clusters were located on this same chromosome, whereas, in O. furvus, P. sureyana, and P. cytripes cytripes, the only rDNA cluster was either proximal or distal on L2 (Fig. 3d, h). In Paranocarodes species, we found higher variation than in Paranocaracris. For instance, in P. straubei and P. fieberi tolunayi, L2, L4 and M6 chromosomes, and the XL arm, carried rDNA clusters, although they were located on different chromosome locations (Fig. 3l , n). In P. fieberi anatoliensis and P. turkmen, the location of rDNA clusters differed significantly compared to other Nocarodeini species (Fig. 3p, r). In P. turkmen, only the M6 chromosome pair carried an rDNA cluster, whereas the cluster on the pericentromeric region of the L3 chromosome was very small and polymorphic. Finally, almost all chromosomes in P. fieberi anatoliensis carried pericentromeric rDNA clusters, except L1, S8, neo-X and neo-Y. In P. karabgi rDNA distribution was similar to P. straubey and P. fieberi tolunai, but no rDNA cluster was observed on L3 chromosome (Fig. 3t).
Telomeric DNA clusters in Pamphagidae grasshoppers
In situ hybridization of the telomeric DNA probe on chromosomes of the species studied here, revealed fluorescent signals on the ends of all chromosomes. However, in five species (three species of Glyphotmethis genus (G. dimorphus, G. efe and G. holtzi pulchripes) (red arrows on Fig. 1d, f, h), P. fieberi tolunayi and P. karabagi (red arrow on Fig. 3n, t)) an additional FISH signal of telomeric repeats was observed on pericentromeric regions of the neo-X chromosome. In addition, G. holtzi pulchripes showed interstitial telomeric sequences (ITS) on the subtelomeric region of the XL arm (Fig. 1h).
Discussion
The neo-sex chromosome systems in Pamphagidae grasshoppers
The X0/XX sex chromosome system had been reported for 15 Trinchinae and 16 Pamphaginae species previously studied, thus being considered the standard sex chromosome system for this grasshopper family [11–13, 15, 17, 20–22, 35–41]. However, only one out of the 12 Pamphagidae species from Western and Central Anatolia analyzed here were X0/XX, the 11 remaining species showing a neo-XY sex chromosome system. As a whole, among the 55 species of Pamphagidae hitherto analyzed, 22 show neo-XY sex chromosome systems [11, 13–22, 35–41]. The high proportion (40%) of species with neo-sex chromosomes in Pamphagidae family is comparable with high portion (48,4%) of species with neo-sex chromosome in neotropical Melanoplinae species [8, 9, 42], thus pointing to a special role of sex chromosome-autosome fusion during the evolution of these groups of grasshoppers.
The high similarity in size and morphology of the neo-X chromosome between all species analyzed suggest the possibility that the ancestral autosomes, engaged in the centric fusion, were similar sized in both lineages. Furthermore, in early publications this fusion was considered monophyletic [19]. However, analysis of mitochondrial COI gene in Pamphagidae family showed that Trinchinae species and Nocarodeini species forms two different clades [43], which supports independent origin of neo-sex chromosomes in these two lineages. Thus, different types of neo-sex chromosomes in Trinchinae and Nocarodeini species could not be considered as different stages of one monophyletic process as it was suggested earlier. Taking in account these data, two independent evolutionary lineages of species with neo-XY sex chromosome systems were revealed in Pamphagidae grasshoppers. One lineage was observed in the studied species of Glyphotmethis (present paper), Asiotmethis [14, 19, 20, 22] and Atrichotmethis [18] genera, (belonging to the Trinchinae subfamily) and the other lineage was observed in all studied species of the Nocarodeini tribe [18, 19, 22] (belonging to the Pamphaginae subfamily). Impressive structural conservatism of neo-X chromosomes was observed in both evolutionary lineages. The submetacentric neo-X probably derived from centric fusion between an ancestral medium sized acrocentric X chromosome and large ancestral acrocentric autosome. The neo-Y chromosomes, by contrast, showed remarkable differences between species belonging to the two different evolutionary lineages. In Glyphotmethis and Asiotmethis genera, the size of the neo-Y chromosome was very similar to that of the XR arm of the neo-X chromosome (both being derived from the ancestral autosome pair), and consistently showed scarce C-banding. This indicates that these neo-Y chromosomes have scarcely evolved in respect to their autosomal ancestral condition, and we can consider that they are at the initial stage of heterochromatinization and differentiation (Fig. 1c, e, g). Additional evidence is provided by chiasma formation along the whole length of the neo-Y and the XR arm [5–7]. In Nocarodeini species, on the other hand, the size of the neo-Y chromosome was conspicuously smaller than that of the XR arm of the neo-X chromosome, and its proximal third is heterochromatic, a feature which is not observed in the corresponding regions of the XR arm (see Figs. 3, 4). The smaller size of the neo-Y suggests that it has lost part of the euchromatin of the original autosome. All these features, along with the fact that chiasma formation between the neo-Y and the XR arm is restricted to a single distal chiasma, indicates more advanced evolutionary stage of these neo-XY sex chromosome systems [5–7]. Initial stage of neo-sex chromosomes in Nocarodeini tribe was observed only in Saxetania cultricollis [18].
Trinchinae species with neo sex chromosomes inhabit Central Asia, China, and Bulgaria [14, 18–20, 22]. However, the highest biodiversity in Trinchinae species has been described in Western Asia [44], for which reason it is worth assuming that neo-XY formation is frequent in this region. The wider geographic distribution of some Trinchinae species with neo-sex chromosomes could be the result of intensive migration through arid planes of Eurasia.
Species from the Nocarodeini tribe are distributed in the Western Asian region and adjacent territories. Neo-sex chromosome systems have been found in all species of this tribe hitherto analyzed, including those previously karyotyped [2–4]. Intensive evolution of neo-sex chromosomes in Nocarodeini grasshoppers has led to variation including several types of neo-Y chromosomes and other derived sex chromosome systems. On the one hand, a neo-Y similar to the XR arm was described in Saxetania cultricollis [18], whereas Paranothrotes opacus shows a neo-X1X1X2X2/neo-X1X2Y sex chromosome system [22]. Remarkably, no species with the basal X0/XX sex chromosome system has yet been found in this tribe. We suggest that the X- autosome fusion took place in a common ancestor of the whole tribe and contributed to the divergence of this taxon. Evolution of the formed neo-sex chromosomes in Nocarodeini species is an ongoing and intensive process, as they appear to be at different evolutionary stages in different species.
Cytogenetic features of Pamphagidae chromosomes
Karyotyping of Orthopteran families performed by White [1] led grasshoppers to be considered a classical example of karyotype stability. The present study in Pamphagidae grasshoppers has shown that about 60% of the species hitherto analyzed still conserve the ancestral X0/XX sex chromosome system, but the remaining 40% carry a derived neo-XY sex chromosome system. Leaving apart sex chromosome differences, the autosomes exhibit a high level of conservatism. The morphology and size of autosomes is rather similar among species. However, minor differences in autosome morphology were found. Small polymorphic short arms were observed in Glyphotmethis species. This feature was previously described in chromosomes of two other species also belonging to the Trinchinae subfamily, i.e. Melanotmethis fuscipenis [18] and Eremopeza festiva [22]. In contrast to the Trinchinae subfamily, in all karyotyped Pamphaginae species no apparent short arms were found. This difference might arise as a result of some events that took place after the divergence of these evolutionary linages.
Chromosome markers are necessary to study karyotype evolution. Unfortunately, the high variability in C-banding patterns does not make them useful markers. C-banding reveals regions enriched in repetitive DNA, but provides no information about the repeats contained in the C-blocks. Similar C-blocks may consist of different repeats. For instance, in a previous paper, we showed that, in the Gomphocerinae subfamily, most rDNA clusters revealed by FISH were located in interstitial C-bands [28]. In Pamphagidae grasshoppers, however, only 9 rDNA clusters co-localized with interstitial C-bands. Many clusters of rDNA were found on a pericentromeric C-positive region and also on C-negative regions (Fig. 4).
In groups of closely related species, such as the Gomphocerini tribe, the location of rDNA clusters can be a useful feature for identification of homeologous chromosomes [28]. However, high evolutionary mobility of the rDNA clusters has been described for many taxa [27, 45–49]. The mechanism of this high evolutionary mobility is unknown; it probably involves different chromosome rearrangements or insertion and amplification of rDNA units [50]. We observed high variation in the location of rDNA clusters in the Pamphagidae family. In all 12 species analyzed here, the only conserved location for rDNA clusters was found in the middle of the M6 autosome. This same location was previously described in Asiotmethis turritus, Nocaracris cyanipes and Paranocaracris rubribes [22]. In G. adaliae (a species with an XX/X0 sex chromosome system) we found an rDNA cluster in the middle of the M7, suggesting the possibility that the M7 chromosome in this species is probably homeologous of the M6 in all 15 species mentioned above. It would be interesting to analyze rDNA location in other X0/XX species to test this possibility.
The location of the rDNA clusters on large chromosomes was very variable, although they showed a tendency to be interstitially located. This variability is indicative of evolutionary changes that took place in these chromosomes but were not detected by the techniques used. Possible mechanisms explaining changes in rDNA cluster location could be paracentric inversions or the insertion of a DNA fragments containing rDNA into the chromosome, with subsequent rDNA amplification and elimination of the old rDNA cluster [50].
We should note that a different distribution of the rDNA clusters was revealed in P. fieberi anatoliensis. In contrast to all other studied species, the rDNA clusters were located in the pericentromeric region of six autosome pairs. This dramatic difference raises the question of its taxonomic status. Both P. fieberi anatoliensis and P. fieberi tolunayi are considered as subspecies of P. fieberi [51]. However, in contrast to P. fieberi anatoliensis, P. fieberi tolunayi showed the standard distribution of the rDNA clusters in the autosomes of other Nocarodeini species. The X chromosome of P. fieberi anatoliensis also differed from the X of other Nocarodeini species. In the P. fieberi anatoliensis XL arm, no rDNA cluster was found, whereas, with one exception (P. turkmen), all species of the Nocarodeini tribe carried an rDNA cluster in the XL arm. It is conceivable that an rDNA cluster was present in the ancestral acrocentric X chromosome, but it has been lost in P. turkmen and P. fieberi anatoliensis during evolution. Alternatively, the existence of other cryptic neo-XY lineages which have gone unnoticed with the present techniques is also possible. The different location of rDNA on the XL arm in the Paranocaracris and Paranocarodes genera might be explained by cryptic rearrangements or other mechanisms.
In the Trinchinae subfamily, analysis of rDNA distribution yielded little information on the evolution of the neo-sex chromosomes and, consequently, no hypothetic pattern of rDNA distribution in the ancestral X chromosome can be suggested.
The absence of an rDNA cluster in the XR arm of most species from both groups and in the neo-Y chromosomes may indicate that the ancestral autosome that entered the fusion and formed the XR arm and neo-Y chromosome did not carry the rDNA.
Apart from the telomeric repeats located in the termini of all chromosomes, we revealed telomeric repeats in the pericentromeric region of the neo-X in five out of 13 species carrying neo-sex chromosomes. The interstitial telomeric sequences (ITS) could be the result of chromosome fusions, unequal crossing-over or double strand break reparation involving telomerase (for review, see [52–54]). We suppose that ITS’s in the neo-sex chromosomes of these five species are the consequence of chromosome fusion. In case of fusion implying the loss of chromosome regions containing telomeric repeats in both chromosomes, no telomeric repeats would be included in the pericentric region of the resulting bi-armed chromosome. However, if the fusion would proceed yielding a dicentric chromosome, the possibility exists that the newly formed bi-armed chromosome would contain ITS’s in the pericentric region as remnants of the telomeric repeats placed beyond the centromere in the acrocentric chromosomes. It would thus be interesting to analyze whether the neo-X chromosome contains two pericentromeric regions and one active centromere in any of these species.
In Trinchinae species, the morphology and C-banding of neo-sex chromosomes indicates they are less evolved, and this might suggest that ITSs might be remnants of these centric fusion, which have been lost in some species. Due to their highly modified neo-Y chromosome, we consider that neo-sex chromosomes in Nocarodeini species are more evolved and therefore the ITSs in the pericentromeric region of the neo-X chromosome in most Nocarodeini species are absent, excepting P. karabagi and P. fieberi. We cannot distinguish whether they are remnants of telomeric sequences captured by the centric fusion yielding the neo-X chromosome or they are result of insertion and subsequent amplification of a DNA fragment containing telomeric repeats, or else the reparation of double strand breaks involving telomerase, however we think that later hypothesis is more probable. Also currently, P. karabagi and P. fieberi are classified into different genera. Therefore, we cannot rule out that these ITS’s may have resulted from independent events.
Conclusion
Taken together, our present results show a high frequency of neo-XY sex chromosome systems in Pamphagidae grasshoppers inhabiting the Anatolian region, with at least two different lineages with independent neo-sex chromosome formation and evolutionary history. The first lineage is species of the Trinchinae subfamily characterized by evolutionary less advanced neo-sex chromosomes; the second lineage includes species of the Nocarodeini tribe and characterized by evolutionary advanced neo-sex chromosome. In the second lineage, the X-chromosome-autosome fusion may probably have taken place in the common ancestor of the Nocarodeini tribe. This family of grasshoppers thus shows one of the highest proportion of species carrying neo-XY sex chromosomes within Acridoidea grasshoppers. However, further research, including additional chromosome markers, is necessary to clarify the mechanisms for sex chromosome evolution in this group of grasshoppers.
Abbreviations
- FISH:
-
Fluorescent in situ hybridization
- ITS:
-
Interstitial telomeric sequences
- rDNA:
-
ribosomal DNA
References
White MJD. Animal cytology and evolution. Cambridge: Cambridge University Press; 1973.
Hewitt GM. Orthoptera: Grasshoppers and crikets (Animal Cytogenetics). Berlin: Stutgart: Gebruder Borntraeger; 1979.
John B. The role of chromosome change in the evolution of orthopteroid insects. In: Sharma A, Sharma AM, editors. Chromosom. Evol. Eukaryot. groups. vol1. Florida: CRS Press; 1983. p. 114.
Fossey A. Cytogenetic research of the short-horned Orthoptera insect from South Africa. Pretoria: Pretoria University; 1985.
White MJD. Karyotypes and Nuclear Size in the Spermatogenesis of Grasshoppers Belonging to the Subfamilies Gomphomastacinae, Chininae and Biroellinae (Orthoptera, Eumastacidae). Caryologia. 1968;21:167–79.
John B, Hewitt GM. Karyotype stability and DNA variability in the Acrididae. Chromosom. 1966;20:155–72.
Bugrov AG, Warchałowska-Śliwa E, Tatsuta H, Akimoto S. Chromosome polymorphism and C-banding variation of the brachypterous grasshopper Podisma sapporensis Shir. (Orthoptera, Acrididae) in Hokkaido, Northern Japan. Folia Biol. 2001;49:137–52.
Castillo ER, Marti DA, Bidau CJ, Castillo ER, Bidau J. Sex and neo-sex chromosomes in Orthoptera: a review Sex and neo-sex chromosomes in Orthoptera : a review. J Orthoptera Res. 2010;19:213–31.
Castillo ERD, Bidau CJ, Martí D. Neo-sex chromosome diversity in Neotropical melanopline grasshoppers (Melanoplinae, Acrididae). Genetica. 2010;138:775–86.
Mesa A, Fontanetti CS, García-Novo P. Does an X-autosome centric fusion in Acridoidea condemn the species to extinction? J Orthoptera Res Orthopterists’ Society. 2001;10:141–6.
Chen ST. Chromosome studies on Haplotropis brunneriana Saussure. Peking nat Hist Bull. 1937;12:79–94.
Camacho JPM, Cabrero J, Viseras E. C-heterochromatin variation in the genus Eumigus (Orthoptera: Pamphagoidea). Genetica Springer. 1981;56:185–8.
Santos JL, Arana P, Giráldez R. Chromosome C-banding patterns in Spanish Acridoidea. Genetica. 1983;61:65–74.
Bugrov AG. Neo-XY sex-chromosome determination in grasshoppers Asiotmethis heptapotamicus heptapotamicus (Zub) and Atrichotmethis semenovi (Zub) (Orthoptera Pamphagidae). Tsitologiya. 1986;28:117–9.
Peng F. C-Banding Karyotypes of Two-Species of Genus Sinotmethis B. Bienko (Orthoptera: Pamphagidae). Hered. 1989;3:7.
Vitturi R, Mansueto C, Di Paola Ficarella F. Heterochromatin variation in four species of the genus Pamphagus (Orthoptera: Pamphagidae) analyzed by C-banding. Biol Zent Bl Fischer. 1993;112:335–41.
Warchałowska-Śliwa E, Maryańska-Nadachowska A, Massa B. Some new data on C-bands and NORs in three species of Pamphagidae (Orthoptera). Folia Biol. 1994;42:13–8.
Bugrov AG, Warchałowska-Śliwa E. Chromosome numbers and C-banding patterns in some Pamphagidae grasshoppers (Orthoptera, Acridoidea) from the Caucasus, Central Asia, and Transbaikalia. Folia Biol. 1997;45:133–8.
Bugrov AG, Grozeva S. Neo-XY chromosome sex determination in four species of the pamphagid grasshoppers (Orthoptera, Acridoidea, Pamphagidae) from Bulgaria. Caryologia. 1998;51:115–21.
Li XJ, Zhang DC, Wang WQ. Chromosomal C-banding karyotype of 2 species of genus Asiotmethis (Acridoidea: Pamphagidae) from China. Hered. 2005;27:735–40.
Buleu OG, Jetybaev IE, Chobanov DP, Bugrov AG. Cytogenetic features of some Pamphagidae grasshoppers from Morocco. Eurasian Entomol J. 2015;14:555–60.
Bugrov AG, Jetybayev IE, Karagyan GH, Rubtsov NB. Cytogenetics Sex chromosome diversity in Armenian toad grasshoppers (Orthoptera, Acridoidea, Pamphagidae). Comp Cytogenet. 2016;10:45–59.
Palacios-Gimenez OM, Marti DA, Cabral-de-Mello DC. Neo-sex chromosomes of Ronderosia bergi: insight into the evolution of sex chromosomes in grasshoppers. Chromosoma. 2015;124:353–65.
Palacios-Gimenez OM, Castillo ER, Martí DA, Cabral-de-Mello DC. Tracking the evolution of sex chromosome systems in Melanoplinae grasshoppers through chromosomal mapping of repetitive DNA sequences. BMC Evol Biol. 2013;13:167.
Cabral-de-Mello DC, Cabrero J, López-León MD, Camacho JPM. Evolutionary dynamics of 5S rDNA location in acridid grasshoppers and its relationship with H3 histone gene and 45S rDNA location. Genetica. 2011;139:921–31.
Cabrero J, López-León MD, Teruel M, Camacho JPM. Chromosome mapping of H3 and H4 histone gene clusters in 35 species of acridid grasshoppers. Chromosome Res. 2009;17:397–404.
Cabrero J, Camacho JPM. Location and expression of ribosomal RNA genes in grasshoppers: abundance of silent and cryptic loci. Chromosom Res. 2008;16:595–607.
Jetybayev I, Bugrov A, Karamysheva T, Camacho J, Rubtsov N. Chromosomal Localization of Ribosomal and Telomeric DNA Provides New Insights on the Evolution of Gomphocerinae Grasshoppers. Cytogenet Genome Res. 2012;138:36–45.
Marshall OJ. PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics England. 2004;20:2471–2.
Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–90.
Sahara K, Marec F, Traut W. TTAGG telomeric repeats in chromosomes of some insects and other arthropods. Chromosome Res. 1999;7:449–60.
Rubtsov NB, Karamisheva TV, Astakhova NM, Liehr T, Claussen U, Zhdanova NS. Zoo-FISH with region-specific paints for mink chromosome 5q: delineation of inter-and intrachromosomal rearrangements in human, pig, and fox. Cytogenet Genome Res Karger Publishers. 2000;90:268–70.
Rubtsov NB, Rubtsova NV, Anopriyenko OV, Karamysheva TV, Shevchenko AI, Mazurok NA, et al. Reorganization of the X chromosome in voles of the genus Microtus. Cytogenet Genome Res Karger Publishers. 2003;99:323–9.
White MJD. The origin and evolution of multiple sex-chromosome mechanisms. J Genet Springer. 1940;40:303–36.
Peng F. C-banding Karyotypes of Two Species of Genus Filchnerella karny (Orthoptera: Pamphagidae). Hered. 1991;5:5.
Mansueto C, Vitturi R. NORs Location and C-Banding Pattern in Spermatogenesis of Pamphagus Ortolanii (Orthoptera, Acrididae). Caryologia. 1989;42:303–11.
Sofradzija A, Miksic S. Characteristic of the chromosomal set of the species Pamphagus tunetanus Vosseler, 1902 (Orthoptera, Pamphagidae). Glas Zemalj mus BiH Saraevu Prir Nauk. 1992;19–20:87–92.
Jian-xue W. Study on the Chromosomal C Banding Karyotype of Filchnerella Rubimargina (Arcidoidea: Pamphagidae). J Anhui Agric Sci. 2011;11:66.
Sun JY, Fu P, Zheng ZM. Researches on C-banding karyotypes of two species of Genus Eotmethis. Hered. 2004;26:870–4.
Li X-J, Zhang D-C, Wang W-Q, Zheng J-Y. The chromosomal C-banding karyotypes of two pamphagid species from China. [J] Chinese Bull Entomol. 2008;4:17.
Li X, Zhang D, Zhang K, Yin C. Study on the Chromosomal C-banding Karyotype of Two Species of Filchnerella from China (Acridoidea: Pamphagidae). Sichuan J Zool. 2011;5:9.
Mesa A, Ferreira A, Carbonell CS. Cariología de los acridoideos neotropicales: estado actual de su conocimiento y nuevas contribuciones. Annls Soc Ent Fr. 1982;18:507–26.
Bugrov AG, Sukhih IS, Unal M, Blinov AG. Phylogenetic relationships of the Pamphagidae grasshoppers with the neo-XY/neo-XX of sex determination based on the analysis of DNA sequences in COI mitochondrial gene. Evraziatskii Entomologicheskii Zhurnal. 2013;12:451–6.
Hodjat SH. An update list of Pamphagidae Brumster 1840 (Insecta : Orthoptera) of Iran with a key to the genera. Journal of Crop Protection. 2012;1:261–70.
Schubert I. Mobile nucleolus organizing regions (NORs) inAllium (Liliaceae s. lat.)?—Inferences from the specifity of silver staining. Plant Syst Evol Springer. 1984;144:291–305.
Schubert I, Wobus U. In situ hybridization confirms jumping nucleolus organizing regions in Allium. Chromosoma Springer. 1985;92:143–8.
Shishido R, Sano Y, Fukui K. Ribosomal DNAs: an exception to the conservation of gene order in rice genomes. Mol Gen Genet MGG Springer. 2000;263:586–91.
Castro J, Rodríguez S, Pardo BG, Sánchez L, Martínez P. Population analysis of an unusual NOR-site polymorphism in brown trout (Salmo trutta L.). Heredity (Edinb) Nature Publishing Group. 2001;86:291–302.
Cai Q, Zhang D, Liu Z, Wang X-R. Chromosomal localization of 5S and 18S rDNA in five species of subgenus Strobus and their implications for genome evolution of Pinus. Ann Bot Annals Botany Co. 2006;97:715–22.
Dubcovsky J, Dvorak J. Ribosomal RNA Multigene Loci: Nomads of the Triticeae Genomes. Genetics. 1995;140:1367–77.
Eades DC, Otte D, Cigliano MM, Braun H, Naskrecki P. Orthoptera species files online [Internet]. Available from: orthoptera.speciesfiles.org
Bolzán AD. Chromosomal aberrations involving telomeres and interstitial telomeric sequences. Mutagenesis. 2012;27:1–15.
Ruiz-Herrera A, Nergadze SG, Santagostino M, Giulotto E. Telomeric repeats far from the ends: Mechanisms of origin and role in evolution. Cytogenet Genome Res. 2009;122:219–28.
Lin KW, Yan J. Endings in the middle: Current knowledge of interstitial telomeric sequences. Mutat Res - Rev Mutat Res. 2008;658:95–110.
Acknowledgement
The authors are grateful to the Center for Microscopy of Biological Subjects (Institute of Cytology and Genetics, Novosibirsk, Russia).
Declarations
This article has been published as part of BMC Evolutionary Biology Vol 17 Suppl 1, 2017: Selected articles from BGRS\SB-2016: evolutionary biology. The full contents of the supplement are available online at https://bmcevolbiol.biomedcentral.com/articles/supplements/volume-17-supplement-1.
Funding
This work (including the publication cost) was funded by the project # 0324-2015-0003 of THE FEDERAL RESEARCH CENTER INSTITUTE OF CYTOLOGY AND GENETICS SB RAS and research grants from the Russian Foundation for Basic Research #15-04-04816-a.
Availability of data and materials
All data generated used and/or analysed during this study are included in this published article.
Authors’ contributions
IJ, AB, NR planned and designed the research. IJ, AB, MU collected and fixed material. MU identified species. OB prepared chromosome spreadings. IJ, OB performed C-banding and FISH experiments and analyzed results. IJ, AB, NR wrote the manuscript. All authors read and approved the final version of the manuscript.
Competing interests
Authors declare that there is no conflict of interest.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not Applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jetybayev, I.Y., Bugrov, A.G., Ünal, M. et al. Molecular cytogenetic analysis reveals the existence of two independent neo-XY sex chromosome systems in Anatolian Pamphagidae grasshoppers. BMC Evol Biol 17 (Suppl 1), 20 (2017). https://doi.org/10.1186/s12862-016-0868-9
Published:
DOI: https://doi.org/10.1186/s12862-016-0868-9