Abstract
Background
Alterations of bioelectrical properties of cells and tissues are known to function as wide-ranging signals during development, regeneration and wound-healing in several species. The Drosophila follicle-cell epithelium provides an appropriate model system for studying the potential role of electrochemical signals, like intracellular pH (pHi) and membrane potential (Vmem), during development. Therefore, we analysed stage-specific gradients of pHi and Vmem as well as their dependence on specific ion-transport mechanisms.
Results
Using fluorescent indicators, we found distinct alterations of pHi- and Vmem-patterns during stages 8 to 12 of oogenesis. To determine the roles of relevant ion-transport mechanisms in regulating pHi and Vmem and in establishing stage-specific antero-posterior and dorso-ventral gradients, we used inhibitors of Na+/H+-exchangers and Na+-channels (amiloride), V-ATPases (bafilomycin), ATP-sensitive K+-channels (glibenclamide), voltage-dependent L-type Ca2+-channels (verapamil), Cl−-channels (9-anthroic acid) and Na+/K+/2Cl−-cotransporters (furosemide). Either pHi or Vmem or both parameters were affected by each tested inhibitor. While the inhibition of Na+/H+-exchangers (NHE) and amiloride-sensitive Na+-channels or of V-ATPases resulted in relative acidification, inhibiting the other ion-transport mechanisms led to relative alkalisation. The most prominent effects on pHi were obtained by inhibiting Na+/K+/2Cl−-cotransporters or ATP-sensitive K+-channels. Vmem was most efficiently hyperpolarised by inhibiting voltage-dependent L-type Ca2+-channels or ATP-sensitive K+-channels, whereas the impact of the other ion-transport mechanisms was smaller. In case of very prominent effects of inhibitors on pHi and/or Vmem, we also found strong influences on the antero-posterior and dorso-ventral pHi- and/or Vmem-gradients. For example, inhibiting ATP-sensitive K+-channels strongly enhanced both pHi-gradients (increasing alkalisation) and reduced both Vmem-gradients (increasing hyperpolarisation). Similarly, inhibiting Na+/K+/2Cl−-cotransporters strongly enhanced both pHi-gradients and reduced the antero-posterior Vmem-gradient. To minor extents, both pHi-gradients were enhanced and both Vmem-gradients were reduced by inhibiting voltage-dependent L-type Ca2+-channels, whereas only both pHi-gradients were reduced (increasing acidification) by inhibiting V-ATPases or NHE and Na+-channels.
Conclusions
Our data show that in the Drosophila follicle-cell epithelium stage-specific pHi- and Vmem-gradients develop which result from the activity of several ion-transport mechanisms. These gradients are supposed to represent important bioelectrical cues during oogenesis, e.g., by serving as electrochemical prepatterns in modifying cell polarity and cytoskeletal organisation.
Similar content being viewed by others
Background
The development and maintenance of complex multicellular structures, like tissues and organs, is controlled by an interplay of various regulatory processes. Besides genetical and biochemical mechanisms, bioelectrical phenomena, i.e. localised ion fluxes, gradients of ion concentrations, intracellular pH (pHi) and membrane potential (Vmem), are known to function as wide-ranging signals to guide polarity in development, regeneration and wound-healing [1,2,3,4,5]. It has been shown that gradual modifications of bioelectrical properties mediate cellular processes like migration [6], proliferation [7], differentiation [8,9,10], and cell-cycle control [11, 12] in various species. For example, Vmem plays a role in specifying the left-right axis in Xenopus and chick embryos [13], in the regeneration of either head or tail in planarians [14], in zebrafish pigment-pattern formation [15], and in planar cell-polarity pathway regulation in Drosophila epithelial tissues [16]. Defects in ion transport have been associated with several human diseases, like e.g. cancer development or Alzheimer’s disease, or with developmental defects caused by human channelopathies [17,18,19,20,21].
The generation and maintenance of electrochemical gradients within cells or tissues requires a polarised distribution and/or activation of specific ion-transport mechanisms [22, 23]. In addition, gap junctions are a prerequisite in coupling groups of cells electrically [24,25,26]. In contrast to classical methods, like e.g., single-cell electrode measurements, new techniques using specific fluorescent probes allow visualisation and analysis of the spatiotemporal characteristics of pHi and Vmem in a large number of cells and in whole tissues [27, 28].
The follicle-cell epithelium (FCE) of the Drosophila ovary provides an appropriate model system for studying bioelectrical phenomena during epithelial development [29,30,31,32,33]. It has been shown that in Drosophila ovarian follicles stage-specific patterns of extracellular currents [29, 30], Vmem [31, 32, 34], and pHi [32] exist that depend mainly on the exchange of protons, potassium ions and sodium ions [31, 34,35,36]. For studying and manipulating pHi and Vmem in the FCE, we used the fluorescent pH-indicator 5-CFDA,AM and the potentiometric dye DiBAC4(3) as well as several inhibitors of ion-transport mechanisms.
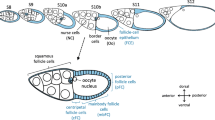
The Drosophila follicle consists of 16 germ-line cells, 15 nurse cells (NC) and one oocyte (Oo), surrounded by a single-layered somatic FCE [37]. During the course of oogenesis, the FCE differentiates into several morphologically distinct follicle-cell (FC) populations (Fig. 1a) [38,39,40]. In addition to establishing embryonic polarity [41] and building up the eggshell [40], the FCE plays a significant role in shaping the elongated egg [42, 43], a process which requires planar cell polarity and a polarised arrangement of the cytoskeleton.
Development of pHi-gradients in the FCE during S8–12 (CFDA; SIM). a Types of germ-line and somatic cells of the Drosophila follicle. At stage 10b (S10b), the oocyte (Oo, posterior) constitutes almost one half of the follicle’s volume. The anterior half is formed by 15 nurse cells (NC). The 16 germ-line cells (Oo and NC) are covered by a single-layered somatic follicle-cell epithelium (FCE). The columnar FCE surrounding the oocyte is subdivided into three follicle-cell (FC) populations: centripetally migrating FC (cFC), mainbody FC (mbFC) and posterior FC (pFC). The dorsal FCE (dFC) is thicker than the ventral FCE (vFC). Squamous FC surround the NC population. The oocyte nucleus and the border cells, a cluster of migratory follicle cells, lie close to each other near the dorsal side (DIC image). b Schematic drawing of an ovariole showing pHi in the analysed stages S8–12 (cf. Additional file 1: Figure S1 for examples; Ger, germarium). c In S8, anterior FC (aFC) are more alkaline compared to pFC. During S9–12, an antero-posterior (a-p) gradient establishes with relatively acidic cFC and relatively alkaline pFC. From S9 and, in particular, from S10b onwards, a dorso-ventral (d-v) gradient with relatively alkaline vFC and relatively acidic dFC develops. To analyse the a-p and d-v gradients, the fluorescence intensities of the different FC types (3 < n < 46 follicles per stage) were measured and normalised using the fluorescence intensity in the whole FCE of the respective follicle (relative intensity). Mean values, shown with their standard deviation (cf. Additional file 2: Table S1), were compared using an unpaired t-test (* p < 0.05; ** p < 0.01; *** p < 0.001). d Optical median sections of a CFDA-stained S10b-follicle. Inset shows a pseudocolour image of the same section. Low fluorescence intensity (blue) indicates lower pHi (relatively acidic) while high fluorescence intensity (pink) indicates higher pHi (relatively alkaline). Asterisk, oocyte nucleus (dorsal)
The purpose of the present study is to clarify the roles that various ion-transport mechanisms play in regulating pHi and Vmem and in generating stage-specific electrochemical gradients in the FCE. Such gradients are likely to be involved in regulating, e.g., cell polarity, cell migration, and the organisation of the cytoskeleton during oogenesis of Drosophila.
Results
Stage-specific pHi-patterns
We analysed the pHi in the FCE during the course of oogenesis. In vitellogenic stages (S8–12), we found distinct alterations of the pHi-patterns (Figs. 1b-d and 2a; for typical follicles, see Additional file 1: Figure S1; for variability between follicles of the same stage, see Additional file 2: Table S1).
Development of Vmem-gradients in the FCE during S8–12 (DiBAC; SIM). a Schematic drawing of a S10b-follicle placed between an object slide and a cover slip. The focal plane of median optical sections generated by the ApoTome (SIM) is shown as dashed green line. b Schematic drawing of an ovariole showing Vmem in the analysed stages S8–12 (cf. Additional file 1: Figure S2 for examples). c During S9–12, an a-p gradient establishes with relatively hyperpolarised cFC and relatively depolarised pFC. From S10b onwards, a d-v gradient with relatively depolarised vFC and relatively hyperpolarised dFC develops (cf. Additional file 2: Table S2). For abbreviations and statistics, see legend to Fig. 1. d Optical median sections of a DiBAC-stained S10b-follicle. Inset shows a pseudocolour image of the same section. Low fluorescence intensity (green) indicates relative hyperpolarisation while high fluorescence intensity (red) indicates relative depolarisation
Antero-posterior gradient
In S8, the pHi in the FCE is relatively acidic compared to the pHi in the germ-line cells. An antero-posterior (a-p) gradient is visible, with more alkaline pHi in the anterior FC (aFC) and more acidic pHi in the posterior FC (pFC). In S9 and 10a, the a-p gradient reverses and the centripetal FC (cFC) show more acidic pHi than the pFC. At S10b, this a-p gradient becomes very distinct and is preserved during S11 and 12. In contrast to earlier stages, the pHi in the FCE is now more alkaline compared to the pHi in the germ-line cells.
Dorso-ventral gradient
In S8, the FCE does not yet exhibit a dorso-ventral (d-v) gradient. A significant gradient with relatively acidic dorsal and relatively alkaline ventral FC arises in S9 and increases during S10b-12.
Stage-specific Vmem-patterns
During the course of vitellogenesis (S8–12), we also observed distinct changes in the Vmem-patterns (Fig. 2; for typical follicles, see Additional file 1: Figure S2; for variability between follicles of the same stage, see Additional file 2: Table S2).
Antero-posterior gradient
Up to S8, all FC show a similar Vmem which is relatively depolarised compared to the germ-line cells. At S9 and 10a, an a-p gradient starts to establish with relatively depolarised FC in the centripetal and posterior regions of the follicle (cFC and pFC) compared to the mainbody FC (mbFC). During S10b-12, the gradient changes so that only the pFC are depolarised compared to mbFC and cFC.
Dorso-ventral gradient
During S8-10a, a significant d-v gradient could not be detected in the FCE. However, a d-v gradient emerges during S10b-12, with relative depolarisation on the ventral side of the follicle (vFC). Dorsal cFC show a striking increase in depolarisation during late S10b and 11. But on average, the dorsal FCE is hyperpolarised compared to the ventral FCE.
Inhibition of ion-transport mechanisms
Six inhibitors were used to determine the roles that specific ion-transport mechanisms play in the regulation of pHi and Vmem as well as in the generation of electrochemical gradients in the FCE during S10b. We found that either pHi or Vmem or both parameters were affected by each tested inhibitor.
Effects of inhibitors on pHi
WFM-experiment
The inhibitors furosemide (Na+/K+/2Cl−-cotransporters), glibenclamide (ATP-sensitive K+-channels), 9-anthroic acid (Cl−-channels) and verapamil (L-type Ca2+-channels) showed significant alkalising effects in the FCE. The inhibitors amiloride (NHEs and Na+-channels) and bafilomycin (V-ATPases) had no significant effects on the pHi in the FCE under these experimental conditions. The strongest effects were observed with both glibenclamide and furosemide (Fig. 3, Additional file 2: Table S3).
Inhibitors of ion-transport mechanisms exert influence on the pHi in the FCE during S10b (WFM-experiment; CFDA). a WFM-fluorescence images were used to measure the fluorescence intensity in the columnar FCE (marked yellow) of every single follicle. While glibenclamide (ATP-sensitive K+-channels) and furosemide (Na+/K+/2Cl−-cotransporters) led to strong alkalisation, the alkalising effects of verapamil (L-type Ca2+-channels) and 9-anthroic acid (Cl−-channels) were smaller. Amiloride (NHEs and Na+-channels) and bafilomycin (V-ATPases) showed no significant effects. To analyse and compare the effects of the inhibitors, averaged values (of 12 time points during 60 min of inhibition) of three experiments per inhibitor were summed up and normalised (mean intensity ratio). Mean values, shown with their standard deviation (cf. Additional file 2: Table S3), were compared using an unpaired t-test (* p < 0.05; ** p < 0.01; *** p < 0.001). b Pseudocolour fluorescence images after 60 min of incubation. Furosemide led to strong alkalisation of the whole follicle. In contrast to the control (DMSO), the columnar FCE exhibits an even stronger fluorescence intensity than the germ-line cells
SIM-experiment
The treatment with the inhibitors furosemide, glibenclamide, 9-anthroic acid and verapamil, respectively, resulted again in alkalisation. These effects were significant in all types of FC and particularly distinct with furosemide and glibenclamide (Figs. 4 and 5a, Additional file 2: Table S5). Amiloride led to slight acidification which was significant in pFC. The increase in fluorescence intensity elicited by bafilomycin was due to granular staining in the FCE (Fig. 5 b). Considering the localisation of V-ATPases in plasma membranes and in vesicle membranes of FC [33, 46], it is obvious that bafilomycin caused alkalisation of vesicles, while the cytoplasm of FC became more acidic. Modifications of the a-p and/or d-v pHi-gradients were observed with all tested inhibitors. The influences were particularly strong with both glibenclamide and furosemide, which enhanced the a-p and d-v gradients (increasing angle; alkalisation), as did verapamil. 9-Anthroic acid resulted in a shallower d-v gradient (decreasing angle; acidification) because of its stronger effect on vFC, whereas the a-p gradient became slightly enhanced (alkalisation). Amiloride, on the other hand, led to both shallower a-p and d-v gradients (acidification), as did bafilomycin (increasing cytoplasmic acidification due to increasing vesicle alkalisation). The inclinations of the gradients were inferred from the mean values of relative fluorescence intensity depicted in Figs. 4b and 5a.
The a-p pHi-gradient in the FCE is affected by all inhibitors during S10b (SIM-experiment; CFDA). a Pseudocolour SIM-fluorescence images of S10b-follicles. Compared to the control (DMSO), blocking with glibenclamide for 20 min resulted in strong alkalisation of the FCE which was most prominent in pFC (arrowhead). b Especially glibenclamide and furosemide, but also verapamil and 9-anthroic acid led to alkalisation in all FC types. Glibenclamide and furosemide resulted in a considerably steeper a-p gradient (enlargement of the angle × 20 and × 150, respectively). Verapamil and 9-anthroic acid enhanced the a-p gradient as well, but to a lesser extent (both × 2). The increase in fluorescence intensity as well as in the inclination of the a-p gradient (× 2) caused by bafilomycin was due to the alkalisation of vesicles (see Fig. 5 b) and, therefore, to the acidification of the cytoplasm resulting in a shallower a-p gradient. Amiloride led to acidification of pFC, thus slightly reducing the angle of the a-p gradient (× 0.5) as well. For each inhibitor, at least five repetitions of the experiment were performed. Normalised values of the single experiments were merged into one evaluation (relative intensity). Mean values, shown with their standard deviation (cf. Additional file 2: Table S5), were compared using an unpaired t-test (* p < 0.05; **p < 0.01; *** p < 0.001)
a All inhibitors exert influence on the d-v pHi-gradient in the FCE during S10b (SIM-experiment; CFDA). Especially glibenclamide and furosemide, but also verapamil and 9-anthroic acid led to alkalisation in both FC types. Verapamil, glibenclamide, and furosemide resulted in a steeper d-v gradient (angle × 2, × 5, and × 100, respectively). The increase in fluorescence intensity as well as in the inclination of the d-v gradient (× 3) caused by bafilomycin was due to the alkalisation of vesicles (see b), leading to increasing cytoplasmic acidification as well as to a shallower d-v gradient. Amiloride and 9-anthroic acid led to a stronger acidification of vFC, thus reducing the angle of the d-v gradient (× 0.5 and × 0.1, respectively) as well (cf. Additional file 2: Table S5). For statistics, see legend to Fig. 4. b Alkalisation of vesicles in the FCE by bafilomycin. SIM-fluorescence images of S10b-follicles incubated in R-14 medium with bafilomycin or with DMSO (control) for 20 min. Inhibition of V-ATPases led to a more granular staining (arrowhead) compared to the controls, indicating alkalisation of vesicles. In addition, the cytoplasm of the FC appeared darker than in the controls, indicating cytoplasmic acidification
Effects of inhibitors on Vmem
WFM-experiment
Almost all tested inhibitors (verapamil, amiloride, 9-anthroic acid, furosemide and bafilomycin) had significant hyperpolarising effects on the Vmem in the FCE. The strongest effect was observed with verapamil, the weakest with bafilomycin. Glibenclamide caused a slight hyperpolarisation which was not significant. Depolarisation was not observed (Fig. 6, Additional file 2: Table S4).
Inhibitors of ion-transport mechanisms exert influence on the Vmem in the FCE during S10b (WFM-experiment; DiBAC). a WFM-fluorescence images were used to measure the fluorescence intensity in the columnar FCE of every single follicle. The measured area was as shown in Fig. 3 a. While verapamil led to strong hyperpolarisation, amiloride, bafilomycin, 9-anthroic acid, and furosemide led to slight hyperpolarisation. The hyperpolarising effect of glibenclamide was not significant (cf. Additional file 2: Table S4). For abbreviations and statistics, see legend to Fig. 3. b Pseudocolour fluorescence images after 60 min of incubation. Verapamil led to a strong decrease in fluorescence intensity (hyperpolarisation) in the whole follicle
SIM-experiment
All inhibitors caused more or less hyperpolarisation in the FCE (Figs. 7 and 8, Additional file 2: Table S6). The strongest effects on Vmem were observed with verapamil and glibenclamide, the weakest with amiloride and bafilomycin (not significant). Glibenclamide and verapamil lowered the a-p gradient as well as the d-v gradient (decreasing angle; hyperpolarisation), whereas furosemide and 9-anthroic acid had prominent decreasing effects only on the a-p gradient. The inclinations of the gradients were inferred from the mean values of relative fluorescence intensity depicted in Figs. 7b and 8.
The a-p Vmem-gradient in the FCE is affected by most inhibitors during S10b (SIM-experiment; DiBAC). a Pseudocolour SIM-fluorescence images of S10b-follicles. Compared to the control (ethanol), blocking with verapamil for 20 min resulted in strong hyperpolarisation of the FCE. b Glibenclamide and verapamil led to strong hyperpolarisation in all FC types, but especially in pFC, thus lowering the inclination of the a-p gradient (angle × 0.5 and × 0.3, respectively). Furosemide and 9-anthroic acid hyperpolarised only the pFC, thereby lowering the a-p gradient as well (both × 0.5). Amiloride and bafilomycin showed no significant effects on the a-p gradient (cf. Additional file 2: Table S6). For statistics, see legend to Fig. 4
Some inhibitors exert influence on the d-v Vmem-gradient in the FCE during S10b (SIM-experiment; DiBAC). Glibenclamide and verapamil led to hyperpolarisation in both FC types, but especially in vFC, thus lowering the inclination of the d-v gradient (angle × 0.1 and × 0.5, respectively). Amiloride, bafilomycin, 9-anthroic acid and furosemide showed no significant effects on the d-v gradient (cf. Additional file 2: Table S6). For statistics, see legend to Fig. 4
Discussion
Stage-specific pHi- and Vmem-gradients are established in the FCE
We have shown that the FCE exhibits stage-specific a-p and d-v gradients of pHi and Vmem, respectively, which are most distinctive during S10b. The pHi- and Vmem-gradients arise approximately at the same stage, but they are not congruent. The pHi increases (alkalisation) from anterior (cFC) to posterior (pFC) and from dorsal (dFC) to ventral (vFC), and, therefore, the dorsal cFC possess the most acidic pHi.
In a-p direction, the developing Vmem-gradient differs from the pHi-gradient. Since the mbFC are relatively hyperpolarised compared to the cFC and pFC, no continuous a-p Vmem-gradient exists. However, from dFC to vFC, the Vmem becomes continuously depolarised. For both pHi and Vmem, the stages differ during which the a-p and d-v gradients become established. Since they start in S9, the a-p gradients are already existing when the d-v gradients become obvious during S10b.
It is expected that the establishment of electrochemical gradients is depending on the asymmetric distribution and/or activity of ion-transport mechanisms in different types of FC. Recent studies have shown that e.g. gap junctions, V-ATPases, Na+/K+-pumps and Na+-channels are enriched in certain regions of the FCE compared to other regions [32, 33, 36, 44,45,46]. To function as temporal and spatial signals during development, electrochemical gradients and/or local changes of pHi and Vmem have to be translated into cellular responses. For example, this could be achieved by voltage-dependent L-type Ca2+-channels that become asymmetrically activated in the FCE [32].
Inhibitors of ion-transport mechanisms affect pHi and Vmem in the FCE
To identify mechanism that are involved in regulating pHi and Vmem in the FCE, we used inhibitors of several ion-transport mechanisms that have already been detected and/or localised in the Drosophila ovary [32, 33, 44, 47,48,49,50]. All used inhibitors affected either pHi or Vmem or both parameters in S10b (for summary, see Fig. 9). If an inhibitory effect was detectable, it could be observed throughout the entire columnar FCE - though, in different FC types, often to varying extents.
Summary of the effects of inhibitors on pHi and Vmem in the WFM- and SIM-experiments. Although both experimental conditions led to somewhat different results (upper part), the effects of the inhibitors can be reliably determined (lower part). While inhibition of both Na+/K+/2Cl−-cotransporters (furosemide) and ATP-sensitive K+-channels (glibenclamide) resulted in the most prominent effects on pHi (strong alkalisation), inhibition of voltage-dependent L-type Ca2+-channels (verapamil) led to the most prominent effect on Vmem (strong hyperpolarisation)
The most prominent effects on pHi were obtained with both glibenclamide (ATP-sensitive K+-channels) and furosemide (Na+/K+/2Cl−-cotransporters). These inhibitors led to strong relative alkalisation in all FC types by blocking H+-transport indirectly. Inhibition of ATP-sensitive K+-channels by glibenclamide reduces K+-efflux. Since the intracellular K+-concentration and the pHi are known to be interrelated, a higher intracellular K+-concentration would reduce H+-influx (K+/H+-antiport [51, 52]). In addition, it has been shown that K+-uptake into Drosophila follicles is strongly dependent on extracellular pH [36]. Na+/K+/2Cl−-cotransport, which can be blocked by furosemide (or bumetanide, an analogue of furosemide), is typically coupled with Cl−/HCO3−-antiport, so that furosemide has no impact on Cl−-transport in total [53, 54]. Due to this coupling, HCO3−-transport might be affected, causing a change of pHi in the FCE [55, 56]. The alkalisation obtained with 9-anthroic acid, which blocks Cl−-channels, is also likely to be due to an effect on Cl−/HCO3−-antiport.
Amiloride (NHEs and Na+-channels) and bafilomycin (V-ATPases), that are both direct inhibitors of H+-transport, led to slight acidification of the FCE. While NHEs are known for their role in pHi-homeostasis [47, 56,57,58], one of the key functions of V-ATPases, besides acidification of cytoplasmic vesicles, is to energise the plasma membranes of most insect cells [59, 60]. Since the generated H+-gradient drives secondary active transport mechanisms like NHEs [51, 60], a strong effect of bafilomycin on pHi was not expected. Similarly, the relatively small impact of amiloride on pHi is supposed to be due to compensatory effects exerted by other ion transporters.
Vmem in the FCE was clearly influenced by glibenclamide (ATP-sensitive K+-channels) and, most strongly, by verapamil (voltage-dependent L-type Ca2+-channels). Although Ca2+-channels are not likely to contribute much to Vmem directly, blocking of Ca2+-influx can result in diverse cellular reactions, since Ca2+ is a second messenger [61]. A strong effect of amiloride on Vmem was not expected, since Na+/H+-antiport is electroneutral. Moreover, the effect of blocking Na+-channels is relatively weak and can be compensated by other ion-transport mechanisms. Compensatory effects always have to be taken into account, and they are supposed to be the reason for the absence of a strong impact of bafilomycin (V-ATPases) on Vmem as well. Similarly, inhibition with furosemide (Na+/K+/2Cl−-cotransporters) and 9-anthroic (Cl−-channels) had only minor effects on Vmem.
Inhibitors of ion-transport mechanisms affect pHi- and Vmem-gradients in the FCE
Since with some inhibitors different types of FC were affected to different extents, the a-p and/or d-v gradients during S10b became modified in several ways (for summary, see Fig. 10).
Summary of the influences of inhibitors on the inclinations of the a-p and/or d-v pHi- and Vmem-gradients. Blocking of ion-transport mechanisms resulted in very different effects on the various gradients of relative fluorescence intensity. While, e.g. for the a-p pHi-gradient, × 0.5 represents a reduction of the angle (decreasing alkalisation, i.e. increasing acidification) by 50% (amiloride; NHEs and Na+-channels), × 150 means a 150fold enlargement (furosemide; Na+/K+/2Cl−-cotransporters) of the angle (increasing alkalisation). The enlargement of the angles caused by bafilomycin (V-ATPases) was due to the alkalisation of vesicles resulting in increased cytoplasmic acidification as well as in shallower pHi-gradients. E.g., for the d-v Vmem-gradient, × 0.1 represents a reduction of the angle (decreasing depolarisation, i.e. increasing hyperpolarisation) by 90% (glibenclamide; ATP-sensitive K+-channels). The inclinations of the gradients were inferred from the mean values of relative fluorescence intensity shown in Figs. 4b and 5a (pHi-gradients) and in Figs. 7b and 8 (Vmem-gradients), respectively
The treatment with glibenclamide (ATP-sensitive K+-channels) resulted in both an enhancement of the a-p and d-v pHi-gradients (increasing alkalisation) and a reduction of the a-p and d-v Vmem-gradients (increasing hyperpolarisation). This could be due to the higher concentrations of ATP-sensitive K+-channels found in dorsal cFC (unpublished results). It has been shown that both acidic pH and low K+-concentrations result in reduced gap-junctional communication in Drosophila follicles [48]. Therefore, glibenclamide is supposed to enhance gap-junctional communication via higher intracellular K+-concentrations as well as alkalisation of pHi, leading to increasing hyperpolarisation in the Vmem-gradients. Furosemide (Na+/K+/2Cl−-cotransporters) also led to markedly steeper a-p and d-v pHi-gradients as well as to a shallower a-p Vmem-gradient. Whether these effects depend on an unequal distribution of Na+/K+/2Cl−-cotransporters remains to be analysed. Likewise, the distributions of NHEs (amiloride) as well as Cl−-channels (9-anthroic acid), which both modified electrochemical gradients in the FCE, are not yet known. However, it has been shown that V-ATPases are accumulated in the membranes of pFC and vFC [32, 33]. A stronger effect of bafilomycin on these FC types led to reduction of a-p and d-v pHi-gradients (increasing cytoplasmic acidification due to increasing vesicle alkalisation). The application of verapamil both enhanced the a-p and d-v pHi-gradients and reduced the a-p and d-v Vmem-gradients (increasing hyperpolarisation). This is in accordance with the observed higher concentrations of activated L-type Ca2+-channels in pFC and vFC [32]. It is known that high intracellular Ca2+-concentrations result in reduced gap-junctional communication in Drosophila follicles [46]. Therefore, verapamil is supposed to enhance gap-junctional communication by lowering intracellular Ca2+-concentrations, leading to both increasing alkalisation in pHi-gradients and increasing hyperpolarisation in Vmem-gradients.
Conclusion
In the FCE of Drosophila, gap junctions and ion-transport mechanisms, like NHEs, Na+-channels, V-ATPases, ATP-sensitive K+-channels, voltage-dependent L-type Ca2+-channels, Cl−-channels and Na+/K+/2Cl−-cotransporters, are involved in the generation of stage-specific pHi- and Vmem-gradients. Since bioelectrical phenomena are known to affect, e.g., cytoskeletal dynamics, such gradients are supposed to serve as electrochemical prepatterns that control planar cell polarity and guide gradual changes in cytoskeletal organisation. In the FCE, a particular arrangement of cytoskeletal elements is involved in shaping the follicle [42, 43, 62], and stage-specific correlations with pHi- and Vmem-gradients can be observed (unpublished results). Therefore, manipulating the electrochemical gradients and analysing related changes in microfilament and microtubule patterns will help to understand the influence of bioelectrical cues during development (I. Weiß and J. Bohrmann, manuscript in prep.).
Methods
Preparation of Drosophila follicles
Oregon R (wild-type) Drosophila melanogaster were reared at 20–23 °C on standard medium with additional fresh yeast. 2–3 days old females were killed by crushing the head and thorax with tweezers without anaesthesia. The ovaries were dissected and single follicles of all stages (S1–14) were isolated. The preparations were carried out in R-14 medium [63] which is best suited for in-vitro culture of Drosophila follicles [64].
Analysis of intracellular pH
Intracellular pH (pHi) of FC was analysed using the pH-sensitive fluorescent dye CFDA (5-carboxyfluorescein diacetate, acetoxymethyl ester; 5-CFDA,AM; Molecular Probes/Thermo Fisher Scientific, USA) [27, 32]. Relative fluorescence intensities were stated, i. e. lower fluorescence intensity indicates lower pHi (more acidic) while higher fluorescence intensity indicates higher pHi (more alkaline). Living follicles were incubated for 20 or 60 min, depending on the type of experiment (see below), in R-14 medium containing 4 μM CFDA (dissolved in dimethyl sulfoxide; DMSO). For controls, see Additional file 1: Fig. S3.
Analysis of membrane potential
To analyse membrane potentials (Vmem) of FC, the voltage-sensitive fluorescent dye DiBAC (bis-(1,3-dibutylbarbituric acid) trimethine oxonol; DiBAC4(3); Molecular Probes) was used [28, 32]. Relative fluorescence intensities were stated, i. e. lower fluorescence intensity indicates relative hyperpolarisation and higher fluorescence intensity indicates relative depolarisation. Living follicles were incubated for 20 or 60 min, depending on the type of experiment (see below), in R-14 medium containing 4 μM DiBAC (dissolved in 70% ethanol).
Fluorescence microscopy and optical sectioning
To investigate pHi and Vmem as well as their changes in detail, two types of experiments were performed. ImageJ (NIH, USA) was used to generate pseudocolour images.
WFM-experiment
Groups of four to ten follicles were imaged for 60 min during staining in covered glass block dishes on a Zeiss Axiovert 200 wide-field fluorescence microscope (WFM), equipped with a Hamamatsu Orca ER camera, using a × 5 objective and a × 1.6 optovar. An image was taken every 5 min. Settings and exposure time remained unchanged.
SIM-experiment
Single follicles were imaged in R-14 medium after staining for 20 min on a Zeiss AxioImager.M2 structured-illumination microscope (SIM), equipped with a Zeiss ApoTome and a Zeiss AxioCamMRm camera, using a × 20 objective. Median optical sections were produced as shown in Fig. 2 a.
Inhibition of ion-transport mechanisms
To compensate for the variability observed between different flies, all S10b-follicles of a single fly (approximately 10–20 follicles) were divided into a control group and an experimental group. Inhibition and staining of living follicles, using either CFDA or DiBAC, were done simultaneously for 20 or 60 min, depending on the type of experiment, in R-14 medium containing the respective inhibitor and the fluorescent probe.
The following inhibitors of ion-transport mechanisms were used: Na+/H+-exchangers (NHE) and amiloride-sensitive Na+-channels were blocked with amiloride (Sigma-Aldrich, Germany; 10 μM; dissolved in DMSO), V-ATPases with bafilomycin A1 (Sigma-Aldrich; 160 nM; dissolved in DMSO), ATP-sensitive K+-channels with glibenclamide (Biomol, Germany; 100 μM; dissolved in DMSO), voltage-dependent L-type Ca2+-channels with verapamil-HCl (Sigma-Aldrich; 50 μM; dissolved in ethanol), Cl−-channels with 9-anthroic acid (Sigma-Aldrich; 100 μM; dissolved in ethanol) and Na+/K+/2Cl−-cotransporters with furosemide (Sigma-Aldrich; 1 mM; dissolved in DMSO). Control experiments were performed in R-14 medium containing 0.1–1% v/v ethanol or DMSO, respectively, without the inhibitor.
Quantitative analysis of fluorescence intensities of CFDA and DiBAC
The original grey-scale images were used to measure the fluorescence intensities in the FCE with ImageJ (“mean grey value”).
WFM-experiment
WFM-images of each point of time were evaluated by measuring the columnar FCE of every single follicle. The mean grey values of the experimental group and the control group, respectively, were averaged (all follicles from the same fly). To compare the long-term effects of the inhibitors on either pHi or Vmem, the averaged values of each point of time were summed up and normalised. Three experiments for each inhibitor were included in this evaluation (mean intensity ratio).
SIM-experiment
SIM-images were used to measure individual regions of different types of FC: cFC, mbFC, pFC, dFC and vFC (see Fig. 1 a). All mean grey values of each FC type of the experimental group and the control group, respectively, were averaged for comparative analysis (all follicles from the same fly). For each inhibitor, at least five repetitions of the experiment were performed. The normalised values of the single experiments were merged into one evaluation (relative intensity).
Multiple t-tests with Holm-Sidak correction, Microsoft Excel and GraphPad Prism were used for statistical analysis. Graphpad Prism was also used for representation of the data.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- aFC:
-
anterior follicle cells
- a-p:
-
antero-posterior
- cFC:
-
centripetal follicle cells
- CFDA :
-
5-carboxyfluorescein diacetate, acetoxymethyl ester
- DiBAC:
-
bis-(1,3-dibutylbarbituric acid) trimethine oxonol
- DIC:
-
differential-interference contrast
- DMSO:
-
dimethyl sulfoxide
- d-v:
-
dorso-ventral
- FC:
-
follicle cells
- FCE:
-
follicle-cell epithelium
- mbFC:
-
mainbody follicle cells
- NC:
-
nurse cells
- NHE:
-
Na+/H+-exchangers
- Oo:
-
oocyte
- pFC:
-
posterior follicle cells
- pHi :
-
intracellular pH
- S:
-
stage
- SIM:
-
structured-illumination microscopy
- Vmem :
-
membrane potential
- WFM:
-
wide-field microscopy
References
McLaughlin KA, Levin M. Bioelectric signaling in regeneration: mechanisms of ionic controls of growth and form. Dev Biol. 2018;433:177–89.
Pietak A, Levin M. Bioelectrical control of positional information in development and regeneration: a review of conceptual and computational advances. Prog Biophys Mol Bio. 2018;137:52–68.
Cervera J, Manzanares JA, Mafe S. Cell-cell bioelectrical interactions and local heterogeneities in genetic networks: a model for the stabilization of single-cell states and multicellular oscillations. Phys Chem Chem Phys. 2018;20:9343–54.
McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol Rev. 2005;85:943–78.
Chang F, Minc N. Electrochemical control of cell and tissue polarity. Annu Rev Cell Dev Biol. 2014;30:317–36.
Stock C, Ludwig FT, Hanley PJ, Schwab A. Roles of ion transport in control of cell motility. Compr Physiol. 2013;3:59–119.
Blackiston DJ, McLaughlin KA, Levin M. Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle. 2009;8:3527–36.
Ulmschneider B, Grillo-Hill BK, Benitez M, Azimova DR, Barber DL, Nystul TG. Increased intracellular pH is necessary for adult epithelial and embryonic stem cell differentiation. J Cell Biol. 2016;215:345–55.
Amith SR, Wilkinson JM, Fliegel L. Na+/H+ exchanger NHE1 regulation modulates metastatic potential and epithelial-mesenchymal transition of triple-negative breast cancer cells. Oncotarget. 2016;7:21091–113.
Tatapudy S, Aloisio F, Barber D, Nystul T. Cell fate decisions: emerging roles for metabolic signals and cell morphology. EMBO Rep. 2017;18:2105–18.
Putney LK, Barber DL. Na-H exchange-dependent increase in intracellular pH times G2/M entry and transition. J Biol Chem. 2003;278:44645–9.
Musgrove E, Seaman M, Hedley D. Relationship between cytoplasmic pH and proliferation during exponential growth and cellular quiescence. Exp Cell Res. 1987;172:65–75.
Aw S, Koster JC, Pearson W, Nichols CG, Shi N-Q, Carneiro K, Levin M. The ATP-sensitive K+-channel (KATP) controls early left-right patterning in Xenopus and chick embryos. Dev Biol. 2010;346:39–53.
Beane WS, Morokuma J, Adams DS, Levin M. A chemical genetics approach reveals H,K-ATPase-mediated membrane voltage is required for planarian head regeneration. Chem Biol. 2011;18:77–89.
Inaba M, Yamanaka H, Kondo S. Pigment pattern formation by contact-dependent depolarization. Science. 2012;335:677.
Simons M, Gault WJ, Gotthardt D, Rohatgi R, Klein TJ, Shao Y, Lee H-J, Wu A-L, Fang Y, Satlin LM, Dow JT, Chen J, Zheng J, Boutros M, Mlodzik M. Electrochemical cues regulate assembly of the frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nat Cell Biol. 2009;11:286–94.
Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671–7.
White KA, Grillo-Hill BK, Barber DL. Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. J Cell Sci. 2017;130:663–9.
Yang M, Brackenbury WJ. Membrane potential and cancer progression. Front Physiol. 2013;4:185.
Harguindey S, Reshkin SJ, Orive G, Arranz JL, Anitua E. Growth and trophic factors, pH and the Na+/H+ exchanger in Alzheimer's disease, other neurodegenerative diseases and cancer: new therapeutic possibilities and potential dangers. Curr Alzheimer Res. 2007;4:53–65.
Levin M, Pezzulo G, Finkelstein JM. Endogenous bioelectric signaling networks: exploiting voltage gradients for control of growth and form. Annu Rev Biomed Eng. 2017;19:353–87.
Levin M. Endogenous bioelectrical networks store non-genetic patterning information during development and regeneration. J Physiol. 2014;592:2295–305.
Adams DS, Levin M. Endogenous voltage gradients as mediators of cell-cell communication: strategies for investigating bioelectrical signals during pattern formation. Cell Tissue Res. 2013;352:95–122.
FitzHarris G, Baltz JM. Granulosa cells regulate intracellular pH of the murine growing oocyte via gap junctions: development of independent homeostasis during oocyte growth. Development. 2006;133:591–9.
Mathews J, Levin M. Gap junctional signaling in pattern regulation: physiological network connectivity instructs growth and form. Dev Neurobiol. 2017;77:643–73.
Cervera J, Pietak A, Levin M, Mafe S. Bioelectrical coupling in multicellular domains regulated by gap junctions: a conceptual approach. Bioelectrochemistry. 2018;123:45–61.
Han J, Burgess K. Fluorescent indicators for intracellular pH. Chem Rev. 2010;110:2709–28.
Adams DS, Levin M. Measuring resting membrane potential using the fluorescent voltage reporters DiBAC4(3) and CC2-DMPE. Cold Spring Harb Protoc. 2012;(4):459–64.
Overall R, Jaffe LF. Patterns of ionic current through Drosophila follicles and eggs. Dev Biol. 1985;108:102–19.
Bohrmann J, Dorn A, Sander K, Gutzeit H. The extracellular electrical current pattern and its variability in vitellogenic Drosophila follicles. J Cell Sci. 1986;81:189–206.
Bohrmann J, Huebner E, Sander K, Gutzeit H. Intracellular electrical potential measurements in Drosophila follicles. J Cell Sci. 1986;81:207–21.
Krüger J, Bohrmann J. Bioelectric patterning during oogenesis: stage-specific distribution of membrane potentials, intracellular pH and ion-transport mechanisms in Drosophila ovarian follicles. BMC Dev Biol. 2015;15:1.
Lautemann J, Bohrmann J. Relating proton pumps with gap junctions: colocalization of ductin, the channel-forming subunit c of V-ATPase, with subunit a and with innexins 2 and 3 during Drosophila oogenesis. BMC Dev Biol. 2016;16:24.
Sun YA, Wyman RJ. Reevaluation of electrophoresis in the Drosophila egg chamber. Dev Biol. 1993;155:206–15.
Munley SM, Kinzeler S, Lizzano R, Woodruff RI. Fractional contribution of major ions to the membrane potential of Drosophila melanogaster oocytes. Arch Insect Biochem. 2009;70:230–43.
Bohrmann J. Potassium uptake into Drosophila ovarian follicles: relevance to physiological and developmental processes. J Insect Physiol. 1991;37:937–46.
Mahowald AP, Kambysellis MP. Oogenesis. In: Ashburner M, Wright TRF, editors. Genetics and biology of Drosophila. New York: Academic Press; 1980. p. 141–224.
González-Reyes A, St Johnston D. Patterning of the follicle cell epithelium along the anterior-posterior axis during Drosophila oogenesis. Development. 1998;125:2837–46.
Wu X, Tanwar PS, Raftery LA. Drosophila follicle cells: morphogenesis in an eggshell. Semin Cell Dev Biol. 2008;19:271–82.
Cavaliere V, Bernardi F, Romani P, Duchi S, Gargiulo G. Building up the Drosophila eggshell: first of all the eggshell genes must be transcribed. Dev Dyn. 2008;237:2061–72.
Riechmann V, Ephrussi A. Axis formation during Drosophila oogenesis. Curr Opin Genet Dev. 2001;11:374–83.
Gates J. Drosophila egg chamber elongation: insights into how tissues and organs are shaped. Fly. 2012;6:213–27.
Viktorinová I, Henry I, Tomancak P. Epithelial rotation is preceded by planar symmetry breaking of actomyosin and protects epithelial tissue from cell deformations. PLoS Genetic. 2017;13:e1007107.
Bohrmann J, Braun B. Na,K-ATPase and V-ATPase in ovarian follicles of Drosophila melanogaster. Biol Cell. 1999;91:85–98.
Bohrmann J, Zimmermann J. Gap junctions in the ovary of Drosophila melanogaster: localization of innexins 1, 2, 3 and 4 and evidence for intercellular communication via innexin-2 containing channels. BMC Dev Biol. 2008;8:111.
Bohrmann J, Haas-Assenbaum A. Gap junctions in ovarian follicles of Drosophila melanogaster: inhibition and promotion of dye-coupling between oocyte and follicle cells. Cell Tissue Res. 1993;273:163–73.
Giannakou ME, Dow JA. Characterization of the Drosophila melanogaster alkali-metal/proton exchanger (NHE) gene family. J Exp Biol. 2001;204:3703–16.
Bohrmann J, Heinrich UR. Localisation of potassium pumps in Drosophila ovarian follicles. Zygote. 1994;2:189–99.
Darboux I, Lingueglia E, Champigny G, Coscoy S, Barbry P, Lazdunski M. dGNaC1, a gonad-specific amiloride-sensitive Na+ channel. J Biol Chem. 1998;273:9424–9.
Robinson SW, Herzyk P, Dow JAT, Leader DP. FlyAtlas: database of gene expression in the tissues of Drosophila melanogaster. Nucleic Acids Res. 2013;41:D744–50.
Wieczorek H, Putzenlechner M, Zeiske W, Klein U. A vacuolar-type proton pump energizes K+/H+ antiport in an animal plasma membrane. J Biol Chem. 1991;266:15340–7.
Adler S, Fraley DS. Potassium and intracellular pH. Kidney Int. 1977;11:433–42.
Sherwood AC, John-Alder K, Sanders MM. Characterization of chloride uptake in Drosophila kc cells. J Cell Physiol. 1988;136:500–6.
Ianowski JP, O'Donnell MJ. Basolateral ion transport mechanisms during fluid secretion by Drosophila Malpighian tubules: Na+ recycling, Na+:K+:2Cl− cotransport and Cl− conductance. J Exp Biol. 2004;207:2599–609.
Hoffmann EK. Anion exchange and anion-cation co-transport systems in mammalian cells. Philos Trans Royal Soc. 1982;299:519–35.
Benitez M, Tatapudy S, Liu Y, Barber DL, Nystul TG. Drosophila anion exchanger 2 is required for proper ovary development and oogenesis. Dev Biol. 2019. https://doi.org/10.1016/j.ydbio.2019.04.018.
Putney LK, Denker SP, Barber DL. The changing face of the Na+/H+ exchanger, NHE1: structure, regulation, and cellular actions. Annu Rev Pharmacol Toxicol. 2002;42:527–52.
Donowitz M, Li X. Regulatory binding partners and complexes of NHE3. Physiol Rev. 2007;87:825–72.
Wang Y, Telfer WH. Cyclic-AMP-induced water uptake in a moth ovary: inhibition by bafilomycin and anthracene-9-carboxylic acid. J Exp Biol. 1998;201:1627–35.
Harvey WR, Wieczorek H. Animal plasma membrane energization by chemiosmotic H+ V-ATPases. J Exp Biol. 1997;200:203–16.
Clapham DE. Calcium signaling. Cell. 2007;131:1047–58.
Gutzeit HO. Organization and in vitro activity of microfilament bundles associated with the basement membrane of Drosophila follicles. Acta Histochem. 1992; Suppl. XLI:201–10.
Robb JA. Maintenance of imaginal discs of Drosophila melanogaster in chemically defined media. J Cell Biol. 1969;41:876–85.
Bohrmann J. In vitro culture of Drosophila ovarian follicles: the influence of different media on development, RNA synthesis, protein synthesis and potassium uptake. Roux Arch Dev Biol. 1991;199:315–26.
Acknowledgements
We are indepted to Susanne Schotthöfer for technical assistance.
Authors´ contributions
IW carried out the experiments and analysed the data under the supervision of JB. JB conceived the study and reviewed the data. Both authors wrote the manuscript and read and approved the final version.
Funding
Financial support by RWTH Aachen University is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1. Development of pHi-gradients in the FCE during S8–12 (CFDA; SIM). Examples corresponding to Fig. 1b. Figure S2. Development of Vmem-gradients in the FCE during S8–12 (DiBAC; SIM). Examples corresponding to Fig. 2b. Figure S3. Controls for pHi (CFDA; WFM). Examples of S10b follicles incubated for 60 min in R-14 medium pH 5.5 (hydrochloric acid) and pH 8.0 (sodium hydroxide), respectively. (PPTX 9873 kb)
Additional file 2:
Table S1. Development of pHi-gradients in the FCE during S8–12 (CFDA; SIM). Numerical values corresponding to Fig. 1 c. Table S2. Development of Vmem-gradients in the FCE during S8–12 (DiBAC; SIM). Numerical values corresponding to Fig. 2 c. Table S3. Inhibitors of ion-transport mechanisms exert influence on the pHi in the FCE during S10b (WFM-experiment; CFDA). Numerical values corresponding to Fig. 3a. Table S4. Inhibitors of ion-transport mechanisms exert influence on the Vmem in the FCE during S10b (WFM-experiment; DiBAC). Numerical values corresponding to Fig. 6a. Table S5. The a-p and d-v pHi-gradients in the FCE are affected by all inhibitors in S10b (SIM-experiment; CFDA). Numerical values corresponding to Figs. 4b and 5a. Table S6. The a-p Vmem-gradient in the FCE is affected by most inhibitors in S10b. Some inhibitors exert influence on the d-v Vmem-gradient (SIM-experiment; DiBAC). Numerical values corresponding to Figs. 7b and 8. (PPTX 78 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Weiß, I., Bohrmann, J. Electrochemical patterns during Drosophila oogenesis: ion-transport mechanisms generate stage-specific gradients of pH and membrane potential in the follicle-cell epithelium. BMC Dev Biol 19, 12 (2019). https://doi.org/10.1186/s12861-019-0192-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12861-019-0192-x